Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- BCLC
Barcelona Clinic Liver Cancer
- CLIP
Cancer of the Liver Italian Program
- CTP
Child‐Turcotte‐Pugh
- CUPI
Chinese University Prognostic Index
- DM
, diabetes mellitus
- GRETCH
Groupe d'ETUDE et de Traitement du Carcinome Hepatocellulaire
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HTN
hypertension
- JIS
Japan Integrated Staging
- TACE
transarterial chemoembolization
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy1 and is one of the leading causes of cancer‐related death in the United States.2 Prognosis of HCC remains poor, driven by advanced tumor burden at diagnosis in two‐thirds of cases.
Cancer staging systems are important for prognostication and determination of therapy. HCC has unique characteristics for which it is more difficult to use standard cancer staging strategies. First, there is enormous heterogeneity with regard to patient characteristics and HCC biology. Second, in the Western world, the majority of HCC occurs in patients with significant underlying liver disease, making liver function and functional status important in determining outcome. Lastly, tissue diagnosis is not often required, and radiological diagnosis is often the standard. Various staging systems (Table 1) have been proposed to incorporate these variables, but there is no universal worldwide consensus.3 Two of these staging systems [Barcelona Clinic Liver Cancer (BCLC) and Groupe d'ETUDE et de Traitement du Carcinome Hepatocellulaire (GRETCH)] include performance status, with the BCLC being validated in various geographical settings and with very large data sets.4 As a result, the BCLC staging system is the most accepted system in the United States, having received the endorsement of both the American Association for the Study of Liver Diseases and the European Association for the Study of Liver diseases (Figure 1).5 Its main advantage is the unique way in which it provides therapy guidelines based on staging, making it a very useful clinical tool.6
Table 1.
Characteristics of Seven Staging Systems for HCC
| Staging System | Hepatic Function | Performance Status | Tumor Variables |
|---|---|---|---|
| BCLC | CTP | Yes | Size, number of nodules and portal vein thrombosis |
| CLIP | CTP | Yes | Number of nodules, tumor greater or less than 50% of liver, portal vein thrombosis, and AFP |
| CUPI | Bilirubin, ascites, alkaline phosphatase | Presence of symptoms | TNM and AFP |
| GRETCH | Bilirubin, alkaline phosphatase | Yes | Portal vein thrombosis and AFP |
| JIS | CTP | No | TNM |
| Okuda | Albumin, ascites, and bilirubin | No | Tumor greater or less than 50% of liver |
| TNM | No | No | Number of nodules, tumor size, presence of portal vein thrombosis, presence of metastasis |
Abbreviations: CLIP, Cancer of the Liver Italian Program; CUPI, Chinese University Prognostic Index; JIS, Japan Integrated Staging; AFP, alpha‐fetoprotein.
Figure 1.
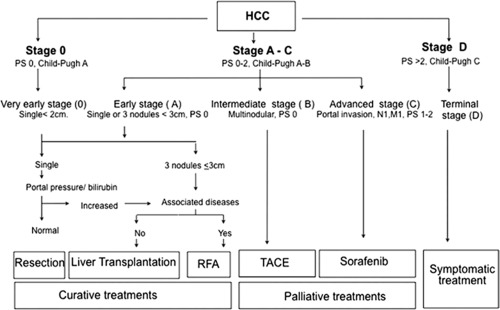
BCLC staging system. Adapted from Hepatology.5 Copyright 2011. American Association for the Study of Liver Diseases.
Although it remains one of the best HCC staging systems, BCLC does have some shortcomings, particularly in regard to intermediate stage (stage B) HCC. In BCLC, transarterial chemoembolization (TACE) is recommended for stage B patients, whereas curative interventions such as surgical resection are recommended only for very early and early disease stage disease (stage 0 or A). Sorafenib and supportive care are recommended for advanced (stage C) and terminal stage (stage D) disease, respectively.5 In practice, these guidelines are not often followed for many reasons.7 One reason is that stage B comprises a highly heterogeneous population with respect to tumor burden, liver function, and cause of underlying liver disease; this latter component is not accounted for in BCLC. Consequently, the prognosis and suitability for treatment are quite variable within this stage. Examples of different stage B patients are listed in Table 2, where all are classified as stage B HCC, but the question remains: Would they benefit from TACE?
Table 2.
Examples of Intermediate‐Stage HCC
| Patient No. | Age (years) | Comorbidities | Child's Score | Cause | Nodules (n) | Size of Largest | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 60 | Tobacco | A (6) | HCV | 5 | 1.4 cm | Needed 1 TACE, with complete response |
| 2 | 65 | DM, HTN, tobacco | B (7) | HCV | 5 | 2 cm | Needed 1 TACE, with progression |
| 3 | 64 | DM, HTN, tobacco | A (5) | HCV | 3 | 5 × 5 cm | Needed 4 TACEs, but only partial response |
| 4 | 62 | Tobacco | B (8) | HCV | 1 | 2 × 5.1 cm | Needed 1 TACE, with complete response |
Abbreviations: DM, diabetes mellitus; HCV, hepatitis C virus; HTN, hypertension.
The cause of the liver disease is also not included in BCLC, yet the presence of comorbid conditions such as diabetes or cardiovascular disease in patients with nonalcoholic steatohepatitis‐related HCC will influence treatment options. Thus, recommendations as prescribed by BCLC in this population may not be feasible. In addition to disease etiology, there are also questions whether the classification of performance status within BCLC is optimized.8 Although performance status is clearly an important component of prognosis in HCC, it is not clear that excluding patients with performance status worse than 0 is ideal for patients with stage B HCC.8
In addition to intrinsic difficulties in staging patients, the recommended intervention is also somewhat controversial. For example, surgical intervention is recommended for only early‐stage disease, but there is now emerging evidence demonstrating surgical resection may have better long‐term outcomes in patients with stage B as compared with TACE,9 prompting the question whether resection should be restricted to stage 0/A disease and not expanded to stage B disease.
For patients with more advanced disease, it is also not clear that TACE is not beneficial. Recommendations of TACE for stage B disease were born from analyses of studies showing negative predictors of survival, such as vascular invasion, performance status >0, and BCLC stage C disease. Much of the data were based on conventional TACE, whereas recent advances have made drug‐eluting beads TACE and Y90 more commonly available and generally better tolerated. There is continued evidence of individual interdisciplinary decisions resulting in treatment stage migration in the nonsurgical population. Although only approximately 10% to 12% of new HCC diagnoses fall into stage B disease, TACE is easily the most commonly used treatment modality, with almost half the treatments worldwide being used in stage C patients.10 This may be explained by both DEB‐TACE and Y90‐TACE demonstrating better efficacy as compared with conventional TACE,11 which may expand the reaches of this treatment modality to patients with stage C HCC.
As a result of a highly heterogeneous patient population in stage B HCC, patient selection has become crucial to the success of TACE. Within stage B, patients with higher tumor burden and less preserved liver function have poorer responses to TACE.7 In our own study, we found that the best predictor of outcome after TACE was not BCLC but Child‐Turcotte‐Pugh (CTP) score and tumor burden. Similar findings have been noted by other groups. In an attempt to improve risk stratification and avoid TACE‐related harm, multiple groups have been working on creating subclassifications within stage B and scoring systems to better guide therapeutic decision making. This includes Bolondi et al.'s work in developing subclasses with stage B,12 as well as the HAP (Hepatoma Arterial‐embolisation Prognostic) and STATE (Selection for TrAnsarterial chemoembolisation TrEatment) scores, which help identify the best patients for initial TACE procedure while limiting procedural harm and lower morbidity and mortality.10 It should be recognized that response to TACE is also an important factor that will not be captured with any pretreatment staging systems.
In addition to routine clinical variables to determine risk stratifications, there is growing interest in biomarkers or alternative patient characterization tools that may predict outcome in HCC. Although functional status is clearly an important patient variable, we and others did not find that Eastern Cooperative Oncology Group (ECOG) score was predictive of outcome after TACE.7 This may be reflective of the intrinsic qualitative nature of the scoring system. In an attempt to better characterize the patient population, we have used a novel methodology (analytic morphomics) to assess patient characteristics by using quantitative image analysis of the readily available computed tomography imaging studies.13, 14 Analytic morphomics is an image‐processing technique that assesses body composition measures such as body dimensions, visceral fat, and muscle mass, and links them to clinical outcomes.13 This provides a more objective manner of assessing functional status. Our results demonstrated that in comparison with the BCLC staging system, a model that included analytic morphomics provided a highly accurate prognosis in the nonsurgical population. Because all patients with HCC generally have cross‐sectional imaging, this method has the potential to provide added information from existing scans that may improve prognostication.
Even though BCLC is the most widely accepted HCC staging system in the Western hemisphere and provides very important guidelines for treatment, there are shortcomings. With regard to recommendations for TACE in intermediate‐stage HCC, there are concerns around a restrictive prescription of therapy to a highly heterogeneous patient population. Thus, it is important to recognize that BCLC should not be used as a conscripted algorithm. Decisions in practice are likely to be affected by local preferences and availability of resources such as transplantation. It should be noted that HCC therapy is best done with multidisciplinary model input from experts in hepatology, hepatobiliary surgery, and interventional radiology to reduce variability and enhance patient selection. Further research in this area will likely yield better predictors of survival and improve patient selection.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. El‐Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res 2007;37(suppl 2):S88‐S94. [DOI] [PubMed] [Google Scholar]
- 2. Mittal S, El‐Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47(suppl):S2‐S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology 2005;41:707‐716. [DOI] [PubMed] [Google Scholar]
- 4. Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol 2006;44:723‐731. [DOI] [PubMed] [Google Scholar]
- 5. Bruix J, Sherman M, American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marrero JA, Kudo M, Bronowicki JP. The challenge of prognosis and staging for hepatocellular carcinoma. Oncologist 2010;15(suppl 4):23‐33. [DOI] [PubMed] [Google Scholar]
- 7. Barman PM, Sharma P, Krishnamurthy V, Willatt J, McCurdy H, Moseley RH, et al. Predictors of mortality in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Dig Dis Sci 2014;59:2821‐2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, Lin HC, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology 2013;57:112‐119. [DOI] [PubMed] [Google Scholar]
- 9. Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, et al. Comparison of long‐term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One 2013;8:e68193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sieghart W, Hucke F, Peck‐Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol 2015;62:1187‐1195. [DOI] [PubMed] [Google Scholar]
- 11. Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate‐stage hepatocellular carcinoma. Nat Rev Clin Oncol 2014;11:525‐535. [DOI] [PubMed] [Google Scholar]
- 12. Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 2012;32:348‐359. [DOI] [PubMed] [Google Scholar]
- 13. Singal AG, Zhang P, Ananthakrishnan L, Waljee AK, Sharma P, Barman P, et al. Analytic morphomics predicts overall survival in patients with hepatocellular carcinoma. Gastroenterology 2015;148:S1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parikh ND, Singal AG, Zhang P, Krishnamurthy V, Barman P, Waljee AK, et al. Analytic morphomics predicts survival in patients with hepatocellular carcinoma treated with transarterial chemoembolization. Hepatology 2015;62:420A‐4021A. [DOI] [PMC free article] [PubMed] [Google Scholar]


