Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- AEA
arachidonyl ethanolamide (anandamide)
- 2‐AG
2‐arachidonoyl glycerol
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- ASH
alcoholic steatohepatitis
- AST
aspartate aminotransferase
- CLD
chronic liver disease
- EC
endocannabinoid
- ECS
endocannabinoid system
- EMT
EC membrane transporter
- HCV
hepatitis C virus
- HDL
high‐density lipoprotein
- HIV
human immunodeficiency virus
- I/R
ischemia/reperfusion
- LDL
low‐density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PBC
primary biliary cirrhosis
- PSC
primary sclerosing cholangitis
- TG
triglyceride
- THC
Δ9‐tetrahydrocannabinol
- VLDL
very low density lipoprotein
Mounting evidence indicates that the endocannabinoid (EC) system (ECS) plays an important role in various liver diseases including viral hepatitis, nonalcoholic fatty liver disease (NAFLD), alcoholic liver disease, hepatic encephalopathy, and autoimmune hepatitis. The ECS also impacts on involved processes such as hepatic hemodynamics, nutrient intake and turnover, and ischemia/reperfusion (I/R) after liver transplantation. Although this involvement is undisputed, therapeutic implications regarding the ECS are just beginning to emerge; so far, no approved drug acting specifically on the ECS is available.
ECS
Research in the ECS began when the most active component of marijuana, Δ9‐tetrahydrocannabinol (THC), was isolated in 1960 to study its psychoactive and neurological effects. Later, endogenously produced analogues were detected and were found to signal in the central nervous system by regulating memory, food intake, motor functions, nociception, and energy balance. Subsequently, components of the ECS were also found to act in the body's periphery on immune cells and various organs, including the liver.
ECs [arachidonyl ethanolamide (anandamide) (AEA) and 2‐arachidonoyl glycerol (2‐AG)], as well as exocannabinoids (THC, cannabidiol, among others), mediate their effects via different cannabinoid receptors. ECs are synthesized on demand, act locally, and are rapidly degraded by specific enzymes (Figure 1).
Figure 1.
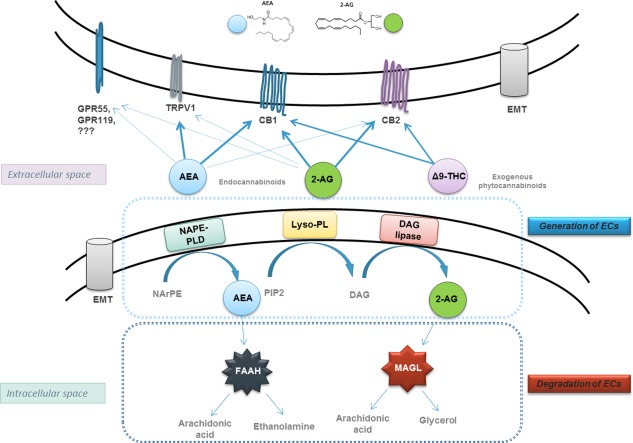
ECS. Anandamide (AEA) is generated by hydrolysis of the membrane lipid N‐arachidonyl‐phosphatidylethanolamine (NArPE), which is catalyzed by a phospholipase D that selectively recognizes N‐acylated species of phosphatidylethanolamine (NAPE‐PLD). 2‐AG is produced from phosphatidylinositol‐4, 5‐bisphosphate (PIP2), converted into 1,2‐diacylglycerol (DAG) by lyso‐phospholipase (Lyso‐PL) and consequent hydrolysis by diacylglycerol lipase‐α (DGL‐α) to yield 2‐AG. CB1, CB2, transient receptor potential cation channel subfamily V member 1 (TRPV1), and G protein‐coupled receptors (GPR) GPR56 and GPR119 are the receptors of EC and exocannabinoid THC. Fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) are degrading enzymes for AEA and 2‐AG, respectively, hydrolyzing them into arachidonic acid, ethanolamine, and glycerol. Abbreviation: EMT, EC membrane transporter.
ECs are involved in numerous physiological and pathophysiological processes in chronic liver diseases (CLDs). The upregulation of cannabinoid receptors CB1 and CB2 in NAFLD, alcoholic liver disease, autoimmune and viral hepatitis, I/R, cirrhosis, and related disturbances (intrahepatic vascular resistance, hyperdynamic circulatory syndrome, hepatic encephalopathy) were convincingly demonstrated, leading to the intriguing hypothesis of functional antagonism of these receptors in liver pathophysiology, suggesting CB1 to promote and CB2 to protect from liver damage.1, 2, 3
ECs in CLD
The role of ECs, AEA and 2‐AG in particular, in the development of CLD is still not completely understood. Most of the studies interrogating the actions of the ECS in the liver have been executed using CB1 and CB2 agonists/antagonists. However, because ECs are ligands for not only EC receptors, but also for other receptors, such as TRPV1, GPR55, and CPR119, it is still largely enigmatic how ECs contribute to liver pathophysiology. One of the major effects of ECs in the liver is its impact on metabolism: secretion of hormones regulating appetite and satiety (leptin, adiponectin), lipogenesis, adipogenesis, obesity, and insulin resistance via CB1. Elevation of certain ECs in plasma and liver tissues has been shown for human and experimental CLD (viral hepatitis, bile duct injury, steatosis, cirrhosis), partly correlating with disease severity. Figure 2 displays the numerous suggested effects and functions of ECs.
Figure 2.
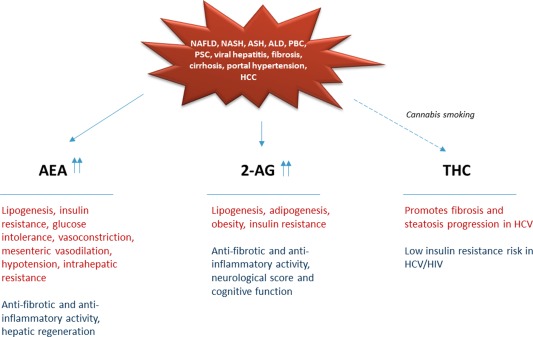
EC and exocannabinoid functions in CLD. Abbreviations: ALD, alcoholic liver disease; HCC, hepatocellular carcinoma; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis.
In rodent models of cirrhosis, AEA appears as a mediator of mesenteric vasodilation and hypotension, promoting intrahepatic resistance, whereas 2‐AG was shown to improve neurological score and cognitive function in hepatic encephalopathy. At the same time, ECs induced proapoptotic effects in myofibroblasts and inflammatory cells in chronic liver damage, indicating potent antifibrotic and anti‐inflammatory activity, whereas in liver regeneration, AEA revealed stimulating effects on hepatocyte proliferation.3
Clinically, patients with chronic hepatitis C who consume cannabis on a daily basis had more severe fibrosis and steatosis compared with nonconsumers or occasional consumers.4 In contrast, others showed no effect of smoked marijuana on hepatitis C virus (HCV) progression in patients with human immunodeficiency virus (HIV).5 Moreover, cannabis use was associated with less insulin resistance in HIV‐HCV‐coinfected patients.6 Thus, more research is required to elucidate the role of ECs in CLD, because ECs might exert positive as well as negative effects, indicating a note of caution in the use of these nonspecifically targeted molecules and their modulators (endocannabinoid‐degrading enzymes inhibitors) or exogenous phytocannabinoids as therapeutics for the treatment of CLD.
Cannabinoid Receptors as Targets in CLD
Many therapeutic approaches have been introduced to target CB1 and CB2 in various settings of chronic hepatitis. With the development of highly specific agonists/antagonists for CB1 and CB2, as well as genetically modified animal models, the research on ECS became clearer and more promising. Thus, in CB1−/− and CB2−/− mice, deletion of CB1 improved hepatic fibrosis and steatosis induced by carbon tetrachloride or high‐fat diet, whereas the lack of CB2 increased collagen deposition, liver fat, and enhanced inflammatory scores (Figure 3).
Figure 3.
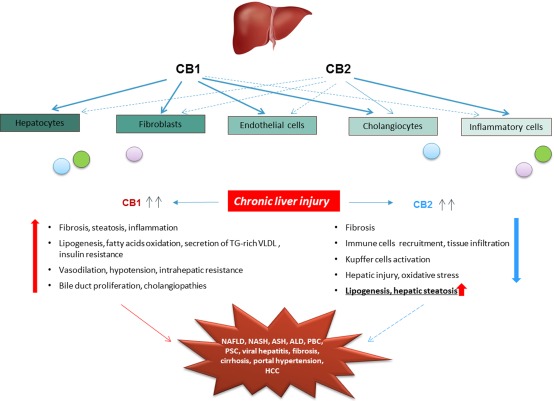
EC receptors expression and functions in the liver. Cannabinoid receptors CB1 and CB2 are expressed in all liver cell types at different basal levels. Both receptors are upregulated during chronic liver damage and mediate opposite functions: CB1 promotes and CB2 protects from liver damage. The experimental and clinical evidences indicate CB1 as a stronger player, contributor and an attractive target in the development of CLD. Abbreviations: ALD, alcoholic liver disease; HCC, hepatocellular carcinoma; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; TG, triglyceride; VLDL, very low density lipoprotein.
Pharmaceutically, CB1 blockage by SR141716 (rimonabant) and AM6545, or CB2 agonism with JWH‐133 and HU‐308 in NAFLD, alcoholic steatohepatitis (ASH), and cholestasis models showed beneficial effects, as reflected by improved hepatic fibrosis, steatosis, inflammation, and liver regeneration, as well as normalization of serum liver enzymes levels, triglycerides, free fatty acids, and cholesterol. In cirrhosis, CB1 blockage positively affected hemodynamics via increased arterial pressure and vascular resistance, and reduced mesenteric blood flow and portal pressure. In cirrhosis‐associated hepatic encephalopathy, rimonabant and HU‐308 improved neurological and cognitive function and activity performance. Alleviated tissue injury, decreased inflammatory cell infiltration, and apoptosis were observed in I/R after CB2 agonism by HU‐308 (Table 1).
Table 1.
Cannabinoid Receptor Agonists/Antagonists Applied in Animal Models and Human Studies of CLD
| Compound | Disease | Effects | |
|---|---|---|---|
| Animal experiments | |||
| CB1 antagonists | SR141716 (rimonabant), CB1−/− mice | Cirrhosis, high‐fat diet, biliary fibrosis, alcoholic hepatitis, I/R | Improved fibrosis, steatosis, ascites, hypotension, portal pressure, cardiac contractility, fatty acids oxidation, insulin and leptin sensitivity, I/R‐related tissue damage, increased adiponectin |
| New, peripherally restricted | AM6545, JD5037, TM38837, VD60 | Improved liver damage, reduced obesity and leptin resistance | |
| CB2 agonists | JWH‐133, HU‐308, HU‐910 | Acute liver injury, alcoholic liver disease, I/R, autoimmune hepatitis, biliary fibrosis, cirrhosis | Suppressed histopathological and apoptotic liver damage, reduced inflammation, oxidative stress, fibrosis and bacterial translocation |
| Metabolic liver disease/high‐fat diet | Induced insulin resistance and steatosis | ||
| HU‐444 (new, peripherally restricted) | Suppressed hepatic inflammation and fibrogenesis | ||
| Human studies | |||
| CB1 and CB2 agonism | Exogenous cannabinoids (THC, cannabis smoking) | HCV/HIV | Promoted fibrosis progression rate, steatosis severity, glucose intolerance (?), lowered insulin resistance risk |
| CB1‐ and CB2‐independent, GPR55 antagonist | Exogenous cannabinoid (cannabidiol) | Hepatic encephalopathy | Improved cognitive and motor function, neuroinflammation |
| CB1 antagonists | Rimonabant | Obesity, metabolic syndrome, NASH, T2D | Reduced the prevalence of metabolic syndrome, body weight, TG, waist circumference, ALT, AST, GGT; increased HDL cholesterol |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; HDL, high‐density lipoprotein; NASH, nonalcoholic steatohepatitis; T2D, type 2 diabetes; TG, triglyceride.
Rimonabant was successfully tested in clinical trials in obese patients, where it reduced the prevalence of the metabolic syndrome (41% versus 26%) after 1 year, improving body weight and waist circumference, HDL cholesterol and triglycerides, and glucose tolerance and insulin levels.7 However, because of a high rate of depression and psychoactive side effects, further development for clinical use was terminated.
In conclusion, ECS modulation seems promising while bearing in mind certain and intended effects, whereas caution is warranted regarding potentially antagonistic activities of the same molecule (Figure 4).
Figure 4.
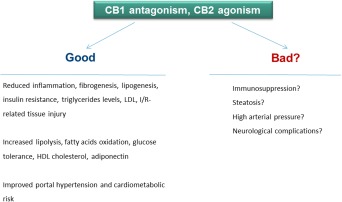
Opposing therapeutic effects of ECS modulation. Abbreviations: HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Future Perspectives
Considering the ubiquity of the ECS in the human body, targeting its components is extremely attractive in many conditions including liver diseases. Although the first CB ligands, such as rimonabant, were effective, they were also encumbered with significant toxicity; novel compounds with improved specificity, restricted to the organ of interest and lacking neuropsychiatric adverse effects, are needed and are currently being developed. Some of them, such as AM6545, TM38837, and HU‐444, have entered clinical testing.8, 9, 10
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Tam J, Liu J, Mukhopadhyay B, Cinar R, Godlewski G, Kunos G. Endocannabinoids in liver disease. Hepatology 2011;53:346‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basu PP, Aloysius MM, Shah NJ, Brown RS. Review article: the endocannabinoid system in liver disease, a potential therapeutic target. Aliment Pharmacol Ther 2014;39:790‐801. [DOI] [PubMed] [Google Scholar]
- 3. Pisanti S, Picardi P, Pallottini V, Martini C, Petrosino S, Proto MC, et al. Anandamide drives cell cycle progression through CB1 receptors in a rat model of synchronized liver regeneration. J Cell Physiol 2015;230:2905‐2914. [DOI] [PubMed] [Google Scholar]
- 4. Hézode C, Zafrani ES, Roudot‐Thoraval F, Costentin C, Hessami A, Bouvier‐Alias M, et al. Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology 2008;134:432‐439. [DOI] [PubMed] [Google Scholar]
- 5. Brunet L, Moodie EE, Rollet K, Cooper C, Walmsley S, Potter M, et al. Marijuana smoking does not accelerate progression of liver disease in HIV‐hepatitis C coinfection: a longitudinal cohort analysis. Clin Infect Dis 2013;57:663‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carrieri MP, Serfaty L, Vilotitch A, Winnock M, Poizot‐Martin I, Loko MA, et al. Cannabis use and reduced risk of insulin resistance in hiv‐hcv infected patients: a longitudinal analysis (ANRS CO13 HEPAVIH). Clin Infect Dis 2015;61:40‐48. [DOI] [PubMed] [Google Scholar]
- 7. Boesten JE, Kaper J, Stoffers HE, Kroon AA, van Schayck OC. Rimonabant improves obesity but not the overall cardiovascular risk and quality of life; results from CARDIO‐REDUSE (CArdiometabolic Risk reDuctIOn by Rimonabant: the Effectiveness in Daily practice and its USE). Fam Pract 2012;29:521‐527. [DOI] [PubMed] [Google Scholar]
- 8. Chorvat RJ. Peripherally restricted CB1 receptor blockers. Bioorg Med Chem Lett 2013;23:4751‐4760. [DOI] [PubMed] [Google Scholar]
- 9. Klumpers LE, Fridberg M, de Kam ML, Little PB, Jensen NO, Kleinloog HD, et al. Peripheral selectivity of the novel cannabinoid receptor antagonist TM38837 in healthy subjects. Br J Clin Pharmacol 2013;76:846‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haj CG, Sumariwalla PF, Hanuš L, Kogan NM, Yektin Z, Mechoulam R, et al. HU‐444, a novel, potent anti‐inflammatory, nonpsychotropic cannabinoid. J Pharmacol Exp Ther 2015;355:66‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]


