Key Points
Question
Does resecting the piriform cortex improve surgical outcome in temporal lobe epilepsy?
Findings
In this multicenter study that included 107 adults with temporal lobe epilepsy in the derivation cohort and 31 in the validation cohort, resecting a larger proportion of the piriform cortex (78% in seizure-free vs 46% in non–seizure-free cases in pooled data) was significantly associated with a favorable outcome. Removal of at least half the piriform cortex significantly increased the odds of becoming seizure free by a factor of 16.
Meaning
These findings support including the piriform cortex in standard anterior temporal lobe resections to achieve seizure freedom.
This multicenter cohort study assesses outcomes of piriform cortex resection among patients with temporal lobe epilepsy.
Abstract
Importance
A functional area associated with the piriform cortex, termed area tempestas, has been implicated in animal studies as having a crucial role in modulating seizures, but similar evidence is limited in humans.
Objective
To assess whether removal of the piriform cortex is associated with postoperative seizure freedom in patients with temporal lobe epilepsy (TLE) as a proof-of-concept for the relevance of this area in human TLE.
Design, Setting, and Participants
This cohort study used voxel-based morphometry and volumetry to assess differences in structural magnetic resonance imaging (MRI) scans in consecutive patients with TLE who underwent epilepsy surgery in a single center from January 1, 2005, through December 31, 2013. Participants underwent presurgical and postsurgical structural MRI and had at least 2 years of postoperative follow-up (median, 5 years; range, 2-11 years). Patients with MRI of insufficient quality were excluded. Findings were validated in 2 independent cohorts from tertiary epilepsy surgery centers. Study follow-up was completed on September 23, 2016, and data were analyzed from September 24, 2016, through April 24, 2018.
Exposures
Standard anterior temporal lobe resection.
Main Outcomes and Measures
Long-term postoperative seizure freedom.
Results
In total, 107 patients with unilateral TLE (left-sided in 68; 63.6% women; median age, 37 years [interquartile range {IQR}, 30-45 years]) were included in the derivation cohort. Reduced postsurgical gray matter volumes were found in the ipsilateral piriform cortex in the postoperative seizure-free group (n = 46) compared with the non–seizure-free group (n = 61). A larger proportion of the piriform cortex was resected in the seizure-free compared with the non–seizure-free groups (median, 83% [IQR, 64%-91%] vs 52% [IQR, 32%-70%]; P < .001). The results were seen in left- and right-sided TLE and after adjusting for clinical variables, presurgical gray matter alterations, presurgical hippocampal volumes, and the proportion of white matter tract disconnection. Findings were externally validated in 2 independent cohorts (31 patients; left-sided TLE in 14; 54.8% women; median age, 41 years [IQR, 31-46 years]). The resected proportion of the piriform cortex was individually associated with seizure outcome after surgery (derivation cohort area under the curve, 0.80 [P < .001]; external validation cohorts area under the curve, 0.89 [P < .001]). Removal of at least half of the piriform cortex increased the odds of becoming seizure free by a factor of 16 (95% CI, 5-47; P < .001). Other mesiotemporal structures (ie, hippocampus, amygdala, and entorhinal cortex) and the overall resection volume were not associated with outcomes.
Conclusions and Relevance
These results support the importance of resecting the piriform cortex in neurosurgical treatment of TLE and suggest that this area has a key role in seizure generation.
Introduction
Approximately 50% of people with refractory focal epilepsy continue to have seizures after surgery.1 Incomplete removal of a crucial area could cause ongoing seizures.2
The targeting of a common critical epileptic trigger zone is thought to prevent the development or progression of epilepsy. In this respect, the identification of the area tempestas in the anterior piriform cortex of rodents and nonhuman primates stands out for several reasons.3 First, injections of chemoconvulsants into this region produced bilateral clonic seizures at much lower concentrations than required when applied to other brain regions.3,4 Second, the piriform cortex was more susceptible to electrical stimulation to generate epileptic seizures than the hippocampus, amygdala, or adjacent cortex.5,6 Third, piriform cortex neurons were most sensitive to damage during prolonged seizures, and injury of this cortex during status epilepticus was associated with the development of spontaneous seizures.7,8 These findings were consistently replicated in animal models but, to date, evidence of a human epileptic trigger zone remains limited.9,10
The human piriform cortex resembles a ribbon, embracing the superior and inferior banks of the entorhinal sulcus, located rostromedially to the amygdala11 (Figure 1A and B). The histologic properties of the piriform cortex are similar to those of the hippocampus.12 The piriform cortex is well connected with the limbic system,13 orbitofrontal and insular cortex,14 and the thalamus,15 hence serving as a corticosubcortical circuit hub.9 Emerging evidence from human imaging data suggests that the piriform cortex may be involved in generating focal seizures arising from the temporal or the frontal lobes.16 Significant abnormalities within the human piriform cortex have been identified using a range of methods, including voxel-based structural magnetic resonance imaging (MRI) analysis,17 positron emission tomography of γ-aminobutyric acid A receptor binding,16 interictal and ictal electroencephalography–correlated functional MRI (fMRI),16,18,19 and most recently, resting state fMRI.20
Figure 1. Localization of the Piriform Cortex and Voxel-Based Morphometry (VBM) Results.
The position of the piriform cortex relative to other mesiotemporal structures is illustrated using 3-dimensional (3D) reconstructions (A) and magnetic resonance imaging (MRI) sections (B). Numbers are Montreal Neurological Institute (MNI) section coordinates. The remnant of the piriform cortex is outlined on postsurgical MRI scans of 2 cases (C). The left image is from a right-handed woman in her 30s with incomplete resection of the piriform cortex who continued having seizures after epilepsy surgery. The right image is from a right-handed man in his 40s with more extensive resection of the piriform cortex who became seizure free. Both cases had right-sided temporal lobe epilepsy (TLE) with neuropathologically confirmed hippocampal sclerosis. The statistical parametric maps for the interaction between seizure-free (SF) vs non–seizure-free (NSF) groups and preoperative vs postoperative scans are displayed in the lower half of the Figure (D). This comparison allows determination of gray matter decreases due to epilepsy surgery (ie, comparing postoperative with preoperative images) that were present in SF but not in NSF participants. The statistical mask of significant gray matter volume decreases (red; threshold, uncorrected P < .001) for left- (n = 68) and right-sided (n = 39) TLE is superimposed on coronal (top), axial (middle), and sagittal (bottom) views of a standard MRI template (numbers below are MNI section coordinates). The probability of a voxel being surgically resected is overlaid in blue (range, 10%-100%). Also displayed are zoomed sections in rectangles and 3D reconstructions of neocortical resection margins.
The piriform cortex likely plays a facilitating and amplifying role in human epileptogenesis and may influence progression to medical intractability.11 We hypothesized that the removal of the piriform cortex could improve outcome after surgery for temporal lobe epilepsy (TLE). Inclusion of the piriform cortex within a successful temporal lobe resection would provide proof-of-concept for the clinical relevance of this area.
Methods
Derivation Cohort
From an ongoing single-center prospective cohort study of long-term outcome after epilepsy surgery,1 we identified consecutive individuals with medically refractory unilateral TLE who underwent anterior temporal lobe resection from January 1, 2005, through December 31, 2013. To maintain population homogeneity, included patients had (1) presurgical and postsurgical high-resolution structural MRI scans performed on the same scanner; (2) underwent surgery by the same neurosurgeon (A.W.M.); and (3) had at least 2 years of postoperative follow-up (median, 5 years; range, 2-11 years; completed September 23, 2016). We also identified 15 healthy control volunteers who underwent scanning using the same sequences on the same scanner. Patients with MRI scans of insufficient quality (ie, patient movement or technical artifacts) were excluded. The standard neurosurgical procedure and MRI acquisition are described in eMethods in the Supplement. The study was classified as a service evaluation involving further anonymized analysis of previously acquired clinical data, not requiring individual consent. Healthy controls and individuals with diffusion MRI provided written consent as part of previous studies approved by the local Research Ethics Committee.
Baseline Characteristics and Follow-up
The methods of data extraction and follow-up were previously described.1 In brief, for each individual, diagnosis of TLE was made by a multidisciplinary epilepsy team evaluation involving neurologists, neurophysiologists, neurosurgeons, neuropsychologists, and psychiatrists specializing in epilepsy based on clinical history, neurologic examination results, seizure semiology, long-term video-electroencephalography telemetry, MRI, and neuropsychological and psychiatric assessments. Seizure outcome was assessed annually using a standard surgery outcome classification.21 Patients were considered to be seizure free only if they never experienced seizures or auras throughout the follow-up period (class IA outcome), excluding acute postoperative seizures within the first week. Conversely, previous studies22 often applied the criterion of terminal remission, which only accounts for seizure control in the last years of follow-up, rather than complete seizure freedom, which has different biological implications.
We categorized presurgical and postsurgical neuropsychiatric outcome according to the DSM-IV-TR, as previously described.23 Verbal and visual memory was assessed preoperatively and 1 year after surgery using the Adult Memory and Information Processing Battery.24 We classified change in neuropsychological performance as improved, no change, or declined according to 80% reliable change indices, as described in detail previously.25
Imaging Analysis
Spatial preprocessing was performed using the voxel-based morphometry (VBM) 8 toolbox (http://www.neuro.uni-jena.de/vbm/) running on Statistical Parametric Mapping (SPM8) (Wellcome Trust Centre for Neuroimaging; https://www.fil.ion.ucl.ac.uk/spm/software/spm8). Our study population was large enough to allow for separate processing and analysis of left- and right-sided TLE without the need to flip images left and right, which may introduce artifacts. Spatially normalizing postsurgical scans can be problematic owing to lack of an appropriate normalization template and owing to shifts caused by surgery. To overcome this, we used a procedure similar to that of Yogarajah et al.26 Briefly, each individual’s postsurgical scan was segmented and nonlinearly coregistered to the individual’s presurgical scan. These deformations were performed within the patients using a patient-specific template and, thus, ensured high spatial accuracy despite the resection of the temporal lobe. Next, spatial normalization deformations were estimated with the VBM8 toolbox for presurgical scans only, and these deformations were applied to the coregistered postsurgical images in each patient. Therefore, all preoperative and postoperative images were aligned in a common space.
To validate the performance of this segmentation and normalization procedure, we determined the correspondence of the preprocessed images masked for the resected area compared with the normalization template using the Cohen κ27 and Dice28 coefficients. No differences were noted in the normalization accuracy between presurgical and postsurgical scans and between scans in seizure-free and non–seizure-free groups. Thus, our group-level VBM findings are unlikely to be explained by image misregistration.
Gray matter volumetric differences were assessed using a 2 × 2 full-factorial model (analysis of covariance) estimating an interaction between seizure-free vs non–seizure-free groups and preoperative vs postoperative scans. This process allowed us to determine gray matter decreases due to surgery (ie, comparing postoperative with preoperative images) seen in seizure-free (SF) but not in non–seizure-free (NSF) groups. Duration of preoperative epilepsy and the interval between presurgical and postsurgical scans were also used as nuisance variables.
Voxel wise corrections for multiple testing implemented in common neuroimaging packages (eg, SPM) have repeatedly been shown to be conservative.29,30 We expected to find small effects within the piriform cortex owing to the small size of this area and the limited variability of the extent of surgical resections. Thus, we decided to report changes at a statistical voxelwise threshold of P < .001 (2 tailed, uncorrected). To reduce false-positive findings, we required a minimum cluster size of 20 continuous voxels and later replicated our results with a regions-of-interest approach using volumetry.
Volumetric Analysis
The estimation of the surgical cavity volumes is described in eMethods in the Supplement. Presurgical volumes of the amygdala and entorhinal cortex were extracted using a parcellation algorithm based on Geodesic Information Flows31 freely available within the NiftyWeb service tool (University College London Centre for Medical Image Computing; http://niftyweb.cs.ucl.ac.uk/). Presurgical hippocampal volumes were obtained using Hipposeg,32 an automated parcellation software based on Similarity and Truth Estimation for Propagated Segmentations.33 This software was specifically developed in people with epilepsy and was shown to be robust in those with atrophic hippocampi. Hipposeg delineates the hippocampus reliably with no more variability than that seen between expert human raters.33 The proportions of resected hippocampus, amygdala, and entorhinal cortex were then estimated as the intersection between an individual’s presurgical regional mask and their resection mask, both in the native space of the presurgical image.
A validated software algorithm for parcellating the piriform cortex is not available. Thus, a blinded rater (I.B. and M.G.) manually outlined the piriform cortex on preoperative and postoperative MRI scans. We used a reliable method with anatomic landmarks based on histologic analysis in controls and patients.11 A detailed description of the outlining algorithm and the intraobserver and interobserver variability is reported in eResults 7 and eFigure 2 in the Supplement.
External Validation
We validated our results in 2 independent external validation cohorts. The first cohort included 18 people with TLE undergoing surgery at the Thomas Jefferson University, Philadelphia, Pennsylvania. The second included 13 undergoing surgery at the University of Pennsylvania. Methods for the external validation cohorts are described in eResults 5 and 6 in the Supplement. The institutional review board of the University of Pennsylvania, Philadelphia, approved this study, and all patients included gave written informed consent.
Statistical Analysis
Data were analyzed from September 24, 2016, through April 24, 2018. Categorical variables are displayed as number (percentage) and were analyzed with the Fisher exact test. Ordinal and continuous variables are displayed as median (interquartile range [IQR]) and were analyzed with the Mann-Whitney test. Paired ipsilateral and contralateral measurements were compared using the Wilcoxon signed-rank test. To correct for covariates, multivariate models were fitted with logistic regression. The outcome variability explained by a model was assessed with Nagelkerke R2 value. Model discrimination was calculated as the area under the receiver operating characteristics curve (AUC). Neuropsychological data was missing in 34 patients and calculations were performed with available data. All other data were complete. Two-sided P < .05 was considered significant. All calculations were performed using SPSS (version 24.0; IBM Corp) and R (version 3.3.3; R Foundation) statistical software.
Results
We included 107 individuals (68 with left-sided TLE) in the derivation cohort (68 women [63.6%] and 39 men [36.4%]; median age, 37 years [IRQ, 30-45 years]). Clinical characteristics are displayed in Table 1. The numbers of eligible or excluded participants were not systematically collected and cannot be determined retrospectively. Forty-six patients (43.0%) remained completely seizure free (outcome scale class IA)21 throughout postoperative follow-up (median, 5 years; range, 2-11 years). We noted no differences in clinical characteristics between outcome groups, except for increased odds of postoperative depression in the NSF group (12 of 46 [26.1%] vs 30 of 61 [49.2%]) (Table 1).
Table 1. Demographic and Clinical Characteristics in the Derivation Cohort.
| Characteristic | SF Group (n = 46) | NSF Group (n = 61) | P Valuea |
|---|---|---|---|
| Demographic | |||
| Sex, No. (%) | |||
| Male | 19 (41.3) | 20 (32.8) | .42 |
| Female | 27 (58.7) | 41 (67.2) | |
| Handedness, No. (%) | |||
| Right | 37 (80.4) | 51 (83.6) | .80 |
| Left | 9 (19.6) | 10 (16.4) | |
| Presurgical seizures, No. (%) | |||
| SPS | 26 (56.5) | 39 (63.9) | .55 |
| CPS | 44 (95.7) | 57 (93.4) | .70 |
| SGS | 33 (71.7) | 48 (78.7) | .50 |
| Status epilepticus | 4 (8.7) | 10 (16.4) | .39 |
| Frequency per month, median (IQR) | |||
| CPS | 7 (3-12) | 8 (3-11) | .93 |
| SGS | 0.3 (0.1-1.0) | 0.3 (0.1-1.8) | .46 |
| Acute postoperative seizures, No. (%) | 1 (2.2) | 3 (4.9) | .63 |
| Age, median (IQR), y | |||
| At seizure onset | 8 (3-17) | 11 (7-19) | .09 |
| At surgery | 35 (28-44) | 37 (31-47) | .23 |
| At middle scan | 34 (28-44) | 37 (31-47) | .20 |
| Duration of epilepsy at surgery, median (IRQ), y | 24 (15-37) | 21 (15-36) | .89 |
| Side of surgery, No. (%) | |||
| Right | 16 (34.8) | 23 (37.7) | .84 |
| Left | 30 (65.2) | 38 (62.3) | |
| Preoperative MRI findings, No. (%) | |||
| Hippocampal sclerosis | 40 (87.0) | 46 (75.4) | .15 |
| Lesion | 9 (19.6) | 17 (27.9) | .37 |
| Normal | 2 (4.3) | 5 (8.2) | .70 |
| Pathologic finding, No. (%) | |||
| Hippocampal sclerosis | 41 (89.1) | 50 (82.0) | .41 |
| Cavernoma | 2 (4.3) | 3 (4.9) | >.99 |
| DNT | 1 (2.2) | 1 (1.6) | >.99 |
| Gliosis | 2 (4.3) | 7 (11.5) | .30 |
| Neuropsychometricb | |||
| Preoperative Verbal IQ, median (IQR)c | 92 (84-107) | 88 (79-102) | .25 |
| Preoperative Performance IQ, median (IQR)c | 98 (76-106) | 96 (80-107) | .70 |
| Verbal memory outcome, No. (%) | |||
| Improved | 1 (0.3) | 7 (16.3) | .22 |
| No change | 20 (66.7) | 26 (60.5) | |
| Declined | 9 (30.0) | 10 (23.2) | |
| Visual memory outcome, No. (%) | |||
| Improved | 3 (10.0) | 4 (9.3) | .67 |
| No change | 24 (80.0) | 31 (72.1) | |
| Declined | 3 (10.0) | 8 (18.6) | |
| Psychiatric | |||
| Preoperative, No. (%) | |||
| Depression | 15 (32.6) | 27 (44.3) | .24 |
| Interictal or postictal psychosis | 3 (6.5) | 6 (9.8) | .73 |
| Anxiety disorder | 5 (10.9) | 11 (18.0) | .41 |
| Postoperative, No. (%) | |||
| Depression | 12 (26.1) | 30 (49.2) | .02 |
| Interictal or postictal psychosis | 2 (4.3) | 6 (9.8) | .46 |
| Anxiety disorder | 8 (17.4) | 14 (23.0) | .63 |
Abbreviations: CPS, complex partial seizure; DNT, dysembryoplastic neuroepithelial tumor; IQR, interquartile range; MRI, magnetic resonance imaging; NSF, non–seizure-free; SF, seizure-free; SGS, secondary generalized seizure; SPS, simple partial seizure.
Analyzed with Fisher exact test or Mann-Whitney test, reporting 2-sided P values.
Neuropsychological data were not available in 34 cases, including 16 patients in the SF group and 18 patients in the NSF group.
Assessed using the Wechler Adult Intelligence Scale III. Each number represents composite function assessed across a number of subtests recorded at a specific point, with higher scores indicating better performance.
Voxel-Based Morphometry
A more marked loss of gray matter occurred in the ipsilateral piriform cortex on postoperative compared with preoperative scans in the postsurgical SF group than in the NSF group (Figure 1D). We saw this effect in separate analyses for left- (cluster size, 222 voxels; t test, 3.85; uncorrected P < .001) and right-sided (cluster size, 133 voxels; t test, 4.07; uncorrected P < .001) TLE. One small significant cluster was also seen in the right thalamus in left-sided TLE (cluster size, 31 voxels; t test, 3.33; uncorrected P = .001) but not in right-sided TLE. Voxel-based comparisons did not reveal any differences in the posterior extent of the resection between the 2 outcome groups.
Volumetry
Having determined the area of significant gray matter differences between outcome groups using VBM, we aimed to confirm these changes in individuals. Thus, we extracted volumes and resected proportions of the piriform cortex and 3 other mesiotemporal regions involved in TLE (ie, hippocampus, amygdala, and entorhinal cortex).
The ipsilateral presurgical volumes of all 4 regions were smaller compared with the contralateral side (eTable 1 in the Supplement). Compared with healthy volunteers (n = 15), the left piriform cortex was smaller in patients with left-sided TLE and the right and left piriform cortices were smaller in patients with right-sided TLE (eResults 1 and eTable 7 in the Supplement). A preoperatively reduced volume of the ipsilateral piriform cortex was significantly associated with postoperative seizures (SF group median, 0.5 mL [IQR, 0.4-0.6 mL]; NSF group median, 0.4 mL [IQR, 0.3-0.5 mL]; P = .02), whereas no association was found for the hippocampus, amygdala, or entorhinal cortex (Table 2).
Table 2. Volumetric Results in the Derivation and Validation Cohorts.
| Cohort | SF Group | NSF Group | P Valuea |
|---|---|---|---|
| Derivation | |||
| No. of patients | 46 | 61 | NA |
| Preoperative ipsilateral volume, median (IQR), ml | |||
| Piriform cortex | 0.5 (0.4-0.6) | 0.4 (0.3-0.5) | .02 |
| Hippocampus | 1.9 (1.7-2.3) | 1.9 (1.6-2.3) | .39 |
| Amygdala | 1.0 (0.9-1.1) | 1.0 (0.8-1.1) | .57 |
| Entorhinal cortex | 2.2 (1.9-2.5) | 2.2 (2.0-2.4) | .75 |
| Resected proportion, median (IQR), % | |||
| Piriform cortex | 83 (64-91) | 52 (32-70) | <.001 |
| Hippocampus | 57 (47-64) | 58 (36-66) | .39 |
| Amygdala | 49 (37-62) | 48 (36-60) | .60 |
| Entorhinal cortex | 82 (72-89) | 82 (71-87) | .51 |
| Resection volume, median (IQR), mL | 49 (42-58) | 50 (42-58) | .92 |
| Validation | |||
| No. of patients | 12 | 19 | NA |
| Piriform cortex resected proportion, median (IQR), % | 67 (55-77) | 26 (6-45) | <.001 |
| Thomas Jefferson University | |||
| No. of patients | 6 | 12 | NA |
| Piriform cortex resected proportion, median (IQR), % | 60 (41-75) | 28 (15-54) | .04 |
| University of Pennsylvania | |||
| No. of patients | 6 | 7 | NA |
| Piriform cortex resected proportion, median (IQR), % | 70 (62-81) | 11 (6-45) | .008 |
Abbreviations: IQR, interquartile range; NA, not applicable; NSF, non–seizure-free; SF, seizure-free.
Analyzed with Mann-Whitney test, reporting 2-sided P values.
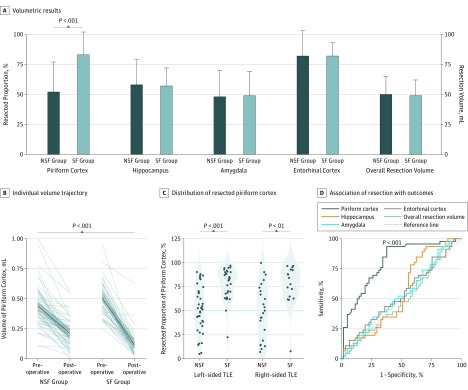
A significantly larger proportion of the piriform cortex had been resected in the SF group compared with the NSF group (median, 83% [IQR, 64%-91%] vs 52% [IQR, 32%-70%]; P < .001) (Figure 2A and B and Table 2). This association remained significant (adjusted odds ratio [OR] per 10% resected, 1.8; 95% CI, 1.4-2.4; P < .001) after correction for presurgical piriform cortex volume and factors previously reported to be associated with postoperative outcome (ie, sex, presence of hippocampal sclerosis, history of generalized seizures, preoperative seizure frequency, and duration of epilepsy)34 (eTable 4 in the Supplement). The variability of the resected proportion of the hippocampus, amygdala, or entorhinal cortex and the overall resection volume variability after standard anterior temporal lobe removal did not correlate with postsurgical outcome (Figure 2A and Table 2). The results were consistent between right- and left-sided temporal lobe surgery (Figure 2C and eResults 2 and eTables 2 and 3 in the Supplement).
Figure 2. Volumetric Results in the Derivation Cohort of Patients Undergoing Surgery for Temporal Lobe Epilepsy (TLE).
Volumetric results (A) are displayed as median (SD) (error bars) of the resected proportion of the piriform cortex, hippocampus, amygdala, and entorhinal cortex and the overall resection volume in the overall cohort (n = 107). Comparing postoperatively seizure-free (SF [n = 46]) with non–seizure-free (NSF [n = 61]) groups, difference in piriform cortex volumes was significant, whereas no differences were observed in all other regions. Gray lines show the individual trajectories of presurgical vs postsurgical volumes of the piriform cortex in the SF vs NSF groups (B). The mean trajectory is illustrated by a dark blue line; SD, by the light blue area. The distribution of the resected proportion of the piriform cortex in the NSF vs SF groups is shown (C); the 50% resection cutoff is displayed as a blue horizontal line. The association of resection area with outcomes (D) used receiver operating characteristics curves to describe individual discrimination. The piriform cortex was the only area to significantly discriminate between SF and NSF groups in the derivation cohort. All other areas remained close to the 45° reference line.
Patients in the NSF group after left-sided temporal lobe resections had gray matter atrophy in several extratemporal areas on their presurgical scans (eFigure 1 and eTables 8 and 9 in the Supplement). Even after controlling for presurgical gray matter volume within these areas, the resected proportion of the piriform cortex remained significantly associated with postsurgical outcome (adjusted OR, 1.8; 95% CI, 1.3-2.5; P = .001) (eTable 9 in the Supplement). Similarly, results were unchanged (adjusted OR, 1.7; 95% CI, 1.4-2.1; P < .001) when adjusting for the presurgical volume of the ipsilateral and contralateral hippocampus (eResults 3 and eTable 10 in the Supplement). We also found that postoperative outcome did not correlate with the disconnected proportion of 55 white matter tracts that we assessed35 (eResults 4 and eTables 11-12 in the Supplement). All these data suggest that our findings remain robust after adjusting for clinical variables, presurgical gray matter alterations, presurgical hippocampal volumes, and the proportion of white matter tract disconnection.
Neuropsychological and Psychiatric Outcomes
The extent of piriform cortex removal did not influence postoperative cognitive outcome and postsurgical anxiety or psychosis (eTable 5 in the Supplement). Larger piriform cortex resections were, however, associated with less postoperative depression (no postoperative depression median, 73% [IQR, 52%-88%]; postoperative depression median, 59% [IQR, 28%-75%]; P = .008). This finding could be due to a cross-correlation with positive seizure outcome in larger piriform cortex resections. Poor verbal memory outcome correlated with the median extent of hippocampal (decline, 58% [IQR, 51%-66%]; no decline, 45% [IQR, 29%-62%]; P = .03), amygdala (decline, 55% [IQR, 44%-67%]; no decline, 45% [IQR, 37%-57%]; P = .04), and entorhinal cortex (decline, 86% [IQR, 80%-90%]; no decline, 77% [IQR, 66%-87%]; P = .01) resections but not with median piriform cortex removal (decline, 69% [IQR, 57%-86%]; no decline, 51% [IQR, 29%-82%]; P = .10) in left-sided TLE (eTable 6 in the Supplement).
Individualized Outcome Associations
The resected proportion of the piriform cortex was associated with individual postoperative seizure outcome with an AUC of 0.80 (P < .001; left AUC, 0.82; right AUC, 0.77) (Figure 2D). All other studied regions were not associated with seizure outcome (hippocampus AUC, 0.55 [P = .39]; amygdala AUC, 0.53 [P = .59]; entorhinal cortex AUC, 0.54 [P = .51]; resection volume AUC, 0.49 [P = .92]).
External Validation
To determine the generalizability of our results, we externally validated them in 31 participants with TLE in 2 independent cohorts at the University of Pennsylvania and Thomas Jefferson University, Philadelphia, Pennsylvania (left-sided TLE in 14; 17 women [54.8%] and 14 men [45.2%]; median age, 41 years [IQR, 31-46 years]). Resection of the piriform cortex was associated with postsurgical outcome in individuals in the external cohorts (combined cohort AUC, 0.89 [P < .001]; Thomas Jefferson University AUC, 0.81 [P = .04]; University of Pennsylvania AUC, 0.93 [P = .01]) (Figure 3 and Table 2). The detailed external validation results are reported in eResults 5 and eTable 13 in the Supplement.
Figure 3. Volumetric Results in the Validation Cohort.
Gray lines show the individual trajectories of presurgical vs postsurgical volumes of the piriform cortex in the seizure-free (SF) vs non–seizure-free (NSF) groups (A). The mean trajectory is illustrated by a dark blue line; SD, by the light blue area. The distribution of the resected proportion of the piriform cortex is compared in the NSF and SF groups (B). The 50% resection cutoff is displayed as a blue horizontal line. The association of piriform cortex resection with outcome (C) used receiver operating characteristics curves to describe individual-subject discrimination. TJU indicates Thomas Jefferson University; UP, University of Pennsylvania.
When we combined the internal derivation data with data from the external validation cohorts (n = 138), only 4 of 47 patients (8.5%) became seizure free if less than 50% of the piriform cortex were resected, compared with 54 of 91 patients (59.3%) if at least 50% was removed (P < .001) (Figure 2C and Figure 3B). Resection of at least half of the piriform cortex increased the odds of complete seizure freedom by a factor of 16 (95% CI, 5-47; P < .001). The proportion of resected piriform cortex explained an estimated 36% of variability in postoperative seizure outcome.
Discussion
We present findings from one of the largest series, to our knowledge, comparing preoperative and postoperative MRI in individuals with epilepsy.22 We demonstrated that larger resections of the piriform cortex were associated with postsurgical seizure freedom using 2 different methods (VBM and volumetry) and validated the results in 2 external cohorts. Our findings suggest that removal of at least 50% of the piriform cortex is required to achieve seizure freedom after temporal lobe resections, because only 9% of individuals became seizure free if less than half of the piriform cortex was removed.
Our results provide evidence suggesting that the human piriform cortex has a role in the generation of seizures that involve the temporal lobe. The piriform cortex is the most susceptible area for epileptogenic stimulation3,4,5,6,8,19 and a node for the spread of epileptic discharges in TLE.9,16,18,19 We infer that seizures originating from different parts of the temporal lobe can involve an epileptic network that includes the piriform cortex.7,8,10
Our findings suggest that if the epileptic network in the piriform cortex is not sufficiently disrupted by removing at least half of this area, an individual has 16 times higher odds of having postoperative seizures. As previously suggested,36 other mesiotemporal regions were not associated with postoperative seizures. Removal of the piriform cortex was safe regarding neuropsychological and psychiatric postsurgical outcome.
Conversely, removal of at least 50% of the piriform cortex does not per se guarantee a favorable outcome. Other factors play a role, and they might explain the remaining two-thirds of outcome variability: mislocalization or incomplete resection of the epileptogenic focus, and the generation of an occult independent epileptogenic area outside of the anterior temporal lobe.2,37
Strengths and Limitations
A strength of our study is that all surgical and imaging procedures in the derivation cohort were performed in a highly standardized way by the same neurosurgeon and on the same MRI scanner. Another strength is that the results were validated in 2 independent external cohorts with a separate scanner and surgeon, supporting the generalizability of our findings. Our findings were robust even after correction for relevant clinical variables, presurgical gray matter alterations, presurgical hippocampal volumes, and the proportion of white matter tract disconnection.
A limitation inherent in all epilepsy surgery studies is that antiepileptic drug dosages cannot be reduced systematically in a randomized or unbiased way after initially successful surgery. Neuropsychometric data were not available in 34 individuals, and we restricted the neuropsychological analyses to available data. We did not see a difference in presurgical hippocampal volumes or the presence of hippocampal sclerosis between postoperatively SF and NSF groups, potentially owing to the small statistical variability of hippocampal findings in our cohort of anterior temporal lobe resections, of whom 86 (80.4%) had hippocampal sclerosis. Voxel-based morphometry identified significant voxels within the piriform cortex in left- and right-sided TLE analyses, although the exact localization of the results did not perfectly overlap. This minimal variability of results can be explained owing to small differences in surgical approaches in the dominant vs the nondominant hemispheres. Information on seizure semiology, including the presence of an olfactory aura, was not prospectively collected and was not available for analysis. Voxel-based morphometry cannot distinguish whether gray matter loss attributes to removal of a structure or to operative disconnection and consecutive atrophy of the area through Wallerian degeneration. For both scenarios, however, our findings support the concept that the piriform cortex is involved in generation or modulation of focal seizure activity. We did not find white matter tract disconnection to be associated with the surgical outcome. The VBM analyses were not corrected for multiple comparisons in view of the presence of an a priori hypothesis about the role of the piriform cortex and of the small size of this area. We have, however, robust confirmatory evidence based on volumetric findings from 1 internal (ie, derivation) and 2 external (ie, validation) cohorts supporting the relevance of this area for postsurgical outcome.
Conclusions
Our results support a critical role of the piriform cortex in generating seizures arising from the temporal lobe. Such knowledge can have implications not only for the understanding of basic mechanisms underlying epileptic networks, but also for the development of pharmacological and nonpharmacological approaches to modulate seizure activity and thus, to modify the course of epilepsy. Our findings provide support for removal of the piriform cortex as a novel outcome biomarker and treatment target for epilepsy surgery. If confirmed in prospective interventional trials, these findings will have practical implications for guiding neurosurgeons about the extent of the surgical resection (Figure 1C) and will provide a novel target for placing electrodes for preoperative recording and deep brain stimulation.
eMethods. Derivation Cohort
eTable 1. Ipsilateral vs Contralateral Volumes of 4 Mesiotemporal Regions in the Derivation Cohort
eTable 2. Volumetric Results in Left-sided TLE in the Derivation Cohort
eTable 3. Volumetric Results in Right-sided TLE in the Derivation Cohort
eTable 4. Logistic Regression Model of Postoperative Seizure Outcome in the Derivation Cohort
eTable 5. Extent of Piriform Cortex Resection and Neuropsychological or Psychiatric Surgical Outcome in the Derivation Cohort
eTable 6. Association of Volumetric Results With Verbal Memory Decline After Left Anterior Temporal Lobectomy in the Derivation Cohort
eTable 7. Volumetric Results in Left- and Right-sided TLE vs Healthy Controls in the Derivation Cohort
eTable 8. Significant Clusters Associated With Lower GM Volume in Left-sided TLE Patients With Postoperative Seizures Compared With Those Who Were Seizure-Free After Surgery
eTable 9. Logistic Regression Model of Postoperative Seizure Outcome Corrected for Presurgical Grey Matter Atrophy
eTable 10. Logistic Regression Model of Postoperative Seizure Outcome Corrected for Ipsilateral and Contralateral Presurgical Hippocampal Volumes
eTable 11. Association of Proportion of Uncinate Fasciculus Resection With Outcome After Epilepsy Surgery
eTable 12. Association of Proportion of Resected White Matter Tracts With Seizure Freedom After Epilepsy Surgery
eTable 13. Demographic and Clinical Characteristics of External Validation Cohorts
eResults 1. Supplementary Analyses of Healthy Controls
eResults 2. Supplementary VBM Analyses
eResults 3. Supplementary Volumetric Analyses
eResults 4. Supplementary Tractography Analyses
eResults 5. Supplementary Notes on External Validation
eResults 6. Validation Cohort
eResults 7. Supplementary Note on Piriform Cortex Outlining
eFigure 1. Glass-Brain Projection of Voxels Significantly Associated With Lower GM Volume in Left-sided TLE Patients With Postoperative Seizures Compared With Those Who Were Seizure-Free After Surgery
eFigure 2. Piriform Cortex Outlining
References
- 1.de Tisi J, Bell GS, Peacock JL, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378(9800):1388-1395. doi: 10.1016/S0140-6736(11)60890-8 [DOI] [PubMed] [Google Scholar]
- 2.Najm I, Jehi L, Palmini A, Gonzalez-Martinez J, Paglioli E, Bingaman W. Temporal patterns and mechanisms of epilepsy surgery failure. Epilepsia. 2013;54(5):772-782. doi: 10.1111/epi.12152 [DOI] [PubMed] [Google Scholar]
- 3.Piredda S, Gale K. A crucial epileptogenic site in the deep prepiriform cortex. Nature. 1985;317(6038):623-625. doi: 10.1038/317623a0 [DOI] [PubMed] [Google Scholar]
- 4.Gale K. Progression and generalization of seizure discharge: anatomical and neurochemical substrates. Epilepsia. 1988;29(suppl 2):S15-S34. doi: 10.1111/j.1528-1157.1988.tb05795.x [DOI] [PubMed] [Google Scholar]
- 5.McIntyre DC, Kelly ME. The parahippocampal cortices and kindling. Ann N Y Acad Sci. 2000;911:343-354. doi: 10.1111/j.1749-6632.2000.tb06736.x [DOI] [PubMed] [Google Scholar]
- 6.Hönack D, Wahnschaffe U, Löscher W. Kindling from stimulation of a highly sensitive locus in the posterior part of the piriform cortex: comparison with amygdala kindling and effects of antiepileptic drugs. Brain Res. 1991;538(2):196-202. doi: 10.1016/0006-8993(91)90430-4 [DOI] [PubMed] [Google Scholar]
- 7.Roch C, Leroy C, Nehlig A, Namer IJ. Predictive value of cortical injury for the development of temporal lobe epilepsy in 21-day-old rats: an MRI approach using the lithium-pilocarpine model. Epilepsia. 2002;43(10):1129-1136. doi: 10.1046/j.1528-1157.2002.17802.x [DOI] [PubMed] [Google Scholar]
- 8.Roch C, Leroy C, Nehlig A, Namer IJ. Magnetic resonance imaging in the study of the lithium-pilocarpine model of temporal lobe epilepsy in adult rats. Epilepsia. 2002;43(4):325-335. doi: 10.1046/j.1528-1157.2002.11301.x [DOI] [PubMed] [Google Scholar]
- 9.Vaughan DN, Jackson GD. The piriform cortex and human focal epilepsy. Front Neurol. 2014;5(suppl 6):259. doi: 10.3389/fneur.2014.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Löscher W, Ebert U. The role of the piriform cortex in kindling. Prog Neurobiol. 1996;50(5-6):427-481. doi: 10.1016/S0301-0082(96)00036-6 [DOI] [PubMed] [Google Scholar]
- 11.Gonçalves Pereira PM, Insausti R, Artacho-Pérula E, Salmenperä T, Kälviäinen R, Pitkänen A. MR volumetric analysis of the piriform cortex and cortical amygdala in drug-refractory temporal lobe epilepsy. AJNR Am J Neuroradiol. 2005;26(2):319-332. [PMC free article] [PubMed] [Google Scholar]
- 12.Allison AC. The secondary olfactory areas in the human brain. J Anat. 1954;88(4):481-488. [PMC free article] [PubMed] [Google Scholar]
- 13.Kajiwara R, Tominaga T, Takashima I. Olfactory information converges in the amygdaloid cortex via the piriform and entorhinal cortices: observations in the guinea pig isolated whole-brain preparation. Eur J Neurosci. 2007;25(12):3648-3658. doi: 10.1111/j.1460-9568.2007.05610.x [DOI] [PubMed] [Google Scholar]
- 14.Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346(3):403-434. doi: 10.1002/cne.903460306 [DOI] [PubMed] [Google Scholar]
- 15.Kuroda M, Murakami K, Kishi K, Price JL. Distribution of the piriform cortical terminals to cells in the central segment of the mediodorsal thalamic nucleus of the rat. Brain Res. 1992;595(1):159-163. doi: 10.1016/0006-8993(92)91468-T [DOI] [PubMed] [Google Scholar]
- 16.Laufs H, Richardson MP, Salek-Haddadi A, et al. Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology. 2011;77(9):904-910. doi: 10.1212/WNL.0b013e31822c90f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centeno M, Vollmar C, Stretton J, et al. Structural changes in the temporal lobe and piriform cortex in frontal lobe epilepsy. Epilepsy Res. 2014;108(5):978-981. doi: 10.1016/j.eplepsyres.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan D, Badawy RAB, Jackson GD. EEG-fMRI in focal epilepsy: local activation and regional networks. Clin Neurophysiol. 2014;125(1):21-31. doi: 10.1016/j.clinph.2013.06.182 [DOI] [PubMed] [Google Scholar]
- 19.Vaudano AE, Carmichael DW, Salek-Haddadi A, et al. Networks involved in seizure initiation: a reading epilepsy case studied with EEG-fMRI and MEG. Neurology. 2012;79(3):249-253. doi: 10.1212/WNL.0b013e31825fdf3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen M, Curwood EK, Vaughan DN, Omidvarnia AH, Jackson GD. Abnormal brain areas common to the focal epilepsies: multivariate pattern analysis of fMRI. Brain Connect. 2016;6(3):208-215. doi: 10.1089/brain.2015.0367 [DOI] [PubMed] [Google Scholar]
- 21.Wieser HG, Blume WT, Fish D, et al. ; Commission on Neurosurgery of the International League Against Epilepsy (ILAE) . ILAE Commission Report: proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42(2):282-286. [PubMed] [Google Scholar]
- 22.Bonilha L, Keller SS. Quantitative MRI in refractory temporal lobe epilepsy: relationship with surgical outcomes. Quant Imaging Med Surg. 2015;5(2):204-224. doi: 10.3978/j.issn.2223-4292.2015.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleary RA, Thompson PJ, Fox Z, Foong J. Predictors of psychiatric and seizure outcome following temporal lobe epilepsy surgery. Epilepsia. 2012;53(10):1705-1712. doi: 10.1111/j.1528-1167.2012.03604.x [DOI] [PubMed] [Google Scholar]
- 24.Coughlan AK, Hollows S. The Adult Memory and Information Processing Battery. Leeds, UK: St James University Hospital; 1985. [Google Scholar]
- 25.Baxendale S, Thompson P. Defining meaningful postoperative change in epilepsy surgery patients: measuring the unmeasurable? Epilepsy Behav. 2005;6(2):207-211. doi: 10.1016/j.yebeh.2004.12.009 [DOI] [PubMed] [Google Scholar]
- 26.Yogarajah M, Focke NK, Bonelli SB, et al. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain. 2010;133(8):2348-2364. doi: 10.1093/brain/awq175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37-46. doi: 10.1177/001316446002000104 [DOI] [Google Scholar]
- 28.Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26(3):297-302. doi: 10.2307/1932409 [DOI] [Google Scholar]
- 29.Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. Neuroimage. 2008;40(2):655-661. doi: 10.1016/j.neuroimage.2007.11.058 [DOI] [PubMed] [Google Scholar]
- 30.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113(28):7900-7905. doi: 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardoso MJ, Modat M, Wolz R, et al. Geodesic Information Flows: spatially-variant graphs and their application to segmentation and fusion. IEEE Trans Med Imaging. 2015;34(9):1976-1988. doi: 10.1109/TMI.2015.2418298 [DOI] [PubMed] [Google Scholar]
- 32.Winston GP, Cardoso MJ, Williams EJ, et al. Automated hippocampal segmentation in patients with epilepsy: available free online. Epilepsia. 2013;54(12):2166-2173. doi: 10.1111/epi.12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jorge Cardoso M, Leung K, Modat M, et al. ; Alzheimer’s Disease Neuroimaging Initiative . STEPS: Similarity and Truth Estimation for Propagated Segmentations and its application to hippocampal segmentation and brain parcelation. Med Image Anal. 2013;17(6):671-684. doi: 10.1016/j.media.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 34.Jehi L, Yardi R, Chagin K, et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 2015;14(3):283-290. doi: 10.1016/S1474-4422(14)70325-4 [DOI] [PubMed] [Google Scholar]
- 35.Rojkova K, Volle E, Urbanski M, Humbert F, Dell’Acqua F, Thiebaut de Schotten M. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Struct Funct. 2016;221(3):1751-1766. doi: 10.1007/s00429-015-1001-3 [DOI] [PubMed] [Google Scholar]
- 36.Keller SS, Richardson MP, Schoene-Bake J-C, et al. Thalamotemporal alteration and postoperative seizures in temporal lobe epilepsy. Ann Neurol. 2015;77(5):760-774. doi: 10.1002/ana.24376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barba C, Rheims S, Minotti L, et al. Temporal plus epilepsy is a major determinant of temporal lobe surgery failures. Brain. 2016;139(pt 2):444-451. doi: 10.1093/brain/awv372 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Derivation Cohort
eTable 1. Ipsilateral vs Contralateral Volumes of 4 Mesiotemporal Regions in the Derivation Cohort
eTable 2. Volumetric Results in Left-sided TLE in the Derivation Cohort
eTable 3. Volumetric Results in Right-sided TLE in the Derivation Cohort
eTable 4. Logistic Regression Model of Postoperative Seizure Outcome in the Derivation Cohort
eTable 5. Extent of Piriform Cortex Resection and Neuropsychological or Psychiatric Surgical Outcome in the Derivation Cohort
eTable 6. Association of Volumetric Results With Verbal Memory Decline After Left Anterior Temporal Lobectomy in the Derivation Cohort
eTable 7. Volumetric Results in Left- and Right-sided TLE vs Healthy Controls in the Derivation Cohort
eTable 8. Significant Clusters Associated With Lower GM Volume in Left-sided TLE Patients With Postoperative Seizures Compared With Those Who Were Seizure-Free After Surgery
eTable 9. Logistic Regression Model of Postoperative Seizure Outcome Corrected for Presurgical Grey Matter Atrophy
eTable 10. Logistic Regression Model of Postoperative Seizure Outcome Corrected for Ipsilateral and Contralateral Presurgical Hippocampal Volumes
eTable 11. Association of Proportion of Uncinate Fasciculus Resection With Outcome After Epilepsy Surgery
eTable 12. Association of Proportion of Resected White Matter Tracts With Seizure Freedom After Epilepsy Surgery
eTable 13. Demographic and Clinical Characteristics of External Validation Cohorts
eResults 1. Supplementary Analyses of Healthy Controls
eResults 2. Supplementary VBM Analyses
eResults 3. Supplementary Volumetric Analyses
eResults 4. Supplementary Tractography Analyses
eResults 5. Supplementary Notes on External Validation
eResults 6. Validation Cohort
eResults 7. Supplementary Note on Piriform Cortex Outlining
eFigure 1. Glass-Brain Projection of Voxels Significantly Associated With Lower GM Volume in Left-sided TLE Patients With Postoperative Seizures Compared With Those Who Were Seizure-Free After Surgery
eFigure 2. Piriform Cortex Outlining