Key Points
Question
Can evidence-based equivalence ratios for late-onset cardiomyopathy be determined between doxorubicin and other anthracyclines or an anthraquinone agent among childhood cancer survivors?
Findings
This large cohort study of 28 423 childhood cancer survivors with detailed cancer treatment exposures found that compared with doxorubicin, the anthraquinone mitoxantrone was associated with more cardiotoxic risk than current guidelines would suggest, whereas the anthracycline daunorubicin was associated with less cardiotoxic risk. The anthracycline epirubicin appeared isoequivalent to doxorubicin.
Meaning
The cardiotoxicity equivalence ratios determined in the present study for the most commonly used cancer treatment agents may influence the choice of agents when designing new protocols.
Abstract
Importance
Anthracyclines are part of many effective pediatric cancer treatment protocols. Most pediatric oncology treatment groups assume that the hematologic toxicity of anthracycline agents is equivalent to their cardiotoxicity; for example, Children’s Oncology Group substitution rules consider daunorubicin and epirubicin isoequivalent to doxorubicin, whereas mitoxantrone and idarubicin are considered 4 to 5 times as toxic as doxorubicin.
Objective
To determine optimal dose equivalence ratios for late-onset cardiomyopathy between doxorubicin and other anthracyclines or the anthraquinone mitoxantrone.
Design, Setting, and Participants
This multicenter cohort study of childhood cancer survivors who survived 5 or more years analyzed data pooled from 20 367 participants in the Childhood Cancer Survivor Study treated from 1970 to 1999, 5741 participants in the Dutch Childhood Oncology Group LATER study diagnosed between 1963 and 2001, and 2315 participants in the St Jude Lifetime study treated from 1962 to 2005.
Exposures
Cumulative doses of each agent (the anthracyclines doxorubicin, daunorubicin, epirubicin, and idarubicin; and the anthraquinone mitoxantrone) along with chest radiotherapy exposure were abstracted from medical records.
Main Outcomes and Measures
Cardiomyopathy (severe, life-threatening, or fatal) by 40 years of age. Agent-specific Cox proportional hazards models evaluated cardiomyopathy risk, adjusting for chest radiotherapy, age at cancer diagnosis, sex, and exposure to anthracyclines or to an anthraquinone. An agent-specific cardiomyopathy equivalence ratio (relative to doxorubicin) was estimated for each dose category as a ratio of the hazard ratios, and then a weighted mean determined the overall agent-specific equivalence ratio across all dose categories.
Results
Of 28 423 survivors (46.4% female; median age at cancer diagnosis 6.1 years [range, 0.0-22.7 years]), 9330 patients received doxorubicin, 4433 received daunorubicin, 342 received epirubicin, 241 received idarubicin, and 265 received mitoxantrone. After a median follow-up of 20.0 years (range, 5.0-40.0 years) following receipt of a cancer diagnosis, 399 cardiomyopathy cases were observed. Relative to doxorubicin, the equivalence ratios were 0.6 (95% CI, 0.4-1.0) for daunorubicin, 0.8 (95% CI, 0.5-2.8) for epirubicin, and 10.5 (95% CI, 6.2-19.1) for mitoxantrone. Outcomes were too rare to generate idarubicin-specific estimates. Ratios based on a continuous linear dose-response relationship were similar for daunorubicin (0.5 [95% CI, 0.4-0.7]) and epirubicin (0.8 [95% CI, 0.3-1.4]). The relationship between mitoxantrone and doxorubicin appeared better characterized by a linear exponential model.
Conclusions and Relevance
In a large data set assembled to examine long-term cardiomyopathy risk in childhood cancer survivors, daunorubicin was associated with decreased cardiomyopathy risk vs doxorubicin, whereas epirubicin was approximately isoequivalent. By contrast, the current hematologic-based doxorubicin dose equivalency of mitoxantrone (4:1) appeared to significantly underestimate the association of mitoxantrone with long-term cardiomyopathy risk.
This multicenter cohort study pools data from more than 28 000 children with cancer who had survived 5 or more years to evaluate the optimal dose equivalence ratio for late-onset cardiomyopathy between doxorubicin and other 3 other anthracyclines (daunorubicin, epirubicin, and idarubicin) and the anthraquinone mitoxantrone.
Introduction
It is estimated that half of children with cancer are currently treated with anthracyclines (doxorubicin, daunorubicin, epirubicin, and idarubicin) or an anthraquinone (mitoxantrone).1 These agents have well-established associations with both acute and chronic cardiomyopathy.2 Depending on the cumulative dose and other risk factors, such as concurrent chest radiation exposure, age at exposure, and in some studies, female sex, the cumulative incidence of clinical cardiomyopathy by 40 or 50 years of age among survivors of childhood cancer can exceed 10% to 25%.3,4 Rates of subclinical cardiomyopathy may be substantially greater, portending further risk as survivors age.5,6,7
With 5-year survival of childhood cancer now reaching nearly 85%,8 balancing the potential long-term adverse effects of otherwise effective cancer treatments is an important consideration. The design of cancer treatment protocols is informed by both the anti-tumor efficacy and toxicity profile of an agent. For many treatments, the traditional assumption has been that the antileukemia potency of an agent is proportional to its acute hematologic toxicity, and by extension, proportional to toxicity in other organ systems.9 However, data in both pediatric and medical oncology supporting this assumption in relation to cardiotoxicity are limited.10,11 Nevertheless, many of the cardiotoxicity conversion ratios for agents featured in the recommendations of various professional societies or clinical trials groups are based on these assumptions (eTable 1 in the Supplement).
In our recent work,12 we investigated the assumption that daunorubicin was approximately isoequivalent to doxorubicin, the most widely used anthracycline agent. Using a large pooled international cohort of long-term childhood cancer survivors, we found that daunorubicin was only half as cardiotoxic as doxorubicin in relation to late (occurring after 5 years) severe, life-threatening, or fatal cardiomyopathy. At the time, we had insufficient numbers of survivors exposed to other less commonly used anthracyclines and mitoxantrone to examine their cardiotoxicity equivalence to doxorubicin. However, with the recent expansions of the Childhood Cancer Survivor Study (CCSS), the Dutch Children’s Oncology Group’s (DCOG) LATER study, and the St Jude Lifetime (SJLIFE) study, we now have access to long-term cardiac outcomes among nearly 30 000 survivors of childhood cancer, double the sample size of our prior analysis. Providing more accurate estimates of late cardiotoxicity profiles of these agents is important as treatment for childhood cancer continues to be refined with the goal of reducing late adverse effects. Increased accuracy of cardiotoxicity estimates may also inform cardiomyopathy surveillance strategies.
Methods
Study Population
Childhood cancer survivor cohorts from the CCSS, DCOG-LATER, and SJLIFE studies have been described in detail previously.13,14,15 In brief, the CCSS cohort included patients younger than 21 years at diagnosis, with the most common types of childhood cancer that were treated at 1 of 27 institutions in North America from 1970 to 1999 and who survived at least 5 years following diagnosis. Those CCSS participants who were also members of SJLIFE were only counted once (in the SJLIFE cohort). The DCOG-LATER cohort was a nationwide cohort of 5-year survivors of cancer diagnosed before 18 years of age recruited from all Dutch pediatric oncology or hematology centers. Eligible diagnosis years ranged from 1963 to 2001. Eligible cancers included those categorized by the International Classification of Childhood Cancer (third edition), plus selected low-grade central nervous system tumors (astrocytoma and ependymoma) and systemic multifocal/polyostotic Langerhans cell histiocytosis. The SJLIFE study enrolled participants who had received a diagnosis of malignancy in childhood and were treated, irrespective of age, at St Jude Children’s Research Hospital located in Memphis, Tennessee, from 1962 to 2005 (at the time of the present analysis). For the present analysis, SJLIFE cohort members survived at least 10 years from diagnosis and were at least 18 years of age at cohort entry. The protocols for CCSS, DCOG-LATER, and SJLIFE were approved by the institutional review boards at all participating institutions. All participants (or their legal guardians) provided informed consent.
For all 3 cohorts, the original medical records were abstracted to obtain the cumulative doses (in units of milligrams per square meter) of each anthracycline or the anthraquinone used to treat the original childhood cancer (and for CCSS, any second cancers that presented within 5 years of original cancer diagnosis). For all 3 cohorts, radiotherapy records were also centrally reviewed, and field-specific maximum total doses were calculated for the chest.4,16 Chest fields included any abdominal fields that extended to the lower part of the chest (ie, above the diaphragm) and also fields that included the thorax (eg, shoulders, ribs, or supraclavicular areas), even if the central chest was not a target.
Cardiomyopathy Assessment
For all 3 cohorts, we restricted cardiomyopathy cases to those occurring after cohort entry and by 40 years of age and defined by the Common Terminology Criteria for Adverse Events (version 4.03)17 as either severe or disabling (grade 3: requiring medical treatment), life-threatening (grade 4), or fatal (grade 5). The CCSS relied on patient or family self-report if corroborated by concurrent use of appropriate cardiac medications, supplemented by death certificates. The DCOG-LATER and SJLIFE studies ascertained cardiomyopathy using medical records, death certificates, and prospective clinical cardiac assessment. Case ascertainment for the present analysis was censored at 40 years of age because follow-up became sparse after that time point.
Statistical Analysis
Cumulative incidence of cardiomyopathy was first estimated as a function of age with staggered entry to account for left truncation, with death from other causes treated as a competing risk. A separate Cox proportional hazards model with age as the time scale was used to determine the dose-response relationships for each drug (daunorubicin, epirubicin, idarubicin, or mitoxantrone) relative to doxorubicin. Because most clinical groups have considered daunorubicin and epirubicin doses to be nearly equivalent to doxorubicin (eTable 1 in the Supplement), the dose ranges (in units of milligrams per square meter) for these drugs were fairly similar to doxorubicin. Therefore, daunorubicin and epirubicin were compared using their original dose values. Because most clinical groups consider idarubicin and mitoxantrone to have much greater hematologic toxicity at isoequivalent doses to doxorubicin, the dose ranges for these drugs (in units of milligrams per square meter) were much lower than doxorubicin. Thus, to facilitate comparability on the same therapeutically administered dose scale (in units of milligrams per square meter) as doxorubicin, we multiplied idarubicin and mitoxantrone doses by a factor of 5 and 4, respectively (eTable 1 in the Supplement). For all 4 drug models, 150-mg/m2 dose categories (ie, 0, <150, 150-299, and ≥300 mg/m2) were used. For each model, doxorubicin and the respective comparative agent were adjusted for each other, along with sex, age at cancer diagnosis, chest radiotherapy exposure (0, <5, 5-14.9, 15-34.9, or ≥35 Gy), and an indicator for exposure to another anthracycline or anthraquinone (besides the 2 being directly compared in the current model). To account for the possibility of any cohort-specific effect, models were stratified by cohort.
If a childhood cancer survivor developed a subsequent cancer before achieving survival for 5 (CCSS and DCOG-LATER) or 10 (SJLIFE) years, the patient was either excluded from the analysis (DCOG-LATER) or the treatment was taken into account (CCSS and SJLIFE). Individuals’ follow-up time began at the age at which they entered the cohort and ended at the time of reported grades 3 through 5 cardiomyopathy (event), 40 years of age, death, subsequent cancer, or the end of cohort follow-up, whichever occurred first. To account for the intentional undersampling of survivors of acute lymphoblastic leukemia who had received a diagnosis from 1987 to 1999, sampling weights were used for CCSS participants, with table percentages and model results reflecting these weights. In each model, interactions of each drug with age at diagnosis, chest radiotherapy dose, and sex were also assessed.
For each drug model, the equivalence ratio of the hazard ratios (HRs) in each dose category was calculated in relation to doxorubicin and then averaged to obtain an equivalence ratio across all dose categories. Bootstrap with replacement from the data with 1000 replications was used to calculate the 95% CIs. The ratios were recomputed from each of these data sets, and the CI was presented based on the 2.5th and 97.5th percentiles of the bootstraps. For each drug, dose-response curves were also modeled for each agent using linear relative rate models, which allowed for the dose contribution to risk to be additive rather than multiplicative. Models were weighted as before and included a log-linear term to adjust for cohort, current age, sex, age at diagnosis, chest radiotherapy category, and exposure to any anthracycline or anthraquinone other than the 2 being compared. A log-linear model with categorical anthracycline doses was first fit because it paralleled the structure of the Cox model most closely. Because it was possible that the relationship between anthracycline dose and cardiomyopathy risk was not linear, a linear exponential model assessed departure from linearity, with the Akaike information criterion and likelihood ratio tests (for nested models) used for model comparisons.
Finally, given concerns that changes in radiotherapy technique over time and lack of heart-specific dosimetry may contribute to our findings, and separately, that survivors with Down syndrome may be more susceptible to cardiotoxicity,18,19,20,21 separate sensitivity analyses were conducted excluding participants with any chest radiotherapy and those with Down syndrome. Data were analyzed using R (version 2.15, The R Foundation) and SAS (version 9.4, SAS Institute Inc). A 2-sided P < .05 was considered statistically significant.
Results
The pooled study population included 28 423 survivors (46.4% female; Table 1 and Table 2) with a median age at cancer diagnosis of 6.1 years (range, 0.0-22.7 years) and a median follow-up after cancer diagnosis of 20.0 years (5.0-40.0 years). In total, 9330 patients (34.8%) received doxorubicin with a median dose of 181 mg/m2 (interquartile range [IQR], 119-320 mg/m2), whereas 4433 patients (18.0%) received daunorubicin with a median dose of 120 mg/m2 (IQR, 99-208 mg/m2). Other drugs were less common: 342 patients received epirubicin (median dose, 300 mg/m2; IQR, 240-400 mg/m2), 241 received idarubicin (median dose, 36 mg/m2; IQR, 20-40 mg/m2; and 265 received mitoxantrone (median dose, 40 mg/m2; IQR, 26-72 mg/m2). Overall 1857 patients (7.4%) received more than 1 type of anthracycline or anthraquinone (eTable 2 in the Supplement), and 87 patients (0.4%) received more than 2 types.
Table 1. Demographic and Clinical Characteristics of the Study Population.
| Characteristic | Participants, No. (%) | |||
|---|---|---|---|---|
| CCSS (n = 20 367)a | DCOG-LATER (n = 5741) | SJLIFE (n = 2315) | Total (N = 28 423) | |
| Female sex | 9570 (46.8) | 2541 (44.3) | 1104 (47.7) | 13 215 (46.4) |
| Age at diagnosis, median (range), y | 6.3 (0.0-21.0) | 5.5 (0.0-17.9) | 6.3 (0.0-22.7) | 6.1 (0.0-22.7) |
| Primary childhood cancer diagnosis | ||||
| Leukemia | 5881 (37.3) | 2012 (35.0) | 847 (36.6) | 8740 (36.3) |
| Lymphoma | 4097 (18.0) | 1004 (17.5) | 376 (16.2) | 5477 (17.7) |
| Brain tumor | 3820 (16.7) | 731 (12.7) | 292 (12.6) | 4843 (15.7) |
| Neuroblastoma | 1600 (7.0) | 302 (5.3) | 121 (5.2) | 2023 (6.6) |
| Kidney tumor | 1814 (8.0) | 566 (9.9) | 167 (7.2) | 2547 (8.3) |
| Soft-tissue sarcoma | 1451 (6.4) | 413 (7.2) | 142 (6.1) | 2006 (6.5) |
| Bone tumor | 1704 (7.5) | 326 (5.7) | 121 (5.3) | 2151 (7.0) |
| Other malignant neoplasm | 0 | 387 (6.7) | 249(10.8) | 636 (2.1) |
| Treatment exposures | ||||
| Doxorubicin | 6686 (35.4) | 1805 (31.8) | 839 (36.3) | 9330 (34.8) |
| Dose, median (IQR), mg/m2 | 198 (114-331) | 150 (100-300) | 185 (151-278) | 181 (119-320) |
| Daunorubicin | 2697 (16.7) | 1084 (19.0) | 652 (28.2) | 4433 (18.0) |
| Dose, median (IQR), mg/m2 | 108 (98-265) | 126 (120-175) | 100 (50-105) | 120 (99-208) |
| Epirubicin | 1 (0.1) | 340 (5.9) | 1 (0.1) | 342 (1.1) |
| Dose, median (IQR), mg/m2 | 175 | 300 (240-400) | 270 | 300 (240-400) |
| Idarubicin | 153 (1.1) | 61 (1.1) | 27 (1.2) | 241 (1.1) |
| Dose, median (IQR), mg/m2 | 30 (20-41) | 36 (35-36) | 20 (11-36) | 36 (20-40) |
| Mitoxantrone | 99 (0.5) | 139 (2.4) | 27 (1.2) | 265 (0.9) |
| Dose, median (IQR), mg/m2 | 47 (32-80) | 40 (20-60) | 50 (36-51) | 40 (26-72) |
| Chest radiotherapy | 4896 (22.9) | 771 (13.4) | 573 (24.8) | 6240 (21.2) |
| Dose, median (IQR), Gy | 26 (15-37) | 24 (16-35) | 25 (15-30) | 25 (15-36) |
| Attained age, median (range), y | 27.1 (5.6-40.0) | 27.3 (5.1-40.0) | 31.6 (18.9-40.0) | 27.5 (5.1-40.0) |
| Cardiomyopathy casesb | 270 (1.4) | 106 (1.8) | 23 (1.0) | 399 (1.4) |
Abbreviations: CCSS, Childhood Cancer Survivor Study; DCOG-LATER, Dutch Children’s Oncology Group LATER cohort; IQR, interquartile range; SJLIFE, St Jude Lifetime Cohort Study.
Percentages may not match numbers because percentages reflect weighting used in the CCSS for patients with acute lymphoblastic leukemia; reported median values and IQR also reflect weighting.
Defined using Common Terminology Criteria for Adverse Events, grade 3 or higher, requiring medical treatment, life-threatening, or fatal.
Table 2. Distribution of Anthracycline, Mitoxantrone, and Chest Radiotherapy Exposures by Cancer Diagnosis.
| Diagnosis | Participants, No. (%)a | |||||
|---|---|---|---|---|---|---|
| Doxorubicin (n = 9330) | Daunorubicin (n = 4433) | Epirubicin (n = 342) | Idarubicin (n = 241) | Mitoxantrone (n = 265) | Chest Radiotherapy (n = 6240) | |
| Leukemia | 2498 (28.6) | 3865 (44.2) | 41 (0.5) | 233 (2.7) | 210 (2.4) | 1126 (12.9) |
| Lymphoma | 2637 (48.1) | 545 (10.0) | 96 (1.8) | 5 (0.1) | 54 (1.0) | 2547 (46.5) |
| Brain tumor | 25 (0.5) | 1 (0.0) | 1 (0.0) | 3 (0.1) | NA | 1156 (23.9) |
| Neuroblastoma | 737 (36.4) | 7 (0.3) | 41 (0.2) | NA | NA | 325 (16.1) |
| Kidney tumor | 945 (37.1) | 3 (0.1) | 125 (4.9) | NA | 1 (0.1) | 567 (22.3) |
| Soft-tissue sarcoma | 700 (34.9) | 3 (0.1) | 67 (3.3) | NA | NA | 187 (9.3) |
| Bone tumor | 1729 (80.4) | 4 (0.2) | 1 (0.0) | NA | NA | 269 (12.5) |
| Other malignant neoplasm | 59 (9.3) | 5 (0.8) | 7 (1.1) | NA | NA | 63 (9.9) |
Abbreviation: NA, not applicable.
Percentages shown are the proportions of exposures across each row (ie, by diagnosis).
The cumulative incidence of grade 3 to 5 cardiomyopathy (n = 399) by 40 years of age was 3.4% (95% CI, 3.1%-3.8%). Of these cases, 229 (56.2%) received only doxorubicin, whereas 81 of the remaining cases were exposed to other anthracyclines or mitoxantrone (Table 3). Forty-five cases received chest radiotherapy without known anthracycline or mitoxantrone exposure, and 44 cases had no known history of chest radiotherapy or cardiotoxic chemotherapy.
Table 3. Cardiomyopathy HRs and Mean Equivalence Ratios for Various Anthracyclines or Mitoxantrone Relative to Doxorubicin.
| Exposure | Participants, No. | HR (95% CI)a | Ratio (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Clinical Cardiomyopathy | Dose Information | <150 mg/m2 | 150-299 mg/m2 | ≥300 mg/m2 | Mean | Linear Dose-Response Model | |
| Daunorubicin | 65 | 4328 | 1.4 (0.9-2.1) | 2.8 (1.7-4.5) | 6.0 (3.8-9.3) | ||
| Doxorubicin | 1.8 (1.2-2.6) | 4.6 (3.3-6.4) | 12.6 (9.8-16.3) | ||||
| Daunorubicin to doxorubicin ratio | 0.8 | 0.6 | 0.5 | 0.6 (0.4-1.0) | 0.5 (0.4-0.7) | ||
| Epirubicin | 9 | 342 | 1.9 (0.3-13.7) | 2.4 (0.6-9.9) | 6.0 (2.6-13.9) | ||
| Doxorubicin | 1.5 (0.99-2.2) | 4.2 (3.1-5.7) | 11.3 (8.8-14.4) | ||||
| Epirubicin to doxorubicin ratio | 1.3 | 0.6 | 0.5 | 0.8 (0.5-2.8) | 0.8 (0.3-1.4) | ||
| Idarubicinb | 5 | 238 | 0 | 3.8 (1.5-9.5) | 0 | ||
| Doxorubicin | 1.4 (0.9-2.1) | 4.1 (3.0-5.7) | 11.1 (8.6-14.1) | ||||
| Idarubicin to doxorubicin ratio | 0 | 0.9 | 0 | NE | NE | ||
| Mitoxantronec | 19 | 261 | 4.2 (1.8-9.9) | 4.2 (1.6-11.4) | 48.3 (24.2-96.5) | ||
| Doxorubicin | 1.5 (1.0-2.3) | 4.4 (3.2-6.0) | 11.6 (9.1-15.0) | ||||
| Mitoxantrone to doxorubicin ratio | 2.8 | 1.0 | 4.2 | 10.5 (6.2-19.1)d | 13.8 (8.0-21.6)d | ||
Abbreviations: HRs, hazard ratios; NE, not estimable.
Patient without exposure to the given anthracycline or anthraquinone as referent; models were adjusted for sex, age at diagnosis, exposure to any other anthracycline or mitoxantrone besides the 2 being compared, and stratified by cohort.
Idarubicin doses multiplied by a factor of 5 to facilitate comparability to doxorubicin doses; at a level of less than 150 mg/m2, 82 patients had dose information (none with cardiomyopathy); at a level of 300 mg/m2 or higher, 28 patients had dose information (none with cardiomyopathy).
Mitoxantrone doses multiplied by a factor of 4 to facilitate comparability to doxorubicin doses.
Multiplied by a conversion factor of 4 (eg, mean ratio of 2.6 × 4 = 10.5).
After multivariable adjustment, the mean equivalence ratio between the HRs of daunorubicin and doxorubicin, in 150 mg/m2 increments, was 0.6 (95% CI, 0.4-1.0) and 0.5 (95% CI, 0.4-0.7) when a linear dose-response model was used (Table 3). The performance of the linear and linear exponential dose-response models was fairly similar in terms of Akaike information criterion and log-likelihood values and appeared to be a better fit than the log-linear model (Table 4).
Table 4. Dose-Response Models Examined and Corresponding Akaike Information Criterion and Log-Likelihood Deviance.
| Description | Modela | Akaike Information Criterion | Log-Likelihood Deviance |
|---|---|---|---|
| Daunorubicin vs doxorubicin | |||
| Log-linear modelb | Risk = exp (ΣαX) exp (0.56 doxoi1 + 1.51 doxoi2 + 2.52 doxoi3 + 0.34 dauni1 + 0.99 dauni2 + 1.76 dauni3) | 6123.1 | 6089.1 |
| Linear modelc | Risk = exp (ΣαX) [1 + 0.02963 doxo_dose + 0.01571 daun_dose] | 6098.8 | 6072.8 |
| Linear exponential modelc | Risk = exp (ΣαX) [1 + 0.01674 doxo_dose × exp (0.00157 doxo_dose) + 0.0129 daun_dose × exp (0.000597 daun_dose)] | 6092.4 | 6062.4 |
| Epirubicin vs doxorubicin | |||
| Log-linear modelb | Risk = exp (ΣαX) exp (0.37 doxoi1 + 1.41 doxoi2 + 2.41 doxoi3 + 0.73 epii1 + 0.71 epii2 + 1.83 epii3) | 6247.5 | 6213.5 |
| Linear modelc | Risk = exp (ΣαX) [1 + 0.02203 doxo_dose +0.01685 epi_dose] | 6235.8 | 6209.8 |
| Linear exponential modelc | Not estimable | ||
| Mitoxantrone vs doxorubicind | |||
| Log-linear modelb | Risk = exp (ΣαX) exp (0.43 doxoi1 + 1.46 doxoi2 + 2.44 doxoi3 + 1.51 mitoxi1 + 1.34 mitoxi2 + 3.89 mitoxi3) | 6197.7 | 6163.7 |
| Linear modelc | Risk = exp (ΣαX) [1 + 0.02313 doxo_dose + 0.07966 mitox_dose] | 6192.0 | 6166.0 |
| Linear exponential modelc | Risk = exp (ΣαX) [1 + 0.01265 doxo_dose × exp (0.0017 doxo_dose) + 0.02498 mitox_dose × exp (0.0036 mitox_dose)] | 6178.7 | 6148.7 |
Abbreviations: daun, daunorubicin; doxo, doxorubicin; epi, epirubicin; mitox, mitoxantrone.
exp (ΣαX) contains these covariates: sex, age at cancer diagnosis, current age, chest radiotherapy exposure, exposure to another anthracycline or anthraquinone, and cohort.
Based on dose categories with no dose as referent, where listed covariates are indicators (i1 = 1-149; i2 = 150-299; and i3 = ≥300 mg/m2).
Based on continuous dose (in units of milligrams per square meter).
Mitoxantrone dose (in units of milligrams per square meter) first multiplied by 4 to facilitate comparability to doxorubicin on the same mg/m2 scale.
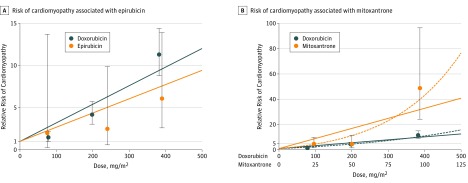
The mean equivalence ratio between the HRs of epirubicin and doxorubicin was 0.8 (95% CI, 0.5-2.8) (Table 3) with similar findings from a linear dose-response model (0.8; 95% CI, 0.3-1.4) (Figure). The linear dose-response model performed better than the less parsimonious log-linear model, whereas the linear exponential model was not estimable (Table 4).
Figure. Risk of Cardiomyopathy Associated With Epirubicin (A) and With Mitoxantrone (B) Relative to Doxorubicin.
The circles (and extending 95% CI error bars) for each agent represent estimates from the Cox proportional hazards model based on categorical dose increments. Each circle is placed at the median dose on the x-axis for each category. Lines represent the linear (solid) and linear exponential (dashed, panel B only) modeled continuous dose-response relationships.
Data for idarubicin were too sparse to generate a mean ratio across the entire dose spectrum, with no cardiomyopathy cases seen in the dose categories of less than 150 mg/m2 or of 300 mg/m2 or greater. For the 150 to 299 mg/m2 dose category, idarubicin (after being multiplied by a factor of 5), appeared to be equivalent to doxorubicin, with a mean ratio of 0.9 (95% CI, 0.2-2.3) (Table 3).
Mitoxantrone, even after multiplying doses by a factor of 4 to account for differences in therapeutic doses, was still associated with a greater risk of cardiomyopathy compared with doxorubicin, with a mean equivalence ratio of 10.5 (95% CI, 6.2-19.1), or approximately 2.6 times more cardiotoxic than what the conventional 4-fold multiplier would suggest (Table 3). Whereas the mitoxantrone to doxorubicin linear dose-response model suggested a ratio of 13.8 (95% CI, 8.0-21.6), there was evidence for nonlinearity beyond the dose category of 300 mg/m2 or more because the exponential term for both drugs was significant in an alternative linear exponential model (P < .05) (Table 4 and Figure). However, even when the analysis was restricted to doxorubicin doses of less than 300 mg/m2 and mitoxantrone doses of less than 75 mg/m2, the linear dose-response ratio remained high albeit imprecise at 8.1 (95% CI, 0.5-16.1).
Although radiotherapy to the chest was associated with the development of grade 3 to 5 cardiomyopathy (15-34.9 Gy vs none, HR 2.1 [95% CI, 1.6-2.8]; ≥35 Gy vs none, HR 3.5 [95% CI, 2.5-4.8], based on a Cox model with daunorubicin), there was no evidence of an interaction between chest radiotherapy and doxorubicin (P = .39), daunorubicin (P = .69) or mitoxantrone (P = .97). Owing to small numbers of cases, we could not estimate the interaction between chest radiotherapy and epirubicin or chest radiotherapy and idarubicin. Given concerns that changes in radiation technique and lack of heart-specific doses may contribute to our findings, we conducted analyses excluding survivors with any chest radiation exposure and generally found similar results (eTable 3 in the Supplement).
In sensitivity analyses, when 208 survivors with Down syndrome were excluded (of whom 7 had grade 3-5 cardiomyopathy), the mean equivalence ratios based on mean HRs were largely similar: daunorubicin (0.6; 95% CI, 0.4-0.9), epirubicin (0.8; 95% CI, 0.5-2.9) and mitoxantrone (8.8; 95% CI, 4.7-18.5). Estimates based on linear dose-response models were also similar to the initial analyses: daunorubicin (0.5; 95% CI, 0.3-0.7), epirubicin (0.8; 95% CI, 0.3-1.3), and mitoxantrone (10.8; 95% CI, 5.7-17.8).
Discussion
Among nearly 30 000 long-term survivors of childhood cancer, we found that by 40 years of age, mitoxantrone was 10 times as cardiotoxic (or more) when compared with the same dose of doxorubicin. By contrast, daunorubicin was approximately half as cardiotoxic when compared with doxorubicin, consistent with our prior finding.12 Epirubicin appeared to be approximately isoequivalent to doxorubicin although the bounds of the 95% CIs ranged from approximately 0.5 to 3.
The discovery and application of mitoxantrone and epirubicin were motivated by the desire to identify effective but less toxic alternatives to doxorubicin. Both agents began to be incorporated into treatment protocols approximately 40 years ago.22,23,24 Initial reports, primarily from breast cancer studies, suggested that mitoxantrone (at one-fifth the mg/m2 dose of doxorubicin) was associated with less acute toxicity, including cardiotoxicity, than doxorubicin.25 A cardiotoxicity equivalence ratio of around 2 has been commonly cited.26 In children, a doxorubicin to mitoxantrone substitution rule of 4:1 has been commonly accepted for both anti-tumor efficacy and toxicity.9,27 Nevertheless, robust data supporting this ratio in terms of long-term cardiotoxicity have been lacking.11,28 Although mitoxantrone has been used much less frequently than doxorubicin or daunorubicin (eTable 2 in the Supplement), mitoxantrone remains an integral component of frontline treatment of acute myeloid leukemia29,30,31,32 and is often used in relapsed acute lymphoblastic leukemia.33
Similar to mitoxantrone, early studies of patients with breast cancer suggested that epirubicin may be less cardiotoxic than doxorubicin,34,35 and a cardiotoxicity equivalence ratio of 0.5 was commonly cited.26 More recent analyses pooling breast cancer treatment cohorts suggested that epirubicin trended toward being less cardiotoxic than doxorubicin, although the CIs were wide (pooled odds ratio, 0.6; 95% CI, 0.3-1.3).36 In pediatrics, a conversion factor of 0.67 has been commonly adopted,9,27 although evidence supporting this ratio with regard to cardiotoxicity is lacking.11 Compared with mitoxantrone, current use of epirubicin in pediatrics is more limited; it is used occasionally as part of therapy for relapsed sarcoma.37
A recent systematic review and harmonization effort of the cardiomyopathy screening guidelines for survivors of childhood cancer established a high-risk cumulative anthracycline dose threshold of 250 mg/m2.11 Although our data were not based on randomized comparisons of children treated with various anthracyclines or anthraquinones, our findings would suggest that the commonly used doxorubicin conversion ratios for daunorubicin (approximately 1.0) and mitoxantrone (approximately 4.0) should be reconsidered (eTable 1 in the Supplement) and perhaps revised (downward and upward, respectively). Insufficient data exist to recommend the revision of the epirubicin to doxorubicin equivalence ratio (typically 0.5 to 1.0). The present results might inform the selection of future anthracyclines in treatment protocols and prompt consideration of potentially less cardiotoxic liposomal formulations or concurrent administration of cardioprotectants, such as dexrazoxane. The adult data suggest that these strategies can be effective,10,38,39 but pediatric data remain limited.11 Finally, our findings may also allow for better personalization of care, when regimens with equivalent anticancer efficacy may be evaluated more accurately with respect to their long-term cardiotoxicity risk.
Limitations
In pediatric cancer survivorship, the relative rarity of significant cardiomyopathy among children and the long latency between exposure and development of cardiac disease makes reliance on randomized clinical trial data difficult. In illustration of this, although our analysis featured one of the largest aggregations of childhood cancer survivors and clinical cardiomyopathy cases ever published, to our knowledge, even after a median follow-up of 20 years following a cancer diagnosis, the number of cardiomyopathy cases associated with epirubicin, idarubicin, and mitoxantrone remained small (<20 per drug). Therefore, it is important to continue follow-up as participants age and the risk of cardiovascular disease increases further. It is also possible that the conversion ratios between different agents may change with further follow-up.
Another potential limitation of our analysis is that the largest proportion of our study participants came from the CCSS, which relies on self-report supplemented with death records. However, prediction models for grade 3-5 cardiomyopathy based on CCSS data worked similarly in other cohorts that ascertained their outcomes via medical records and clinical assessment.3 Consistent with our findings, prior data from the Netherlands (including approximately 500 patients in this analysis) found that when examined by echocardiography, there was a suggestion that patients treated with daunorubicin were more likely to have preserved left ventricular systolic function vs those treated with doxorubicin, whereas epirubicin-associated changes were similar in magnitude to doxorubicin, albeit after exposure to higher mean epirubicin doses.5
Conclusions
In summary, even as there is growing interest and development of more targeted therapies that rely on activating the immune system, for the foreseeable future, conventional cytotoxic agents, such as anthracyclines and mitoxantrone, will remain an integral part of treatment of many childhood cancers (eg, leukemia, lymphoma, sarcoma, neuroblastoma, and more advanced stage Wilms tumor). Although cardiac mortality among survivors of childhood cancer has decreased in recent decades,40 overall cardiac morbidity, including the incidence of heart failure, has not changed significantly from the 1970s to the 1990s.41 To reduce morbidity in future generations of long-term survivors, we will need to continue to test the efficacy of combination chemotherapy that incorporates less cardiotoxic agents as well as investigate new formulations and other cardioprotective strategies.42 Among contemporary agents, in relation to doxorubicin, our estimates for mitoxantrone suggest that a conversion ratio of 4 may substantially underestimate its long-term cardiotoxicity risk in children, whereas a daunorubicin ratio of 1 may overestimate cardiotoxicity. A better understanding of the long-term effects of these agents may enhance long-term overall survival without necessarily compromising cancer-free survival.
eTable 1. Toxicity Equivalence Ratios Used by Various Clinical Groups for Assessment of Cardiotoxicity
eTable 2. Number of Survivors (%) Exposed to Chest Radiation and One or More Anthracyclines or Anthraquinone, N = 28 423
eTable 3. Equivalence Ratios for Different Agents Relative to Doxorubicin After Excluding Survivors With any Chest Radiation
References
- 1.van Dalen EC, Raphaël MF, Caron HN, Kremer LC. Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database Syst Rev. 2014;9(9):CD006647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128(17):1927-1995. doi: 10.1161/CIR.0b013e3182a88099 [DOI] [PubMed] [Google Scholar]
- 3.Chow EJ, Chen Y, Kremer LC, et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33(5):394-402. doi: 10.1200/JCO.2014.56.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow EJ, Chen Y, Hudson MM, et al. Prediction of ischemic heart disease and stroke in survivors of childhood cancer. J Clin Oncol. 2018;36(1):44-52. doi: 10.1200/JCO.2017.74.8673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Pal HJ, van Dalen EC, Hauptmann M, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170(14):1247-1255. doi: 10.1001/archinternmed.2010.233 [DOI] [PubMed] [Google Scholar]
- 6.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673-3680. doi: 10.1200/JCO.2013.49.3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulrooney DA, Armstrong GT, Huang S, et al. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross-sectional study. Ann Intern Med. 2016;164(2):93-101. doi: 10.7326/M15-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noone AM, Howlader N, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975-2015. https://seer.cancer.gov/csr/1975_2015/. Accessed October 22, 2018.
- 9.Le Deley MC, Leblanc T, Shamsaldin A, et al. ; Société Française d’Oncologie Pédiatrique . Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case-control study by the Société Française d’Oncologie Pédiatrique. J Clin Oncol. 2003;21(6):1074-1081. doi: 10.1200/JCO.2003.04.100 [DOI] [PubMed] [Google Scholar]
- 10.van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. 2010;(5):CD005006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armenian SH, Hudson MM, Mulder RL, et al. ; International Late Effects of Childhood Cancer Guideline Harmonization Group . Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16(3):e123-e136. doi: 10.1016/S1470-2045(14)70409-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feijen EA, Leisenring WM, Stratton KL, et al. Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol. 2015;33(32):3774-3780. doi: 10.1200/JCO.2015.61.5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308-2318. doi: 10.1200/JCO.2009.22.3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feijen EAM, Font-Gonzalez A, van der Pal HJH, et al. ; DCOG LATER Study Group . Risk and temporal changes of heart failure among 5-year childhood cancer survivors: a DCOG-LATER study. J Am Heart Assoc. doi: 10.1161/JAHA.118.009122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(5):666-674. doi: 10.1158/1055-9965.EPI-16-0812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166(1, pt 2):141-157. doi: 10.1667/RR3525.1 [DOI] [PubMed] [Google Scholar]
- 17.Nation Cancer Institute Division of Cancer Treatment and Diagnosis. Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed October 22, 2018.
- 18.Pui CH, Burghen GA, Bowman WP, Aur RJ. Risk factors for hyperglycemia in children with leukemia receiving L-asparaginase and prednisone. J Pediatr. 1981;99(1):46-50. doi: 10.1016/S0022-3476(81)80955-9 [DOI] [PubMed] [Google Scholar]
- 19.Rao A, Hills RK, Stiller C, et al. Treatment for myeloid leukaemia of Down syndrome: population-based experience in the UK and results from the Medical Research Council AML 10 and AML 12 trials. Br J Haematol. 2006;132(5):576-583. doi: 10.1111/j.1365-2141.2005.05906.x [DOI] [PubMed] [Google Scholar]
- 20.O’Brien MM, Taub JW, Chang MN, et al. ; Children’s Oncology Group Study POG 9421 . Cardiomyopathy in children with Down syndrome treated for acute myeloid leukemia: a report from the Children’s Oncology Group Study POG 9421. J Clin Oncol. 2008;26(3):414-420. doi: 10.1200/JCO.2007.13.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldsby RE, Stratton KL, Raber S, et al. Long-term sequelae in survivors of childhood leukemia with Down syndrome: a childhood cancer survivor study report. Cancer. 2018;124(3):617-625. doi: 10.1002/cncr.31065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonfante V, Bonadonna G, Villani F, Martini A. Preliminary clinical experience with 4-epidoxorubicin in advanced human neoplasia. Recent Results Cancer Res. 1980;74:192-199. doi: 10.1007/978-3-642-81488-4_24 [DOI] [PubMed] [Google Scholar]
- 23.Von Hoff DD, Pollard E, Kuhn J, Murray E, Coltman CA Jr. Phase I clinical investigation of 1,4-dihydroxy-5,8-bis {{{2-[(2-hydroxyethyl)amino]ethyl} amino}}-9,10-anthracenedione dihydrochloride (NSC 301739), a new anthracenedione. Cancer Res. 1980;40(5):1516-1518. [PubMed] [Google Scholar]
- 24.Alberts DS, Griffith KS, Goodman GE, Herman TS, Murray E. Phase I clinical trial of mitoxantrone: a new anthracenedione anticancer drug. Cancer Chemother Pharmacol. 1980;5(1):11-15. doi: 10.1007/BF00578556 [DOI] [PubMed] [Google Scholar]
- 25.Posner LE, Dukart G, Goldberg J, Bernstein T, Cartwright K. Mitoxantrone: an overview of safety and toxicity. Invest New Drugs. 1985;3(2):123-132. doi: 10.1007/BF00174159 [DOI] [PubMed] [Google Scholar]
- 26.Keefe DL. Anthracycline-induced cardiomyopathy. Semin Oncol. 2001;28(4)(suppl 12):2-7. doi: 10.1053/sonc.2001.26431 [DOI] [PubMed] [Google Scholar]
- 27.Shankar SM, Marina N, Hudson MM, et al. ; Cardiovascular Disease Task Force of the Children’s Oncology Group . Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121(2):e387-e396. doi: 10.1542/peds.2007-0575 [DOI] [PubMed] [Google Scholar]
- 28.van Dalen EC, van der Pal HJ, Bakker PJ, Caron HN, Kremer LC. Cumulative incidence and risk factors of mitoxantrone-induced cardiotoxicity in children: a systematic review. Eur J Cancer. 2004;40(5):643-652. doi: 10.1016/j.ejca.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 29.Burnett AK, Hills RK, Milligan DW, et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 2010;28(4):586-595. doi: 10.1200/JCO.2009.22.9088 [DOI] [PubMed] [Google Scholar]
- 30.Aplenc R, Alonzo TA, Gerbing RB, et al. ; Children’s Oncology Group . Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2008;26(14):2390-3295. doi: 10.1200/JCO.2007.13.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarfelt M, Andersen NH, Glosli H, et al. ; Nordic Society of Pediatric Hematology and Oncology (NOPHO) . Cardiac function in survivors of childhood acute myeloid leukemia treated with chemotherapy only: a NOPHO-AML study. Eur J Haematol. 2016;97(1):55-62. doi: 10.1111/ejh.12683 [DOI] [PubMed] [Google Scholar]
- 32.Alexander TB, Wang L, Inaba H, et al. Decreased relapsed rate and treatment-related mortality contribute to improved outcomes for pediatric acute myeloid leukemia in successive clinical trials. Cancer. 2017;123(19):3791-3798. doi: 10.1002/cncr.30791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376(9757):2009-2017. doi: 10.1016/S0140-6736(10)62002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain KK, Casper ES, Geller NL, et al. A prospective randomized comparison of epirubicin and doxorubicin in patients with advanced breast cancer. J Clin Oncol. 1985;3(6):818-826. doi: 10.1200/JCO.1985.3.6.818 [DOI] [PubMed] [Google Scholar]
- 35.Hortobagyi GN, Yap HY, Kau SW, et al. A comparative study of doxorubicin and epirubicin in patients with metastatic breast cancer. Am J Clin Oncol. 1989;12(1):57-62. doi: 10.1097/00000421-198902000-00014 [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi N, Fujii T, Aoi S, Kozuch PS, Hortobagyi GN, Blum RH. Comparison of cardiac events associated with liposomal doxorubicin, epirubicin and doxorubicin in breast cancer: a Bayesian network meta-analysis. Eur J Cancer. 2015;51(16):2314-2320. doi: 10.1016/j.ejca.2015.07.031 [DOI] [PubMed] [Google Scholar]
- 37.Winter S, Fasola S, Brisse H, Mosseri V, Orbach D. Relapse after localized rhabdomyosarcoma: Evaluation of the efficacy of second-line chemotherapy. Pediatr Blood Cancer. 2015;62(11):1935-1941. doi: 10.1002/pbc.25622 [DOI] [PubMed] [Google Scholar]
- 38.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011;(6):CD003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35(8):893-911. doi: 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 40.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833-842. doi: 10.1056/NEJMoa1510795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions among survivors of childhood cancer diagnosed 1970-1999: a report from the Childhood Cancer Survivor Study [published online November 8, 2018]. Lancet Oncol. doi: 10.1016/S1470-2045(18)30537-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armenian SH, Armstrong GT, Aune G, et al. Cardiovascular disease in survivors of childhood cancer: insights into epidemiology, pathophysiology, and prevention. J Clin Oncol. 2018;36(21):2135-2144. doi: 10.1200/JCO.2017.76.3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Toxicity Equivalence Ratios Used by Various Clinical Groups for Assessment of Cardiotoxicity
eTable 2. Number of Survivors (%) Exposed to Chest Radiation and One or More Anthracyclines or Anthraquinone, N = 28 423
eTable 3. Equivalence Ratios for Different Agents Relative to Doxorubicin After Excluding Survivors With any Chest Radiation