Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- AOR
adjusted odds ratio
- CI
confidence interval
- HBV
hepatitis viral B
- HCC
hepatocellular carcinoma
- HCV
hepatitis viral C
- HR
hazard ratio
- IGF
insulin growth factor
- IGFBP
IGF binding protein
- IGF1R
IGF1 receptor
- IL‐6
interleukin‐6
- JNK1
c‐jun amino terminal kinase 1
- LPS
lipopolysaccharide
- MAPK
mitogen‐activated protein kinase
- MS
metabolic syndrome
- mTOR
mammalian target of rapamycin
- NAFLD
nonalcoholic fatty liver disease
- NF‐κB
nuclear factor‐κB
- OR
odds ratio
- ROS
reactive oxygen species
- TNF‐α
tumor necrosis factor‐α
- VEGF
vascular endothelial growth factor
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer‐related deaths worldwide. HCC develops mainly on a background of chronic liver disease, with an incidence rate of 2% to 4% per year in cirrhotic patients. The main causative agents of the underlying liver disease and HCC are viral infections by hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcohol abuse. Recently, new risk factors for HCC have emerged in developed countries, including nonalcoholic fatty liver disease (NAFLD) caused by metabolic syndrome (MS). Among the different components of the MS, type 2 diabetes, characterized by hyperglycemia, hyperinsulinemia, and insulin resistance, has been identified as an independent risk factor for HCC. The aim of this brief review is to describe the impact of insulin resistance and type 2 diabetes on the incidence of HCC, the underlying molecular defect linked to tumor development, and potential future therapeutic strategies identified by analysis of epidemiological studies and in vitro experiments.
Epidemiological Evidence
Epidemiological studies have revealed that type 2 diabetes plays an independent role in HCC development (Table 1). Diabetes has been associated with a 2‐ to 3‐fold increase in the risk for HCC occurrence; this risk did not differ between ethnic populations in the United States. The risk is even higher with duration of diabetes, with an odds ratio (OR) of 2.2 in patients having a longer than 10‐year duration (Table 1). New‐onset diabetes has also been significantly associated with higher incidence of HCC. Strong evidence also describes a synergistic interaction between type 2 diabetes and other risk factors for HCC, such as chronic HCV and HBV infection and alcohol abuse. In a prospective cohort of patients with HCV‐related cirrhosis, insulin resistance was also an independent risk factor for HCC development.1 Finally, a subset of HCC related to MS developed on noncirrhotic liver, suggesting a direct oncogenic mechanism.2
Table 1.
Risk for HCC and Type 2 Diabetes
| Authors | Association | Description of the Study |
|---|---|---|
|
Fu et al.11
Aliment Pharmacol Therapy 2015 |
HR 1798 (95% CI 1.194–2.707) |
Cohort study New‐onset diabetes, patients with HBV |
|
Raff et al.12
J Clin Transl Hepatol 2015 |
HR 3.0 (95% CI 1.3–6.9) |
Cohort study Patients with NAFLD or alcoholic liver disease |
|
Setiawan et al.13
J Natl Cancer Inst 2014 |
RR 2.62 (95% CI 2.13–3.23) |
Cohort study Increased risk in all ethnic groups |
|
Arase et al.14
Hepatology 2013 |
HR 1.73 (95% CI 1.3–2.3) |
Cohort study Patients with HCV |
|
Turati et al.15
Br J Cancer 2013 |
OR 4.33 (95% CI 1.89–9.86) | Case–control study |
|
Koh et al.16
Br J cancer 2013 |
HR 2.14 (95% CI 1.69–2.71) | Cohort study |
|
Schlesinger et al.17
Ann Oncol 2013 |
RR 2.17 (95% CI 1.36–3.47) | Cohort study |
|
Atchison et al.18
Int J Cancer 2011 |
RR 1.95 (95% CI 1.82–20.9) | Cohort study |
|
Hassan et al.19
Cancer 2010 |
AOR 4.2 (95% CI 3.0–5.9) |
Case–control study Decreased risk for HCC using metformin |
|
Veldt BJ et al.20
Hepatology 2008 |
HR 3.28 (95% CI 1.35–7.97) |
Cohort study Patients with HCV |
|
Rousseau et al.21
Int J cancer 2006 |
OR 3.1 (95% CI 1.1–8.8) | Case–control study |
|
Davila et al.22
Gut 2005 |
OR 3.8 (95% CI 2.74–3.46) |
Case–control study Increased risk regardless of other HCC risk factors |
|
El‐Serag et al.23
Gastroenterology 2004 |
HR 2.16 (95% CI 1.86–2.52) |
Cohort study Increased risk for chronic liver diseases |
|
Hassan et al.24
Hepatology 2002 |
OR 4.3 (95% CI 1.9–9.9) |
Case–control study Synergism with alcohol and viral hepatitis |
AOR, adjusted odds ratio; CI, confidence interval; HR, hazard ratio.
Physiopathology and Molecular Pathways
In cellulo models, as well as mouse models, have helped to dissect the molecular pathway involved in liver carcinogenesis caused by insulin resistance and obesity (Fig. 1).
Figure 1.
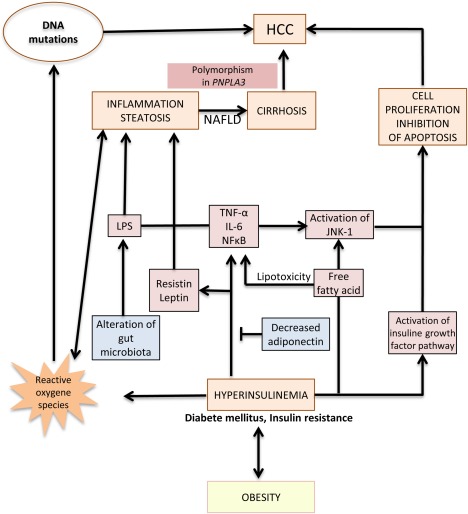
Pathogenesis of HCC developed on NAFLD. Hyperinsulinemia and obesity lead to NAFLD, cirrhosis, and finally, HCC development. However, a subset of NAFLD‐related HCC could also develop on noncirrhotic liver. Chronic liver inflammation, alteration of gut microbiota, lipotoxicity of free fatty acid, production of ROS, and activation of the IGF pathway and the JNK1 pathway promote NAFLD and HCC development. A polymorphism of PNPLA3 (Patatin‐like phospholipase domain containing 3, rs738409 c444C>G, I148M minor allele) increased the risk for NAFLD‐related cirrhosis and NAFLD‐related HCC. Abbreviation: LPS, lipopolysaccharide.
Hyperinsulinemia and obesity promote chronic liver inflammation and steatosis by the release of proinflammatory cytokines such as tumor necrosis factor‐α (TNF‐α), interleukin‐6 (IL‐6), and nuclear factor‐κB (NF‐κB) (Fig. 1). This inflammatory state is a conjunction of the action of hepatocytes, Kupffer cells, and adipocytes.3 Moreover, MS increases the circulating level of leptin, a proinflammatory cytokine, but also decreases the production of adiponectin, an anti‐inflammatory polypeptide that also inhibits angiogenesis in animal models.4 Hyperglycemia leads to free fatty acid release that induces accumulation of reactive oxygen species (ROS) (Fig. 1). ROS promote carcinogenesis through uncontrolled liver inflammation, steatosis, and cell proliferation, and may induce cancer‐promoting mutations in tumor suppressor gene TP53.5 Moreover, free fatty acid production activates c‐jun amino terminal kinase 1 (JNK1) that induces cellular proliferation and inhibition of apoptosis.4
In human HCC, a subset of tumors have been shown to harbor activation of the insulin growth factor (IGF) pathway.6 IGF1 induces phosphorylation of insulin receptor substrate 1, leading to activation of AKT/mammalian target of rapamycin (MTOR) and mitogen‐activated protein kinase (MAPK) pathways that inhibit apoptosis and promote cell proliferation (Fig. 2).7 Upregulation of the IGF pathway by hyperinsulinemia is the consequence of overexpression of ligands such as IGF1 and aberrant expression of fetal IGF2.6 Concomitant downregulation of negative regulators of the pathway, such as the suppressor of cytokine signaling protein, is also observed in tumors, along with decreased production of IGF binding protein (IGFBP) type 1 and IGFBP2 that increases IGF1 bioavailability (Fig. 2). Consequently, the IGF axis is a key signaling pathway involved in liver carcinogenesis related to type 2 diabetes and obesity. Moreover, each human HCC is a unique combination of somatic genetic alterations, with a mean number of 40 to 60 mutations per tumor in the coding sequence.8 Somatic mutations in driver genes were also closely related to risk factors and etiology: TP53 mutations with HBV infection, the TP53 R249S mutation with aflatoxin B1 exposure, and ARID1A and CTNNB1 mutations with alcohol consumption.8 However, no clear association between genetic defects or nucleotide signatures and type 2 diabetes and MS have thus far been identified.
Figure 2.
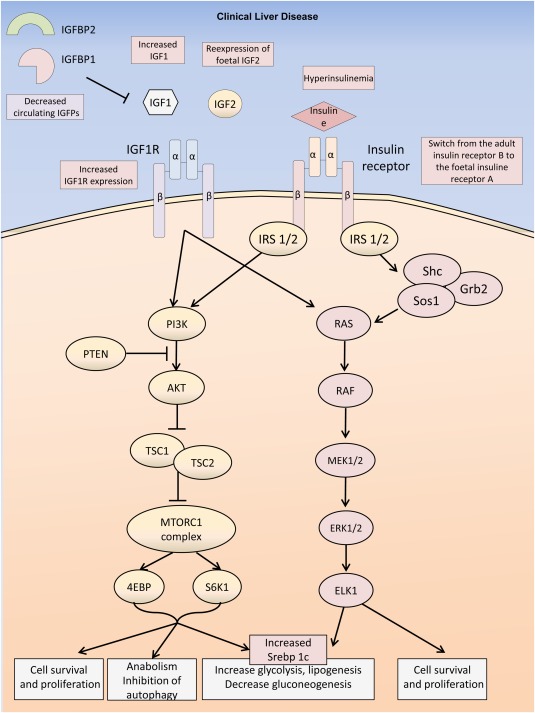
Insulin signaling pathways in HCC. Different mechanisms (decreased circulating level of IGFBPs, increased circulating level of IGF1, reexpression of the foetal IGF2, overexpression of IGF1R, hyperinsulinemia, reexpression of the foetal form of the insulin receptor (IR) type A instead of the adult form IRB) lead to the activation of the IGF1 receptor (IGF1R) and insulin receptor signaling pathways. Most of the metabolic and proliferative consequences are mediated through the downstream activation of Akt/Mtor and ras/raf/map kinase signaling.
Insulin Resistance and Liver Carcinogenesis: A New Therapeutic Strategy
Human epidemiological studies have shown that statins confer protection against development of steatohepatitis, fibrosis, and HCC, mainly in patients with diabetes and obesity. El‐Serag et al.9 compared 1303 patients with diabetes and 5212 control subjects, and identified a 25% reduction in the risk for development of HCC in patients with diabetes using statins. Statins have no direct effect on insulin resistance, but the antitumor action could be explained by an anti‐inflammatory property mediated by inhibition of the JNK and MAPK pathways.
Moreover, in epidemiological studies performed on a general population, HBV‐ or HCV‐infected patients showed that metformin intake was also associated with a reduced incidence of HCC in a time‐ and dose‐dependent manner.10 Metabolic and antiangiogenic effects have been proposed as potential antitumor mechanisms. Metformin activated AMP‐activated protein kinase that inhibited the mTOR complex and fostered cell cycle arrest in vitro. Metformin also exhibited antiangiogenic effects through downregulation of vascular endothelial growth factor.4 Moreover, different signaling pathways such as Wnt/ß‐catenin or transcription factors such as Myc proto‐oncogene protein were shut down by metformin in cellulo.
Finally, an anti‐IGF1 receptor (anti‐IGF1R) antibody, cixutumumab, has been tested in patients with advanced HCC, as well as a small tyrosine kinase inhibitor, OSI‐906, targeting IGF1R/IR. However, they demonstrated no antitumor activity and have metabolic toxicities. An antibody against IGF2 is currently tested in solid cancers including HCC.7
In conclusion, type 2 diabetes and obesity are key players in NAFLD and HCC development. Chronic liver inflammation, modulation of the immune response, lipotoxicity, production of ROS, and activation of the IGF pathway are some of the mechanisms that promote tumorigenesis in this setting. Finally, epidemiological and in vitro studies suggested a chemopreventive effect of statin and metformin on HCC development. This will require confirmation by randomized controlled trials.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Nkontchou G, Bastard JP, Ziol M, Aout M, Cosson E, Ganne‐Carrie N, et al. Insulin resistance, serum leptin, and adiponectin levels and outcomes of viral hepatitis C cirrhosis. J Hepatol 2010;53:827–833. [DOI] [PubMed] [Google Scholar]
- 2. Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology 2009;49:851–859. [DOI] [PubMed] [Google Scholar]
- 3. Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL‐6 and TNF expression. Cell 2010;140:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 2013;10:656–665. [DOI] [PubMed] [Google Scholar]
- 5. He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, et al. Hepatocyte IKKbeta/NF‐kappaB inhibits tumor promotion and progression by preventing oxidative stress‐driven STAT3 activation. Cancer Cell 2010;17:286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tovar V, Alsinet C, Villanueva A, Hoshida Y, Chiang DY, Sole M, et al. IGF activation in a molecular subclass of hepatocellular carcinoma and pre‐clinical efficacy of IGF‐1R blockage. J Hepatol 2010;52:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chettouh H, Lequoy M, Fartoux L, Vigouroux C, Desbois‐Mouthon C. Hyperinsulinaemia and insulin signalling in the pathogenesis and the clinical course of hepatocellular carcinoma. Liver Int 2015;35:2203–2217. [DOI] [PubMed] [Google Scholar]
- 8. Zucman‐Rossi J, Villanueva A, Nault JC, Llovet JM. The genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 2015;149:1226–1239.e4. [DOI] [PubMed] [Google Scholar]
- 9. El‐Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 2009;136:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose‐dependent manner: population‐based and in vitro studies. Gut 2013;62:606–615. [DOI] [PubMed] [Google Scholar]
- 11. SC Fu, YW Huang, TC Wang, JT Hu, DS Chen, SS Yang. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with new onset diabetes: a nationwide cohort study. Aliment Pharmacol Ther 2015;41:1200–1209. [DOI] [PubMed] [Google Scholar]
- 12. EJ Raff, D Kakati, JR Bloomer, M Shoreibah, K Rasheed, AK Singal. Diabetes Mellitus Predicts Occurrence of Cirrhosis and Hepatocellular Cancer in Alcoholic Liver and Non-alcoholic Fatty Liver Diseases. J Clin Transl Hepatol 2015;3:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. VW Setiawan, BY Hernandez, SC Lu, DO Stram, LR Wilkens, L Le Marchand, et al. Diabetes and racial/ethnic differences in hepatocellular carcinoma risk: the multiethnic cohort. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Y Arase, M Kobayashi, F Suzuki, Y Suzuki, Y Kawamura, N Akuta, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology 2013;57:964–973. [DOI] [PubMed] [Google Scholar]
- 15. F Turati, R Talamini, C Pelucchi, J Polesel, S Franceschi, A Crispo, et al. Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer 2013;108:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. WP Koh, R Wang, A Jin, MC Yu, JM Yuan. Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer 2013;108:1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. S Schlesinger, K Aleksandrova, T Pischon, M Jenab, V Fedirko, E Trepo, et al. Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol 2013;24:2449–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. EA Atchison, G Gridley, JD Carreon, MF Leitzmann, KA McGlynn. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer 2011;128:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. MM Hassan, SA Curley, D Li, A Kaseb, M Davila, EK Abdalla, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer 2010;116:1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. BJ Veldt, W Chen, EJ Heathcote, H Wedemeyer, J Reichen, WP Hofmann, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology 2008;47:1856–1862. [DOI] [PubMed] [Google Scholar]
- 21. MC Rousseau, ME Parent, MN Pollak, J Siemiatycki. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal, Canada. Int J Cancer 2006;118:2105–2109. [DOI] [PubMed] [Google Scholar]
- 22. JA Davila, RO Morgan, Y Shaib, KA McGlynn, HB El-Serag. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut 2005;54:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. HB El-Serag, T Tran, JE Everhart. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004;126:460–468. [DOI] [PubMed] [Google Scholar]
- 24. MM Hassan, LY Hwang, CJ Hatten, M Swaim, D Li, JL Abbruzzese, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002;36:1206–1213. [DOI] [PubMed] [Google Scholar]


