Watch a video presentation of this article
Abbreviations
- BCLC
Barcelona Clinic Liver Cancer Staging
- BMI
body mass index
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NR
not reported
Predicted Impact of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis‐Hepatocellular Carcinoma in the Hepatology Clinic
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disorder in Western countries. It is estimated that NAFLD affects more than 10% of the adult population in the United States,1 but this number can increase up to 35% when considering fatty liver diagnosed by imaging.2 NAFLD prevalence has increased in the last decades,1 across all ethnic groups. This also affects the pediatric population, with a 2‐fold increase in the last 15 years.3 Insulin resistance and metabolic syndrome are almost invariably associated to NAFLD and to certain extent, NAFLD is viewed as the hepatic manifestation of metabolic syndrome. The spectrum of NAFLD includes a variety of different clinical conditions from simple steatosis with normal liver function to active inflammation [nonalcoholic steatohepatitis (NASH)] and subsequent fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). Arterial hypertension and type 2 diabetes are frequently associated with fibrosis progression4 (Fig. 1), but many other factors are involved, including gut microbiota, dietary habits, and genetic factors, such as PNPLA3 and TM6SF2 polymorphisms. In patients with simple steatosis, the average progression of one stage of fibrosis is estimated to be 14 years, whereas for patients with NASH this time is shorter, being around 7 years.4 NASH is a leading cause of liver cirrhosis in Western countries, and considering the recent advancements in the field of anti–hepatitis C virus (HCV) therapies, it is likely that in the next 30 years NASH will become a major cause of advanced liver disease. Parallel to the increase in NASH‐cirrhosis cases, there has been an increase in the prevalence of NASH‐related HCC.5
Figure 1.
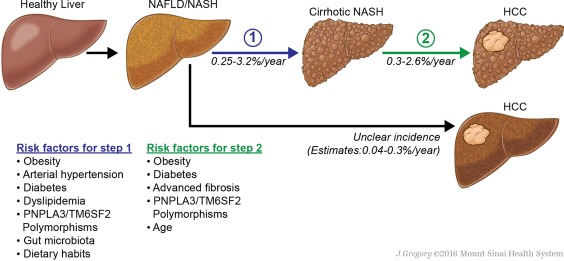
Natural history of NASH/NAFLD‐related HCC. Illustration by Jill K. Gregory, CMI. Mount Sinai Health System.
The pathogenesis of HCC in the context of NAFLD is only partially understood; interestingly, close to 30% of NASH‐related HCCs are diagnosed in the setting of noncirrhotic liver.5, 6 Older age, diabetes, advanced fibrosis, and obesity are the main risk factors associated with HCC development (Table 1). Typical patients with HCC developing in a setting of noncirrhotic NASH are males, older, and display criteria of metabolic syndrome.7, 8 Obesity is known to increase significantly the risk for different types of cancer including liver cancer. In obese men, the excess risk of death for liver cancer is 4 times higher than that of nonoverweight individuals.9 The association of diabetes and HCC has also been reported in different series. A US study showed that the relative risk of HCC in patients with diabetes is 2 compared with those without diabetes, and similar figures have been reported in Europe.10, 11 Among the genetic factors, the PNPLA3 rs738409 C>G polymorphism is associated with an increased risk for HCC development through a mechanism yet ill‐defined.12 Overall, these data suggest that NASH will become the dominant cause of HCC in Western countries in the next decades.
Table 1.
Studies Reporting HCC Incidence in NASH
| Author, Year, Publication | NASH/NAFLD Diagnostic Criteria | Study Population | Follow‐up (years) | Annual HCC Incidence | Risk Factors for HCC Development |
|---|---|---|---|---|---|
| Sanyal, 2006, Hepatology17 | Biopsy proven, alcohol intake <40 g/week, negative tests for other causes of cirrhosis | 152 NASH‐cirrhosis | 10 | 0.2% | Not identified |
| Bhala, 2011, Hepatology13 | Biopsy proven | 247 NASH (cirrhosis 52%, advanced fibrosis 48%) | 7.1 | 0.3% | Not identified |
| Kawamura, 2012, Am J Gastroenterol15 | Fatty liver at ultrasound, alcohol intake <20 g/day, negative tests for other causes of cirrhosis | 6508 NAFLD (not reported % of significant fibrosis/cirrhosis) | 5.6 | 0.04% |
Age Elevated alanine aminotransferase Low platelet count Diabetes |
| Adams, 2005, Gastroenterology18 | Fatty liver at ultrasound or biopsy, alcohol intake <140 g/week, HCV/HBV‐negative, or cryptogenic cirrhosis with criteria of metabolic syndrome | 420 NAFLD (cirrhosis 2%) | 7.6 | 0.06% | Not analyzed |
| Ascha, 2010, Hepatology14 | Biopsy‐proven or cryptogenic cirrhosis with metabolic syndrome without history of significant alcohol intake | 195 NASH‐cirrhosis | 3.2 | 2.6% |
Older age Any alcohol consumption |
Trends and Clinical Singularities of NASH‐HCC
Most NASH‐related HCC cases are diagnosed in the context of cirrhosis. However, the incidence of HCC in these patients varies widely depending on the population studied and the diagnostic criteria used. Indeed, patients with advanced cirrhosis may lose the typical histological features of NASH, and in many retrospective series, patients with cryptogenic cirrhosis and clinical features of metabolic syndrome are considered to have NASH‐cirrhosis. The cumulative incidence rate of HCC in NASH‐cirrhosis ranges between 0.3% and 2.6% per year.13, 14 This risk increases with older age at diagnosis of cirrhosis and concomitant diabetes and obesity.14, 15
Although HCC predominantly occurs in a cirrhotic background liver, its incidence in noncirrhotic patients has been increasingly reported. An analysis of 128 HCC patients compared cases caused by metabolic syndrome versus other chronic liver disease.16 The authors observed that patients with metabolic syndrome tended to be older and were less likely to have cirrhosis. Subsequent studies conducted mostly in Japan showed consistent results. In a study of 87 HCC patients with underlying NASH, cirrhosis was intriguingly less frequent in male compared with female patients.7 Using the aspartate aminotransferase/platelet ratio index as a surrogate marker of fibrosis, a Japanese report described the cumulative rate of HCC in a cohort of 6324 patients without significant fibrosis. Estimated HCC rates were 0.02%, 0.06%, and 0.39% at 4, 8, and 12 years of follow‐up, respectively.15 Strikingly, 184 patients with significant fibrosis enrolled in the same study showed HCC rates up to 4% at 12 years. Although data from the United States are still scarce, two recent studies evaluated this using data from the Veterans Administration hospitals. The first study showed that compared with HCV‐HCC, patients with NASH‐HCC were significantly less likely to be cirrhotic.6 A higher proportion of patients with NASH‐HCC did not receive HCC surveillance during the 3 years preceding diagnosis. The second study sought to identify risk factors of HCC in the absence of cirrhosis.8 In this cohort of 1500 patients with HCC, NASH and metabolic syndrome was the main risk factor in patients without cirrhosis.
The epidemiology of NASH‐HCC is changing as the number of patients with metabolic syndrome increases every year. A significant proportion of these patients develop HCC in the setting of a noncirrhotic liver, and hence outside surveillance programs.6 Compared with patients with other causative factors, patients with NAFLD‐HCC tend to be older, with less severe liver dysfunction,6 and more frequently display comorbidities such as diabetes, obesity, dyslipidemia, and hypertension. These factors increase the clinical complexity of these patients, and ultimately, this makes more challenging their clinical management (Table 2).13, 14 Indeed, patients with NASH‐related HCC are less likely to receive potentially curative treatment compared with patients with HCV.6 There are still many uncertainties in terms of HCC risk at the different stages of NAFLD/NASH (Fig. 1) and whether other genetic factors could contribute to an increased risk in this population. Tailored surveillance programs using robust risk biomarkers could be an option to make surveillance in noncirrhotic NASH‐HCC cost‐effective, especially considering the increasing incidence of NASH. In summary, the irruption of NASH in the hepatology clinic will have a strong impact in the epidemiological landscape and clinical management of HCC. This may translate in older patients with frequent comorbidities and less advanced liver dysfunction that could impact the applicability of potentially curative therapies.
Table 2.
Main Clinical Characteristics of Patients With NASH‐Related HCC Compared With Other Causative Factors
| Author, Year, Publication | Study Population | Age (years) | Diabetes | Dyslipidemia | Hypertension | BMI (kg/m2) | Cirrhosis | Tumor Burden at Diagnosis | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Nodules | Size (cm) | |||||||||
| Dyson J, 2014, J Hepatol5 | 136 NAFLD/NASH | 71 | 80% | NR | NR | 32 | 77% | 2.3 | 5.3 | |
| 469 other causative factors | 68.2 | 30% | 26.4 | 70.9% | 2.4 | 5.7 | ||||
| Beste L, 2015, Gastroenterology19 | 1029 NAFLD/NASH | 70.5 | 76% | NR | NR | 31 | NR | NR | ||
| 6641 other causative factors | 66.2 | 39.2% | 28.2 | |||||||
| BCLC | ||||||||||
| 0‐A | B | C | ||||||||
| Piscaglia F, 2016, Hepatology20 | 145 NAFLD/NASH | 67.8 | 73.1% | 57% | 73.1% | 29.1 | 53.8% | 42.8% | 19.3% | 33.1% |
| 611 HCV | 71.1 | 24.9% | 8.3% | 37.1% | 27.6 | 97.2% | 53% | 14.6% | 23.9% | |
| BCLC | ||||||||||
| A | B | C | ||||||||
| Weinmann A, 2015, BMC Cancer21 | 45 NAFLD/NASH | 67.6 | 66.7% | 0% | 71.1% | 29 | 77.8% | 20% | 24.4% | 42.2% |
| 1074 other causative factors | 65 | 37.8% | 19.6% | 45.2% | 26.6 | 79.9% | 24% | 16.7% | 42.6% | |
| Wong RJ, 2014, Hepatology22 | 807 NAFLD/NASH | 59.3 | 42.8% | NR | NR | 33.6 | NR | NR | ||
| 7066 other causative factors | 57.2 | 20.8% | 27.3 | |||||||
| >3 Nodules | Size (cm) | Vascular inv. or Metastasis | ||||||||
| Tateishi R, 2015, J Gastroenterol23 | 596 NAFLD/NASH | 69.7 | 32.7% | 22.9% | 55.5% | NR | 64.9% | 16.8% | 3.0 | 6% |
| 4730 other causative factors | 72 | 41.9% | 12.5% | 36.9% | 63.4% | 23% | 3.06 | 11.6% | ||
Abbreviations: BCLC, Barcelona Clinic Liver Cancer Staging; BMI, body mass index; NR, not reported.
This study was supported by a Grant for Studies Broadening from the Spanish Association for the Study of the Liver (Asociación Española para el Estudio del Hígado to D.D.), a Cancer Research Grant from the Nuovo Soldati Foundation (to D.D.), a grant from the Swiss National Science Foundation (to I.L.), Foundations Roberto & Gianna Gonella (I.L.), SICPA (I.L.), and an American Association for the Study of Liver Diseases Foundation Alan Hofmann Clinical and Translational Award (to A.V.).
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011;9:524–530.e1; quiz e60. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 3. Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988‐1994 to 2007‐2010. J Pediatr 2013;162:496–500.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta‐analysis of paired‐biopsy studies. Clin Gastroenterol Hepatol 2015;13:643–654.e1‐9; quiz e39‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol 2014;60:110–117. [DOI] [PubMed] [Google Scholar]
- 6. Mittal S, Sada YH, El‐Serag HB, Kanwal F, Duan Z, Temple S, et al. Temporal trends of nonalcoholic fatty liver disease‐related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol 2015:594–601.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol 2011;9:428–433, quiz e50. [DOI] [PubMed] [Google Scholar]
- 8. Mittal S, El‐Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2016;14:124–131.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–1638. [DOI] [PubMed] [Google Scholar]
- 10. Adami HO, Chow WH, Nyren O, Berne C, Linet MS, Ekbom A, et al. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst 1996;88:1472–1477. [DOI] [PubMed] [Google Scholar]
- 11. Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, et al. Cancer incidence in a population‐based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst 1997;89:1360–1365. [DOI] [PubMed] [Google Scholar]
- 12. Falleti E, Fabris C, Cmet S, Cussigh A, Bitetto D, Fontanini E, et al. PNPLA3 rs738409C/G polymorphism in cirrhosis: relationship with the aetiology of liver disease and hepatocellular carcinoma occurrence. Liver Int 2011;31:1137–1143. [DOI] [PubMed] [Google Scholar]
- 13. Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 2011;54:1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972–1978. [DOI] [PubMed] [Google Scholar]
- 15. Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, et al. Large‐scale long‐term follow‐up study of Japanese patients with non‐alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol 2012;107:253–261. [DOI] [PubMed] [Google Scholar]
- 16. Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology 2009;49:851–859. [DOI] [PubMed] [Google Scholar]
- 17. Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682–689. [DOI] [PubMed] [Google Scholar]
- 18. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–121. [DOI] [PubMed] [Google Scholar]
- 19. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 2015;149:1471–1482. [DOI] [PubMed] [Google Scholar]
- 20. Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016;63:827–838. [DOI] [PubMed] [Google Scholar]
- 21. Weinmann A, Alt Y, Koch S, Nelles C, Düber C, Lang H, et al. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer 2015;15:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188–2195. [DOI] [PubMed] [Google Scholar]
- 23. Tateishi R, Okanoue T, Fujiwara N, Okita K, Kiyosawa K, Omata M, et al. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J Gastroenterol 2015;50:350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]


