Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- AEAT
acyl‐coenzyme a‐ethanol O‐acyltransferase
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CDT
carbohydrate‐deficient transferrin
- EtG
ethyl‐glucuronide
- EtS
ethyl sulfate
- FAEE
fatty acid ethyl esters
- GGT
gamma-glutamyl transpeptidase
- GluA
glucuronic acid
- 5HIAA
5‐hydroxyindol‐3‐acetic acid
- 5HTOL
5‐hydroxytriptophol
- LFT
liver function tests
- LT
liver transplantation
- OHdet
ethanol detecto
- PEth
phosphatidylethanol
- PLD
phospholipase D
- UDP‐GT
uridine diphosphate
Importance of Abstinence in Patients With Alcoholic Liver Disease
Alcoholic liver disease (ALD) represents half of the cases of liver cirrhosis, making it the dominant cause of advanced liver disease globally. Moreover, ALD is one of the main indications for liver transplantation (LT) in both Europe and the United States. To date, the most effective therapeutic approach in patients with ALD has been prolonged alcohol abstinence.1 Few reports have investigated the efficacy of targeted therapies in patients with ALD. Most ongoing clinical trials are focused on alcoholic hepatitis, which is characterized not only by fibrosis, but also by profound liver failure and cholestasis. In this severe condition, prednisolone improves only 1‐month survival, whereas the long‐term survival is heavily influenced by alcohol relapse.2 In patients with compensated ALD, few attempts have been made to attenuate disease progression. Most approaches have focused on antioxidant and antifibrogenic drugs (i.e., angiotensin blockers), but the results have mostly been negative. Establishing total abstinence from alcohol consumption leads to better clinical outcomes for all forms of ALD and is the most important factor determining long‐term survival in patients with more severe forms such as alcoholic hepatitis and/or alcohol‐related cirrhosis.2, 3 Few studies have assessed the efficacy of motivational and/or pharmacological interventions to promote abstinence in patients with ALD. In addition to counseling, anticraving drugs such as baclofen (a gamma‐aminobutyric acid B agonist) have been shown to be effective and safe in patients with ALD.4 In transplant patients with ALD, alcohol relapse increases the risk for cirrhosis and graft loss, leading to early mortality.
Existing Biomarkers to Estimate Alcohol Intake
The role of an array of alcohol biomarkers has been extensively studied in alcoholic patients,5, 6 although their usefulness in patients with ALD is largely unknown.7 There is no single ideal biomarker (Fig. 1) because all of them have pros and cons (Table 1). The first tool for assessing alcohol misuse is self‐reported questionnaires [i.e., CAGE, Alcohol Use Disorders Identification Test (AUDIT‐C)]; however, many patients tend to underreport, particularly in the pre‐ or post‐LT interval, for fear of reprisal by the transplant program. Other patients with ALD with end‐stage liver disease may have cognitive impairment during hospitalization, which would impede their ability to accurately complete the questionnaires. Most studies assessing biomarkers compare their results against data from questionnaires, thereby limiting the assessment of the validity of the biomarker in question.
Figure 1.
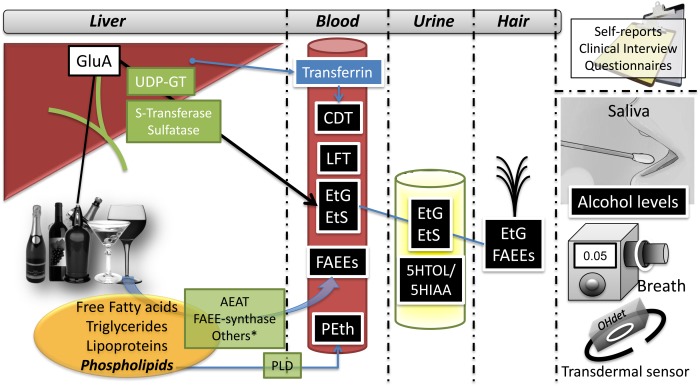
Sources and biological samples of biomarkers of alcohol consumption.
*Refers to other enzymes with FAEE‐synthase activity: pancreatic lipase, lipoprotein lipase, glutathione transferase. Abbreviations: AEAT, acyl‐coenzyme a‐ethanol O‐acyltransferase; CDT, carbohydrate‐deficient transferrin; EtS, ethyl sulfate; GluA, glucuronic acid; LFT, liver function tests; PLD, phospholipase D; UDP‐GT, uridine diphosphate.
Table 1.
Advantages and Disadvantages of Alcohol Biomarkers
| Advantages | Disadvantages | |
|---|---|---|
| Self‐report, clinical interviews, questionnaires | Inexpensive, easy, and quick | Dependent on patient candor |
| Routine laboratory parameters | ||
|
Liver function tests (ALT, AST, GGT) Mean corpuscular volume |
Inexpensive, widely available AST/ALT ratio is useful |
Nonspecific; severe liver disease can interfere |
| Carbohydrate‐deficient transferrin | Long‐term consumption | Confounders: liver disease, smoking, low body mass index, female sex |
| Direct markers | ||
| Ethanol detection in serum, urine, body fluids | Gold standard | Short half‐life of ethanol |
| Ethanol levels in saliva | Quick and cheap | No good correlation with blood alcohol concentration |
| Ethanol breath detection | Rapid results | Only acute consumption; sensitive to breath pattern and meals |
| Transdermal alcohol sensor | Continuous monitoring | Not tested in liver disease |
| Methanol detection in blood | Measurable when ethanol is no longer available | Endogenous production |
| Indirect alcohol biomarkers | ||
| EtG |
Age, sex, ethnicity, and severity of liver disease have no influence Long‐term consumption |
Unable to detect low levels of alcohol consumption Bacterial degradation (urine) Laboratory sample Influencing factors: age, cannabis consumption, renal impairment |
| Ethyl sulfate |
Age, sex, ethnicity, and severity of liver disease have no influence No degradation by microbes |
Reduced kidney function |
| FAEE | Measured with EtG increases validity of hair analysis |
Fat production may depend on age/sex/hormones Capillary treatments/cosmetic can induce false determination |
| PEth |
Good correlation with amount consumed No influence of liver disease |
Generation postsampling in presence of ethanol (solved with dried blood spots) |
The first sets of biomarkers of alcohol abuse consist of routine laboratory parameters. These include gamma‐glutamyl transpeptidase (GGT), aspartate aminotransferase/alanine aminotransferase (AST/ALT) ratio, and mean corpuscular volume. Although these tests are relatively inexpensive and widely available, their specificity is limited. Direct alcohol biomarkers, such as the detection of ethanol in the serum, urine, breath, or bodily fluids, are considered to be the gold standard in alcohol detection. However, because alcohol is rapidly cleared from the body, these biomarkers are valid only to test for recent alcohol intake. Some other potential disadvantages to alcohol detection include the possibility of false positives, poor storage of samples, incorrect sampling, or postsampling production of ethanol because of microbes.
As a result of the narrow time window for ethanol detection, indirect alcohol biomarkers are increasingly being used. They mostly consist of the measurement of ethanol metabolites. Alcohol is metabolized by transferases in the liver and can develop ethyl‐glucuronide (EtG) or ethyl sulfate, which can be measured in blood, urine, or hair. When alcohol interacts with lipids, fatty acid ethyl esters (FAEE) and phosphatidylethanol (PEth) are generated. Alcohol ingestion can modify serotonin catabolism, which implies an increase in the 5‐hydroxytriptophol (5HTOL)/5‐hydroxyindol‐3‐acetic acid (5HIAA) ratio. Remarkably, serum PEth has a high correlation to the amount of alcohol consumption, yet the role of confounders such as age, sex, or concomitant diseases are unclear. Table 2 summarizes the main alcohol biomarkers through their characteristics, time frame of detection, and proposed cutoffs for determined circumstances. Finally, with recent technological advances, the use of wearables has been proposed (i.e., SCRAM). These devices are able to perform transdermal alcohol measurements.8 Future research should evaluate its usefulness and feasibility in patients with ALD.
Table 2.
Main Characteristics of Existing Alcohol Biomarkers
| Marker | Sample | Time Frame | Cutoff Value |
|---|---|---|---|
| CDT | Blood | 2–3 weeks | 1.7% (percentage of transferrin) Sensitivity 46%‐73%, specificity 70% |
| EtG and EtS | Hair | Up to several months | Abstinence <7 pg/mg Excessive drink >30 pg/g |
| Urine | Up to 3–5 days after complete elimination of alcohol from the body | Abstinence <0.5mg/L (sensitivity 89.2%, specificity 98.8%) Active alcohol >1mg/l | |
| Blood | Up to 48 hours | 100–250 μg/L | |
| FAEEa | Hair | 1‐month period |
Excessive intake: • >200 pg/mg (hair measuring 0–3 cm) • >400 pg/mg (hair measuring 0–6 cm) |
| PEth | Blood (capillary blood sampling and analysis on dry blood spot) | 2–4 weeks |
Abstinence: 20–150 ng/mlb
Excessive drinking: 210–800 ng/mlb |
Abbreviations: CDT, carbohydrate‐deficient transferrin; EtS, ethyl sulfate.
The sum of four FAEE: ethyl myristate, palmitate, stearate, and oleate.
Depends on the methods (liquid chromatography‐tandem mass spectrometry or high‐performance liquid chromatography).
Use of Alcohol Biomarkers in Clinical Practice
Monitoring alcohol intake theoretically could be useful in patients with earlier stages of ALD receiving counseling or anticraving therapy. However, because these patients are not commonly seen in most liver centers, no studies have assessed the role of alcohol biomarkers at this stage of the disease. The diagnosis of ALD is usually made in its advanced stages with higher rates of complications and mortality.9 In practical terms, alcohol biomarkers are mainly used in advanced ALD during transplant evaluation or while in the waiting list and, in some centers, after the LT has been made. Many of these biomarkers have been shown to be useful in the detection of recent alcohol intake, but most of them have not been extensively validated in the setting of advanced liver disease.10 Therefore, their clinical use should be considered with caution, especially when assessing patients for LT. Given that, in many transplant centers, patients with severe ALD require a period of 6 months of abstinence to be listed for an LT, many centers use alcohol biomarkers to monitor abstinence and rule out relapse. In this particular setting, the results from alcohol biomarkers have to be interpreted in conjunction with other methods to avoid false positives that can be misleading in the final decision on a patient's candidacy.
The most frequently used biomarkers in patients being evaluated for an LT or while in the waiting list are: (1) routine biochemical test (i.e., GOT and GGT), which only increase after prolonged and intense alcohol relapse and can be influenced by factors other than alcohol intake; (2) direct alcohol biomarkers, the most used of which is ethanol detection in urine and/or blood, but they capture only very recent consumption; and (3) indirect alcohol biomarkers, which are strongly recommended to be able to identify patients with alcohol intake the days before the test. The most used biomarker is EtG, which is relatively inexpensive and can capture alcohol intake in an extended window (up to 2 days in blood and 3 days in urine). Other biomarkers such as PEth are also used in some centers, but they have not been adequately studied in cirrhotic patients. The use of more sophisticated methods (i.e., EtG in the hair) could capture consumption 4 weeks before the test, but they are costly and have not been extensively validated in patients with ALD.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol 2015;62(suppl 1):S38–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Potts JR, Goubet S, Heneghan MA, Verma S. Determinants of long‐term outcome in severe alcoholic hepatitis. Aliment Pharmacol Ther 2013;38:584–595. [DOI] [PubMed] [Google Scholar]
- 3. Cuadrado A, Fabrega E, Casafont F, Pons‐Romero F. Alcohol recidivism impairs long‐term patient survival after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl 2005;11:420–426. [DOI] [PubMed] [Google Scholar]
- 4. Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol‐dependent patients with liver cirrhosis: randomised, double‐blind controlled study. Lancet 2007;370:1915–1922. [DOI] [PubMed] [Google Scholar]
- 5. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules 2015;5:1339–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wurst FM, Thon N, Yegles M, Schruck A, Preuss UW, Weinmann W. Ethanol metabolites: their role in the assessment of alcohol intake. Alcohol Clin Exp Res 2015;39:2060–2072. [DOI] [PubMed] [Google Scholar]
- 7. Allen JP, Wurst FM, Thon N, Litten RZ. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl 2013;19:369–376. [DOI] [PubMed] [Google Scholar]
- 8. Simons JS, Wills TA, Emery NN, Marks RM. Quantifying alcohol consumption: self‐report, transdermal assessment, and prediction of dependence symptoms. Addict Behav 2015;50:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011;141:1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loiselle M, Bataller R. Liver: detecting alcohol intake in patients with ALD. Nat Rev Gastroenterol Hepatol 2012;9:432–434. [DOI] [PubMed] [Google Scholar]


