Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- anti-HBs
antibody to hepatitis B surface antigen
- cccDNA
covalently closed circular DNA
- HAPs
heteroaryldihydropyrimidine
- HBcAg
hepatitis B core antigen
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- mRNA
messenger RNA
- NTCP
sodium/taurocholate cotransporting polypeptide
- pDC
plasmacytoid dendritic cell
- siRNA
small interfering RNA
- SMAC
second mitochondria‐derived activator of caspases
- TLR7
toll‐like receptor 7
Chronic hepatitis B virus (HBV) infection is now a treatable disease, with long‐term nucleoside analogue therapy attaining sustained rates of virological suppression. It, however, remains an incurable disease, with withdrawal of nucleos(t)ide analogue therapy resulting in high rates of virological relapse.1 Even achieving hepatitis B surface antigen (HBsAg) seroclearance, the ultimate treatment endpoint of chronic HBV infection, HBV remains present because of the persistence of intrahepatic covalently closed circular DNA (cccDNA),2 and liver‐related complications can still develop especially if HBsAg seroclearance occurs after age 50 years or cirrhosis is already established. New therapeutic approaches will be needed to accomplish a functional cure of chronic HBV infection, implying the achievement of HBsAg seroclearance with or without seroconversion of antibody to HBsAg (anti‐HBs). This should be best achieved as early as possible in the lifelong disease course to reduce the risk for disease complications. Functional cure is now seen as a pragmatic treatment endpoint for HBV clinical trials, although it is important to note functional cure does not equal total cure as long as cccDNA persists. As depicted in Table 1 and Figure 1, multiple clinical trials in phases 1 and 2 have commenced recently aiming at investigating different therapeutic agents to attain a functional cure of HBV.
Table 1.
New HBV Therapeutics Not Acting Through HBV Polymerase Inhibition Undergoing Clinical Trials in Humans
| Target | Name | Compounds | Sponsor | Stage of Development | Reference | |
|---|---|---|---|---|---|---|
| Viral antigen | HBV mRNA | ARC‐520 | siRNA | Arrowhead Pharmaceuticals | Phase 2 |
NCT02065336 NCT02604212 |
| ARB‐1467 | siRNA | Arbutus Biopharma | Phase 2a | NCT02631096 | ||
| GSK 3228836 | Antisense oligonucleotide | GlaxoSmithKline | Phase 1 | Company Web site | ||
| RO7020322 | Small‐molecule viral expression inhibitor | Roche | Phase 1 | NCT02604355 | ||
| Nucleocapsid assembly | NVR 3‐778 | HBV core inhibitor | Johnson & Johnson | Phase 1b |
NCT02112799 NCT02401737 |
|
| JNJ379 | Capsid assembly modulator | Johnson & Johnson | Phase 1 | NCT02662712 | ||
| GLS4 (Morphothiadin) | HAPs | HEC Pharm | Phase 2 | Company Web site | ||
| HBV entry | Myrcludex‐B | HBV pre‐S1‐derived lipopeptide affecting NTCP | Hepatera | Phase 2 | Company Web site | |
| HBsAg release | REP 2139 | Phosphorothioated oligonucleotides | Replicor Inc. | Phase 2 |
NCT02646189 NCT02565719 |
|
| GC 1102 | Recombinant hepatitis B human immunoglobulin that neutralizes HBsAg | Green Cross Corporation | Phase 2 | NCT02304315 | ||
| Immune modulation | Therapeutic vaccine | GS‐4774 | Recombinant antigen containing X, Env, Core epitopes | Gilead | Phase 2 |
NCT01943799 NCT02174276 |
| ABX‐203 | Recombinant antigen containing HBsAg and HBcAg | Abivax | Phase 2 | NCT02249988 | ||
| TG‐1050 | Nonreplicative adenovirus encoding a large fusion protein (truncated Core, modified Pol, and t wo Env domains) | Transgene | Phase 1 | NCT02428400 | ||
| INO‐1800 | DNA plasmids encoding HBsAg and HBcAg | Inovio | Phase 1 | NCT02431312 | ||
| FP‐02.2 (HepTCell) | Peptide encoding CD4+ and CD8+ epitopes | Altimmune | Phase 1 | NCT02496897 | ||
| pDC stimulation | GS‐9620 | Oral TLR7 agonist | Gilead | Phase 2 |
NCT02166047 NCT02579382 |
|
| Immune stimulation | SB‐9200 | Small molecular nucleic acid hybrid activating RIG‐I and NOD2 pathways | Spring Bank Pharmaceuticals | Phase 2 | NCT02751996 | |
| AIC649 | Proprietary inactivated parapox virus | AiCuris | Phase 1 | Company Web site | ||
| Apoptosis protein cellular inhibitor | Birinapant | SMAC inhibitor | Tetralogic | Phase 1 | NCT02288208 |
Abbreviations: HAPs, heteroaryldihydropyrimidine; HBcAg, hepatitis B core antigen; NOD, nucleotide‐binding oligomerization domain; RIG‐I, retinoic acid‐inducible gene‐I; SMAC, second mitochondria‐derived activator of caspases.
Figure 1.
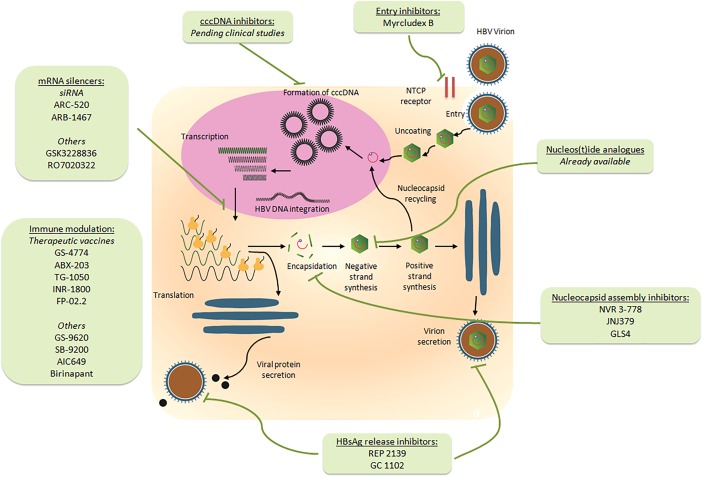
The HBV life cycle and therapeutics currently undergoing clinical trials in humans.
Targeting HBV Messenger RNA Transcription
One promising antiviral target is viral messenger RNA (mRNA) transcription. Chronic HBV infection is characterized by excess HBsAg‐containing subviral particle production. The continued exposure of T cells to viral antigens results in the functional T cell impairment of immune response commonly seen in HBV infection. If viral mRNA transcription were controlled, this will lead to a profound reduction in viral antigens, followed by host immune reconstitution, HBsAg seroclearance, and finally a functional cure.3 This whole action can be augmented with the simultaneous suppression of viral replication via nucleos(t)ide therapy, which indirectly controls cccDNA amplification. One such example is ARC‐520, which is a small interfering RNA (siRNA) that can be successfully delivered to the cytosol of hepatocytes. Viral RNAs contain overlapping sequences, and a single RNA interference can theoretically suppress all related viral protein production. A phase 2a study involving one to two doses of intravenous ARC‐520, when in combination with entecavir, resulted in a profound and durable reduction of viral antigens (Fig. 2).4 Other siRNAs (e.g., ARB‐1467) and other viral mRNA inhibitors achieving satisfactory suppression of HBV viral antigens in preclinical studies are also entering clinical development (Table 1).5
Figure 2.
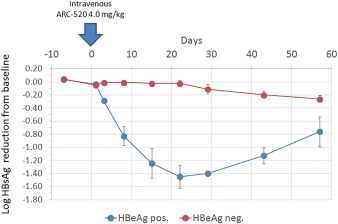
Reduction in serum HBsAg levels after one dose of ARC‐520 in treatment‐naive chronic hepatitis B patients.4 Entecavir was also given in combination. Reproduced with permission from Arrowhead Pharmaceuticals.
HBV Core Protein
Another potential target is the HBV core protein. This unique viral protein is essential to the HBV nucleocapsid assembly, and hence its inhibition not only suppresses the production of HBV virions, but also reduces cccDNA replenishment (Fig. 1). In addition, because the HBV core protein may also exert inhibitory effects on interferon‐stimulated gene, restoration of host innate immune response may be possible by its inhibition. Because of the natural pressure linked with capsid assembly, inhibitors of the HBV core protein are less likely to foster any development of specific resistance. Currently in development is NVR 3‐778, which binds to the core protein resulting in the formation of structurally abnormal capsids that are empty and noninfectious. A recent phase 1b study showed that NVR 3‐778 alone or in combination with pegylated alpha 2a in hepatitis B e antigen (HBeAg)–positive patients for 4 weeks could achieve good reductions in HBV DNA, HBV RNA, and HBeAg levels.6
Viral Entry and Release
Other potential viral antigen targets include the suppression of HBV hepatocyte entry. Myrcludex B is a novel HBV viral entry inhibitor that interacts with the sodium/taurocholate cotransporting polypeptide (NTCP) and the HBV L‐surface protein. A phase 2a study showed Myrcludex B achieving excellent tolerability in human subjects with no serious or relevant adverse effects, with 75% of patients achieving more than 1 log decline of HBV DNA after 12 weeks.7 Myrcludex B was also effective against hepatitis D virus, with virus kinetic modeling suggesting a strong synergistic effect of Myrcludex B and pegylated interferon on both hepatitis D virus and HBV.8 Another target is the suppression of HBsAg secretion. The HBsAg release inhibitor REP‐2139 prevents subviral particle formation and HBsAg release, and when in combination with pegylated interferon, is able to achieve significant declines in HBV DNA and HBsAg and increased rates of anti‐HBs seroconversion.9
Immune Modulation
Immune modulation remains an important target of investigation, aiming at restoring host adaptive or innate immunity and attaining control of HBV infection. Several therapeutic vaccines are currently under development, aiming to activate HBV‐specific immune responses. Different therapeutic vaccines use different viral targets. For example, GS4774 is a recombinant antigen containing HBV surface, core, envelope, and X epitopes, ABX‐203 contains HBsAg and hepatitis B core antigen, whereas TG1050 contains a nonreplicative adenovirus that encodes the core, polymerase, and envelop proteins (Table 1).10 Another method of immune modulation involves the activation of toll‐like receptor 7 (TLR7), which stimulates plasmacytoid dendritic cells (pDCs) and enhances both adaptive and innate immune response. Other immunostimulants with satisfactory results from preclinical studies have also recently commenced phases 1 to 2 clinical trials (Table 1). With abundant immune modulators currently in development, the role of pegylated interferon in HBV therapeutics will likely be diminished in the long run.
Functional Cure Versus Complete Cure
The wide variety of emerging HBV therapeutics offers optimism in achieving a functional cure of HBV, likely through a combination of virological suppression via nucleos(t)ide analogue therapy, viral antigen (e.g., mRNA, nucleocapsid) inhibition, and effective immune modulation (Fig. 3). Nonetheless, these new approaches do not directly target cccDNA, the most important element of the HBV life cycle. Elimination of cccDNA would not only bring about functional cure, but potentially a complete cure from HBV. Various cccDNA inhibitors are now in preclinical development, including the transcription activator‐like effector nucleases, which are able to cleave sequence‐specific DNA targets,11 and the clustered regularly interspaced short palindromic repeats/Cas9 system, which directly cleaves cccDNA.12 As research in new pharmacological approaches continues, a cure for HBV infection will soon be within our grasps.
Figure 3.
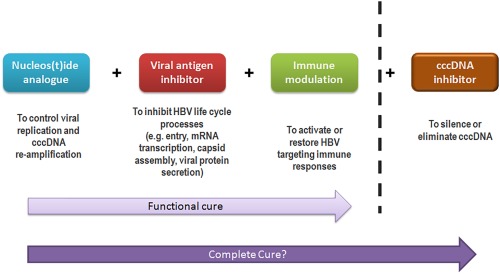
The possible future curative regimen for hepatitis B.
Acknowledgment
The authors acknowledge the assistance of Mr. Spencer Ng in the generation of graphics for this article.
Potential conflict of interest: W.K.S. is an advisory board member of Gilead Sciences and Bristol Myers Squibb and received speaker's fees from Gilead Sciences, Bristol Myers Squibb, AbbVie, and Novartis. M.F.Y. is an advisory board member and received speaker's fees from Gilead Sciences, Bristol Myers Squibb, AbbVie, Janssen, Sysmex Corporation, and Biocartis NV.
REFERENCES
- 1. Seto WK, Hui AJ, Wong VW, Wong GL, Liu KS, Lai CL, et al. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicentre prospective study. Gut 2015;64:667‐672. [DOI] [PubMed] [Google Scholar]
- 2. Yuen MF, Wong DK, Fung J, Ip P, But D, Hung I, et al. HBsAg seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology 2008;135:1192‐1199. [DOI] [PubMed] [Google Scholar]
- 3. Gish RG, Yuen MF, Chan HL, Given BD, Lai CL, Locarnini SA, et al. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent. Antiviral Res 2015;121:97‐108. [DOI] [PubMed] [Google Scholar]
- 4. Yuen MF, Chan HL, Liu K, Given BD, Schluep T, Hamilton J, et al. Differential reductions in viral antigens expressed from cccDNA vs integrated DNA in treatment naive HBeAg positive and negative patients with chronic HBV after RNA interference therapy with ARC‐520 (abstract). J Hepatol 2016;64:S390‐S391. [Google Scholar]
- 5. Lee AC, Thi EP, Pei L, Ye X, Cross J, Du SY, et al. TKM‐HBV, a novel RNA interference treatment for chronic hepatitis B, rapidly reduces surface antigen and other viral proteins in both intrahepatic and peripheral compartments (abstract). Hepatology 2015;62:1186A. [Google Scholar]
- 6. Yuen MF, Kim DJ, Weilert F, Chan HL, Lalezari JP, Hwang SG, et al. NVR 3–778, a first‐in‐class HBV core inhibitor, alone and in combination with peg‐interferon (PegIFN), in treatment‐naive HBeAg‐positive patients: early reductions in HBV DNA and HBeAg (abstract). J Hepatol 2016;64:S210‐S211. [Google Scholar]
- 7. Bogomolov P, Voronkova N, Allweiss L, Dandri M, Schwab M, Lempp FA, et al. A proof‐of‐concept phase 2a clinical trial with HBV/HDV entry inhibitor Myrcludex B (abstract). Hepatology 2014;60:1279A‐1280A. [Google Scholar]
- 8. Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: first results of a phase Ib/IIa study. J Hepatol 2016;65:490‐498. [DOI] [PubMed] [Google Scholar]
- 9. Jansen L, Vaillant A, Stelma F, Kootstra NA, Bazinet M, Al‐Mahtab M, et al. Serum HBV‐RNA levels decline significantly in chronic hepatitis B patients dosed with the nucleic‐acid polymer REP 2139‐CA (abstract). J Hepatol 2015;62:S250. [Google Scholar]
- 10. Liang TJ, Block TM, McMahon BJ, Ghany MG, Urban S, Guo JT, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology 2015;62:1893‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen J, Zhang W, Lin J, Wang F, Wu M, Chen C, et al. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator‐like effector nucleases. Mol Ther 2014;22:303‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramanan V, Shlomai A, Cox DB, Schwartz RE, Michailidis E, Bhatta A, et al. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus. Sci Rep 2015;5:10833. [DOI] [PMC free article] [PubMed] [Google Scholar]


