Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- AASLD
American Association for the Study of Liver Disease
- DAC
daclatasvir
- FDA
US Food and Drug Administration
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IDSA
Infectious Disease Society of America
- IFN
interferon
- PEG
peginterferon
- RBV
ribavirin
- SOF
sofosbuvir
- SVR
sustained virological response
Therapy for hepatitis C virus (HCV) infection has progressed dramatically with well‐tolerated and highly effective regimens. Daclatasvir, an NS5A replication complex inhibitor with in vitro activity against genotypes 1 to 6, is the latest antiviral to enter practice.1 Daclatasvir in combination with sofosbuvir was approved by the US Food and Drug Administration (FDA) in July 2015 for genotype 3. The daclatasvir and sofosbuvir regimen addresses limitations in HCV treatment, particularly among patients with genotype 3, but also for human immunodeficiency virus (HIV)‐HCV coinfection and those patients who are unable to tolerate ribavirin.
HCV Genotype 3
Before daclatasvir, sofosbuvir with ribavirin was the only interferon‐free option for genotype 3. The VALENCE trial established higher sustained virological response (SVR) rates for genotype 3 by extending sofosbuvir and ribavirin to 24 weeks. SVR12 rates in VALENCE was 93% among treatment‐naive patients and 77% among patients who did not respond positively to interferon.2 However, the long duration, need for ribavirin, and low rates with cirrhosis were limitations for sofosbuvir and ribavirin. Furthermore, the BOSON trial found that peginterferon, ribavirin, and sofosbuvir for 12 weeks led to higher SVR rates than 16 or 24 weeks of sofosbuvir and ribavirin.3 Accordingly, the American Association for the Study of Liver Disease (AASLD) and the Infectious Disease Society of America (IDSA) recommended peginterferon, ribavirin, and sofosbuvir for genotype 3 and relegated sofosbuvir and ribavirin to alternative regimen status (Table 1).4
Table 1.
American Association for the Study of Liver Disease/Infectious Disease Society of America 2015 Treatment Recommendations for Genotype 3
| Genotype and Treatment History | Peginterferon + Sofosbuvir + Ribavirin | Sofosbuvir + Ribavirin | Daclatasvir + Sofosbuvir |
|---|---|---|---|
| Naive |
Recommended 12 weeks |
Alternative 24 weeks |
Recommended, 12 weeks Cirrhosis: 24 weeks ± ribavirin |
| Peginterferon/Ribavirin failure |
Recommended 12 weeks |
Recommended if IFN ineligible, 12 weeks Cirrhosis: 24 weeks + ribavirin |
|
| Sofosbuvir failure |
Recommended 12 weeks |
Recommended if IFN ineligible, 24 weeks + ribavirin |
Abbreviation: IFN, interferon.
In the ALLY‐3 trial, daclatasvir and sofosbuvir without ribavirin for 12 weeks achieved SVR12 in 90% of treatment‐naive and 86% of treatment‐experienced genotype 3 patients.5 The AASLD/IDSA recommends daclatasvir and sofosbuvir for 12 weeks for noncirrhotic genotype 3 patients, including treatment‐naive patients and patients with unsuccessful peginterferon therapy.4 In ALLY‐3, lower SVR occurred with cirrhosis (Fig. 1). The ALLY‐3+ study was reported at the 2015 Liver Meeting and treated patients with bridging fibrosis or cirrhosis with daclatasvir, sofosbuvir, and ribavirin for 12 or 16 weeks. All patients with bridging fibrosis were cured, and SVR12 rates for patients with cirrhosis were 83% for 12 weeks and 89% for 16 weeks.6 The AASLD/IDSA guidance panel has recommended 24 weeks' duration with sofosbuvir, daclatasvir, and ribavirin for cirrhotic genotype 3 patients.
Figure 1.

ALLY‐3 study results of daclatasvir and sofosbuvir for 12 weeks for genotype 3. Abbreviation: SVR, sustained virological response.
HCV Genotype 1
The daclatasvir plus sofosbuvir combination is pangenotypic, and the AASLD/IDSA recommends this regimen for genotype 1 patients. The duration is 12 weeks without ribavirin if no cirrhosis and 24 weeks with or without ribavirin in patients with cirrhosis (Table 2).4 Although no large phase 3 trial evaluated genotype 1–naive patients, these recommendations are supported from a phase 2 trial (Fig. 2), ALLY‐1 in advanced cirrhosis and post–liver transplantation, and ALLY‐2 in HIV/HCV coinfection (Fig. 3).7, 8, 9 The phase 2 study was an early demonstration of the potency of this regimen with SVR in 40 of 41 (98%) patients who did not respond successfully to a first‐generation protease inhibitor regimen.7 ALLY‐1 added ribavirin and demonstrated high SVR rates in all patient groups except Child‐Pugh class C.8 Daclatasvir and sofosbuvir is not FDA‐approved for genotype 1, and the likelihood of coverage by payers may determine whether clinicians can offer this regimen to patients.
Table 2.
American Association for the Study of Liver Disease/Infectious Disease Society of America 2015 Treatment Recommendations for Genotypes 1 and 2
| Genotype and Treatment History | Daclatasvir + Sofosbuvir |
|---|---|
| Genotype 1, naive or PEG/PI failure | |
| No cirrhosis | Recommended, 12 weeks |
| Cirrhosis | Recommended, 24 weeks ± RBV |
| Genotype 2 | |
| Naive | Recommended, 12 weeks |
| PEG/RBV failure | Limited data |
| Sofosbuvir failure | Recommended if interferon ineligible, 24 weeks ± RBV |
Abbreviations: PEG, peginterferon; RBV, ribavirin; PI, protease inhibitor.
Figure 2.

Phase 2 results of daclatasvir (DAC) and sofosbuvir (SOF) for genotype 1. Abbreviations: RBV, ribavirin; SVR, sustained virological response.
Figure 3.
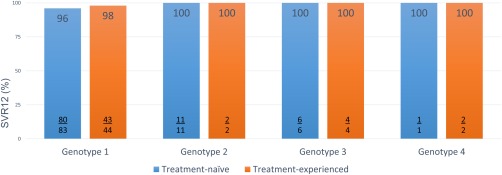
ALLY‐2 study 12‐week arm results of daclatasvir and sofosbuvir for human immunodeficiency virus/hepatitis C virus coinfection. Abbreviation: SVR, sustained virological response.
HCV Genotype 2
The first‐line treatment option for treatment‐native patients with genotype 2 is sofosbuvir and ribavirin for 12 weeks with 16 weeks if cirrhotic.4 No large phase 3 study was conducted for daclatasvir and sofosbuvir for genotype 2 patients, and the combination is not FDA‐approved for genotype 2. In the phase 2 study, genotype 2 treatment‐naive patients achieved SVR12 in 24 of 26 (92%) patients with 24 weeks of sofosbuvir and daclatasvir.7 In the HIV/HCV coinfection ALLY‐2 study, all 11 treatment‐naive patients and both treatment‐experienced genotype 2 patients achieved SVR12 with 12 weeks of daclatasvir and sofosbuvir.9 The AASLD/IDSA recommends 12 weeks of daclatasvir and sofosbuvir for treatment‐naive patients who cannot tolerate ribavirin (Table 2).4 No specific recommendation was made for peginterferon and ribavirin failures because of limited data. Patients with genotype 2 with unsuccessful sofosbuvir therapy are recommended to receive sofosbuvir in combination with peginterferon and ribavirin. If interferon‐ineligible, daclatasvir and sofosbuvir with or without ribavirin is recommended.
HIV/HCV Coinfection
The ALLY‐2 study evaluated daclatasvir and sofosbuvir in HIV/HCV coinfection. A key finding of the study was that 8 weeks' duration was ineffective. In the 12‐week arms, daclatasvir and sofosbuvir for 12 weeks achieved high SVR rates in HIV/HCV genotypes 1 to 4 regardless of previous HCV treatment or cirrhosis (Fig. 3).9 Treatment of HIV/HCV coinfected patients requires management of the drug–drug interactions between the HCV and HIV. Daclatasvir offers a potential advantage over other agents with limited drug–drug interactions (Table 3) and flexibility in dosing. Daclatasvir comes in the standard dose of 60 mg but also 30‐mg tablets. As an example, patients receiving moderate CYP3A inducers like efavirenz would require increased dosing of daclatasvir from 60 mg to 90 mg. Patients on strong CYP3A inhibitors like atazanavir/ritonavir would require reduced dosing of daclatasvir to 30 mg. This flexibility potentially would allow some patients to avoid alterations in their HIV antiretroviral regimens.
Table 3.
| Nucleoside reverse‐transcriptase inhibitors | |
| Abacavir | a, b |
| Didanosine | b |
| Emtricitabine | a, b |
| Lamivudine | b |
| Stavudine | b |
| Tenofovir | b |
| Zidovudine | b |
| Non‐nucleoside reverse‐transcriptase inhibitor | |
| Efavirenz | Daclatasvir ↓c |
| Etravirine | Daclatasvir ↓c |
| Nevirapine | c |
| Rilpivirine | a, b |
| Protease inhibitors | |
| Atazanavir/Ritonavir | Daclatasvir ↑c |
| Darunavir/Ritonavir | Daclatasvir ↑b |
| Fosamprenavir | c |
| Lopinavir/Ritonavir | Daclatasvir ↑b |
| Saquinavir | c |
| Integrase inhibitors | |
| Dolutegravir | Dolutegravir ↑b |
| Elvitegravir/Cobicistat | a, c |
| Maraviroc | a, b |
| Raltegravir | a, b |
Not studied/no data.
No clinically significant interaction expected.
Potential interaction that may require a dosage adjustment, altered timing of administration, or additional monitoring.
Conclusion
Daclatasvir in combination with sofosbuvir offers major advantages for genotype 3 patients with a once‐daily, ribavirin‐free regimen for noncirrhotic patients and improved response rates for patients with cirrhosis through extended duration and the addition of ribavirin. Although the FDA approval for only genotype 3 may limit access, the pangenotypic nature of the regimen offers other options to patients who cannot tolerate ribavirin. The favorable drug–drug interaction profile and flexible dosing may be preferable to patients with HIV/HCV coinfection.
Potential conflict of interest: A.J.M. has the following potential conflicts of interest: Abbvie, research grants and advisory boards; BMS, research grants and advisory boards; Gilead, research grants and advisory boards; Janssen, research grants and advisory boards; and Merck, research grants and advisory boards.
References
- 1. Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 2010;465:96‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al.; VALENCE Investigators . Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014;370:1993‐2001. [DOI] [PubMed] [Google Scholar]
- 3. Foster GR, Pianko S, Cooper C, Agarwal K. Sofosbuvir + peginterferon/ribavirin for 12 weeks vs sofosbuvir + ribavirin for 16 or 24 weeks in genotype 3 HCV infected patients and treatment‐experienced cirrhotic patients with genotype 2 HCV: the BOSON study. In: 50th Annual Meeting of the European Association for the Study of the Liver (EASL). New York: Gastroenterology and Hepatology; 2015. Abstract L05.
- 4. American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society of America (IDSA) . HCV guidance: recommendations for testing, managing, and treating hepatitis C. 2015. Retrieved from http://hcvguidelines.org/ on October, 2015.
- 5. Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N; ALLY‐3 Study Team . All‐oral 12‐week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY‐3 phase III study. Hepatology 2015;61:1127‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leroy V, Angus PW, Bronowicki JP, Dore G, Hezode C, Pianko S, et al. All‐oral treatment with Daclatasvir plus Sofosbuvir plus Ribavirin for 12 or 16 weeks in HCV genotype 3 infected patients with advanced fibrosis or cirrhosis: the ALLY‐3+ Phase Study. In: The American Association for the Study of Liver Disease (AASLD) Liver Meeting; November 13–17, 2015; San Francisco, CA. Abstract LB3.
- 7. Sulkowski MS, Gardiner DF, Rodriguez‐Torres M, Reddy KR, Hassanein T, Jacobson I, et al.; AI444040 Study Group . Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014;370:211‐221. [DOI] [PubMed] [Google Scholar]
- 8. Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR, et al.; ALLY‐2 Investigators . Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV‐1. N Engl J Med 2015;373:714‐725. [DOI] [PubMed] [Google Scholar]
- 9. Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, et al. Daclatasvir, sofosbuvir, and ribavirin combination for HCV patients with advanced cirrhosis or post‐transplant recurrence: ALLY‐1 Phase 3 Study. Gastroenterol Hepatol 2015:1‐23. [Google Scholar]
- 10. Eley T, You X, Wang R, Luo W‐L, Huang S‐P, Kandoussi H, et al. Daclatasvir: overview of drug‐drug interactions with antiretroviral agents and other common concomitant drugs. HIV DART 2014; December 9–12, 2014; Miami, FL. Abstract 63.
- 11. Bifano M, Hwang C, Oosterhuis B, Hartstra J, Grasela D, Tiessen R, et al. Assessment of pharmacokinetic interactions of the HCV NS5A replication complex inhibitor daclatasvir with antiretroviral agents: ritonavir‐boosted atazanavir, efavirenz and tenofovir. Antivir Ther 2013;18:931‐940. [DOI] [PubMed] [Google Scholar]


