Watch a video presentation of this article
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CC
cryptogenic cirrhosis
- GGT
gamma‐glutamyltransferase
- HCC
hepatocellular carcinoma
- IBD
inflammatory bowel disease
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OLT
orthotopic liver transplantation
Cryptogenic cirrhosis (CC) is the end stage of a chronic liver disease in which its underlying etiology remains unknown after extensive clinical, serological, and pathological evaluations have been performed. CC contributes to morbidity and mortality associated with liver disease worldwide and represents a significant and increasing indication for orthotopic liver transplantation (OLT) in the United States according to data from the Organ Procurement and Transplantation Network (Figure 1).1 The prevalence rate of CC ranged from 5% to 30% in patients with cirrhosis in earlier studies but has decreased to an estimated 5% with advances in the field and the development of viral hepatitis testing and serology, as well as other biomarkers.1
Figure 1.
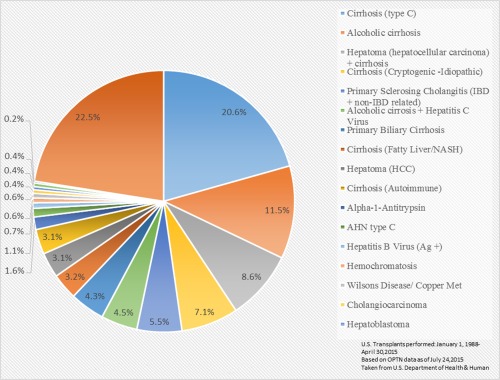
Liver transplants by diagnosis from 1988 to 205 (n = 135,191). Abbreviations: HCC, hepatocellular carcinoma; IBD, inflammatory bowel disease. Abbreviations: AHN, acute hepatic necrosis.
In an attempt to identify possible causative factors, early studies focused on describing unrecognized prevalence of viral and nonviral hepatitis including hepatitis A, B, and C, autoimmune hepatitis, and occult alcohol use, but shifted years later as testing and diagnostic yield improved and research elucidated other causative factors. Metabolic causes including nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH), metabolic syndrome, type 2 diabetes, and obesity became of interest because they were identified more commonly in patients with CC in comparison with other well‐described etiologies (i.e., chronic viral hepatitis, primary biliary cirrhosis, autoimmune hepatitis, and alcoholic liver disease).2, 3
An extensive evaluation must be performed before the diagnosis of CC, including work‐up for chronic viral hepatitis, autoimmune conditions, alcohol abuse, toxin exposure, vascular and biliary tract diseases, congenital causes, and progression of NAFLD/NASH. Many causes of chronic liver injury can lead to cirrhosis and it is important to determine the specific cause in view of the implications in the management of patients and the long‐term outcomes including liver transplantation (Table 1). Histological assessment is important, because specific tissue diagnosis can detect clinically silent advanced disease and provide an assessment of grade and fibrosis, thus providing staging information for the clinician. In those identified with NAFLD/NASH, aggressive management of metabolic comorbidities such as type 2 diabetes mellitus, dyslipidemia, and obesity is important and may be helpful, because no particular proven treatment or drug has been developed for fatty liver disease (Table 2). In view of an increased proportion of the current population with NASH risk factors such as obesity and metabolic syndrome, this underlying cause should be suspected when evaluation renders clinicians with no identifiable causes despite presence of metabolic syndrome in the cirrhotic patient.
Table 1.
Various Indications for Liver Transplantation
| Fulminant hepatic failure |
| Complications of cirrhosis (ascites, synthetic dysfunction, encephalopathy, liver malignancy, variceal hemorrhage refractory to standard therapy, chronic gastrointestinal bleeding secondary to portal hypertension) |
| Systemic complications of chronic liver disease (hepatopulmonary syndrome and portopulmonary hypertension) |
| Metabolic conditions that cause systemic disease (NASH, alpha1‐antitrypsin deficiency, Wilson's disease, amyloidosis, hemochromatosis, glycogen storage diseases, urea cycle enzyme deficiencies) |
Table 2.
Nonalcoholic Fatty Liver Disease
| Diagnosis |
| Elevated serum liver enzymes (AST, ALT, GGT) or |
| Imaging study with evidence of fatty content and minimal to no alcohol intake and |
| Negative results for viral hepatitis, autoimmune disease, congenital liver disease, and any other plausible explanation |
| Liver biopsy with evidence of fatty content with or without inflammation or fibrosis and minimal to no alcohol intake |
| Management |
| Lifestyle modification (weight loss, physical exercise, diet with calories restriction) |
| Insulin‐sensitizing agents (e.g., biguanides and thiazolidinedione) |
| Others (e.g., antioxidants, lipid‐lowering agents, and ursodeoxycholic acid) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyltransferase.
It has been demonstrated that patients with NASH and CC with low Model for End‐Stage Liver Disease scores have slower progression in their clinical course and are less likely to be OLT recipients when compared with those with hepatitis C virus–associated cirrhosis (O'Leary, 2011).4 A single‐center study aimed at evaluating long‐term outcomes and survival in those transplant‐free patients reported similar survival rates in CC patients compared with those with alcoholic cirrhosis, and mortality was influenced mainly by age at diagnosis and Child's class.4 In comparison with patients with hepatitis C virus, hepatocellular carcinoma is detected at a more advanced stage in those with CC, limiting therapeutic options and shortening survival time. This may be attributed to lack of surveillance programs in those with CC.6 Therefore, increased liver‐related morbidity and complications in those with CC is an important issue for primary clinicians caring for these patients. Adequate follow‐up, screening measures, comorbidity management, and early pre‐OLT referral must ensue to ensure adequate care for patients with CC.
Histopathological examination of the post‐OLT explant may help identify hidden etiologies and lesions, yet in almost 50% to 60%, the cause remains undetermined.7, 8 A few studies have examined long‐term clinical and graft outcomes after OLT. Four of these studies have shown a low prevalence of recurrent disease, favorable outcomes, and survival comparable with other recipients.8, 9, 10, 11 However, in a study by Álamo et al.,12 chronic rejection and postoperative mortality rates were higher and survival rates at 5, 10, and 15 years were lower than other OLT etiologies. Another retrospective study revealed higher recurrence of CC and NASH in post‐OLT patients and was associated with the presence of metabolic syndrome, hypertension, and use of insulin.13 However, the possibility of developing NASH in the post‐OLT allograft in a proportion of transplanted patients with CC suggests this cause may have been the underlying liver disease now presenting with recurrence, although de novo NASH has to be considered as well. Studies have demonstrated that histological changes of NASH may no longer be identified at the time of liver biopsy or explant evaluation; thus, a significant number of patients with CC may have burnt‐out liver disease (“burnt‐out NASH”) once cirrhosis has been established.9, 13
Recent work in the role of genetic factors and thrombogenetic and fibrogenetic mechanisms in the development of cirrhosis is under investigation. A study in a Caucasian population with cirrhosis (including CC) involving thrombophilic genetic factors suggested that PAI‐1 4G‐4G and MTHFR 677TT may have roles as risk factors in the development of liver fibrosis and thrombosis. Thus, the genetics of cirrhosis is yet to be further clarified as a possible link to the development and outcomes specifically of patients with CC.14
Cryptogenic liver disease remains a multifactorial and heterogeneous condition, a challenge for clinicians and investigators alike, and merits further investigation to describe its pathophysiology and natural history. Research is warranted in an attempt to define risk factors, improve characterization, and determine causal relationship. The evolving field of genetics and gene–environment interactions may provide ground to determine cause–effect relationships. More data regarding post‐OLT outcomes and survival are needed in different population settings. Explant analysis and histopathology reevaluation may provide clinical clues to reconsider previously ruled out causative factors. With new advancements in the field of hepatology, we may be able to elucidate this condition and improve the management of affected patients, as well as the long‐term results in those who undergo transplantation.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Charlton MR, Kondo M, Robert SK, Steers JL, Krom RA, Wiesner RH. Liver transplantation for cryptogenic cirrhosis. Liver Transpl Surg 1997;3:359‐364. [DOI] [PubMed] [Google Scholar]
- 2. Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology 1999;29:664‐669. [DOI] [PubMed] [Google Scholar]
- 3. Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case‐control study. Hepatology 2000;32:689‐692. [DOI] [PubMed] [Google Scholar]
- 4. JG O'Leary, C Landaverde, L Jennings, RM Goldstein, GL Davis. Patients with NASH and cryptogenic cirrhosis are less likely than those with hepatitis C to receive liver transplants. Clin Gastroenterol Hepatol. 2011;9:700‐704.e1. doi: 10.1016/j.cgh.2011.04.007. Epub 2011 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Senanayake SM, Niriella MA, Weerasinghe SK, Kasturiratne A, De Alwis JP, De Silva AP, et al. Survival of patients with alcoholic and cryptogenic cirrhosis without liver transplantation: a single center retrospective study. BMC Res Notes 2012;5:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giannini EG, Marabotto E, Savarino V, Trevisani F, Di Nolfo MA, Del Poggio P, et al. Hepatocellular carcinoma in patients with cryptogenic cirrhosis. Clin Gastroent Hepatol 2009;7:580‐585. [DOI] [PubMed] [Google Scholar]
- 7. Tardu A, Karagul S, Yagci MA, Ertugrul I, Sumer D, Kirmizi S, et al. Histopathological examination of explanted liver after transplantation in patients with cryptogenic cirrhosis. Transplant Proc 2015;47:1450‐1452. [DOI] [PubMed] [Google Scholar]
- 8. Marmur J, Bergquist A, Stal P. Liver transplantation of patients with cryptogenic cirrhosis: clinical characteristics and outcome. Scand J Gastroenterol 2010;45:60‐69. [DOI] [PubMed] [Google Scholar]
- 9. Ayata G, Gordon FD, Lewis WD, Pomfret E, Pomposelli JJ, Jenkins RL, et al. Cryptogenic cirrhosis: clinicopathologic findings at and after liver transplantation. Hum Pathol 2002;33:1098‐1104. [DOI] [PubMed] [Google Scholar]
- 10. Sanjeevi A, Lyden E, Sunderman B, Weseman R, Ashwathnarayan R, Mukherjee S. Outcomes of liver transplantation for cryptogenic cirrhosis: a single‐center study of 71 patients. Transplant Proc 2003;35:2977‐2980. [DOI] [PubMed] [Google Scholar]
- 11. Duclos‐Vallée JC, Yilmaz F, Johanet C, Roque‐Afonso AM, Gigou M, Trichet C, et al. Could post‐liver transplantation course be helpful for the diagnosis of so called cryptogenic cirrhosis? Clin Transplant 2005;19:591‐599. [DOI] [PubMed] [Google Scholar]
- 12. Álamo JM, Bernal C, Barrera L, Marín LM, Suárez G, Serrano J, et al. Liver transplantation in patients with cryptogenic cirrhosis: long‐term follow‐up. Transplant Proc 2011;43:2230‐2232. [DOI] [PubMed] [Google Scholar]
- 13. El Atrache MM, Abouljoud MS, Divine G, Yoshida A, Kim DY, Kazimi MM, et al. Recurrence of non‐alcoholic steatohepatitis and cryptogenic cirrhosis following orthotopic liver transplantation in the context of the metabolic syndrome. Clin Transplant 2012;26:E505‐E512. [DOI] [PubMed] [Google Scholar]
- 14. Amico MD, Pasta F, Pasta L. Thrombophilic genetic factors PAI‐1, 4G‐4G and MTHFR 677TT as risk factors of alcohol, cryptogenic liver cirrhosis and portal vein thrombosis in a Caucasian population. Gene 2015;568:85‐88. [DOI] [PubMed] [Google Scholar]


