Abstract
To study negative interactions between phytochromes, phytochrome B (phyB) overexpressor lines, the mutants phyA-201, phyB-4, phyB-5, phyD-1, phyA-201 phyB-5, phyA-201 phyD-1, and phyB-5 phyD-1 of Arabidopsis were used. Endogenous phyB, but not phytochrome D (phyD), partly suppressed phytochrome A (phyA)-dependent inhibition of hypocotyl elongation in far-red light (FR). Dichromatic irradiation demonstrated that the negative effect of phyB was largely independent of the photoequilibrium, i.e. far-red light absorbing form of phytochrome formation. Moreover, phyB-4, a mutant impaired in signal transduction, did not show a loss of inhibition of phyA by phyB. Overexpression of phyB, conversely, resulted in an enhanced inhibition of phyA function, even in the absence of supplementary carbohydrates. However, overexpression of a mutated phyB, which cannot incorporate the chromophore, had no detectable effect on phyA action. In addition to seedling growth, accumulation of anthocyanins in FR, another manifestation of the high irradiance response, was strongly influenced by phyB holoprotein. Induction of seed germination by FR, a very low fluence response, was suppressed by both endogenous phyB and phyD. In conclusion, we show that both classical response modes of phyA, high irradiance response, and very low fluence response are subject to an inhibitory action of phyB-like phytochromes. Possible mechanisms of the negative interference are discussed.
Phytochromes are the best characterized of the plant informational photoreceptors. The N-terminal one-half of the protein contains the chromophore phytochromobilin (PΦB), which is covalently linked to a conserved Cys residue (Quail, 1997). The chromoprotein can adopt two spectroscopically distinct forms: the red light (R) absorbing form of phytochrome (Pr)- and the far-red light (FR) absorbing form of phytochrome (Pfr)-form. Generally speaking, only the latter is regarded to be physiologically active (Batschauer, 1998).
In Arabidopsis, there are five phytochrome genes PHYA-E (Sharrock and Quail, 1989; Clack et al., 1994). Both PHYA and PHYC are evolutionary clearly separated from the group of PHYB/D/E. Of these, PHYB and PHYD share approximately 80% sequence identity and are believed to be the result of a recent gene duplication (Mathews and Sharrock, 1997). Mutants and overexpressor lines confirmed distinct and common functions of individual family members (Whitelam and Devlin, 1997). In germination and seedling development phytochrome A (phyA) and phytochrome B (phyB) play a predominant role (Nagatani et al., 1993; Reed et al., 1993; Shinomura et al., 1996). Extremely low fluences of both R and FR can induce germination and partial seedling deetiolation by the very low fluence response (VLFR). This response is mediated by phyA (Neff et al., 2000). Furthermore, phyA can cause efficient inhibition of hypocotyl elongation and induction of anthocyanin synthesis in continuous FR via the high irradiance response (HIR) (Casal et al., 1998). It is interesting that VLFR and HIR appear to correspond to two branches of phyA signal transduction that can be genetically separated (Yanovsky et al., 1997). In contrast to these observations, phyB leads to germination and inhibition of growth after pulses of low fluences of R (low fluence response, LFR). Whereas the LFR is completely reversible by FR pulses, the phyA-mediated VLFR and HIR are usually not (Casal et al., 1998). The importance of phyC-E has been characterized much less extensively. However, phyB like phytochromes play a major role in adult plants (Whitelam and Devlin, 1997). The involvement of phytochrome D (phyD) and phytochrome E (phyE) in internodial elongation and induction of flowering becomes apparent especially in phyB mutants (Aukerman et al., 1997; Devlin et al., 1998, 1999). Moreover, also phyD can control seedling growth via a LFR (Aukerman et al., 1997; Hennig et al., 1999a). Nevertheless, there appears to exist some functional diversifications between the very similar phyB and phyD proteins (Hennig et al., 1999a)
Individual phytochromes do not act independently but are part of an elaborate network of interactions with other members of the phytochrome family and with the blue-light photoreceptors of the cryptochrome type (Casal, 2000; Neff et al., 2000). Several groups demonstrated synergistic effects of phyA on phyB action (Casal, 1995; Neff and Chory, 1998; Hennig et al., 1999b). Likewise, cry1 interacts with phyB and phyD (Casal and Boccalandro, 1995; Casal and Mazzella, 1998; Hennig et al., 1999a). In addition, inhibitory interactions of photoreceptors have been reported. Overexpression of phyB decreases inhibition of hypocotyl elongation in FR (Wagner et al., 1996). Moreover, phyB can mediate growth responses to single R pulses in Arabidopsis seedlings only in the absence of phyA (Casal, 1995; Hennig et al., 1999b). phyA, consequently, displays both positive and negative interactions with phyB. Casal and coworkers suggested that phyA acting in the VLFR mode is antagonistic while phyA acting in the HIR mode is synergistic with phyB function (Cerdán et al., 1999). Furthermore, the blue light receptor cryptochrome 2 suppresses initiation of floral induction by phyB (Mockler et al., 1999).
The influence of carbohydrates on the inhibition of phyA function by phyB has been reported recently (Short, 1999), thus explaining some previous conflicting observations. Nevertheless, a systematic study on negative functional interactions of phytochromes is needed for a detailed understanding of plant responses toward light. Therefore, we analyzed the influence of phyB and phyD on phyA function. Using both mutants and overexpressor lines we characterized seed germination, accumulation of anthocyanin, and inhibition of hypocotyl elongation in Arabidopsis.
RESULTS
C7g Is a Strong Overexpressor
In this study we used the Arabidopsis phyB overexpressing lines Arabidopsis phyB overexpressing (ABO)-1 and ABO-13. In addition we used the line C7g, which overexpresses the C357S mutation of phyB preventing incorporation of the chromophore. To facilitate comparison of the physiological results we investigated phyB protein levels in ABO-1, ABO-13, C7g, and the corresponding wild type (Arabidopsis ecotype Nossen [No-0]) by immunoblotting. The results in Figure 1a show that under our conditions expression in C7g is comparable with that in ABO-13 and considerable higher than that of ABO-1. The endogenous content of phyB in No-0 was below the detection limit. Figure 1b contains the results of a dilution analysis with samples of ABO-1, C7g, and No-0. The data indicate an overexpression of at least 8-fold for ABO-1 and of approximately 20-fold for ABO-13 and C7g.
Figure 1.
Contents of phyB in overexpressor lines. a, Samples of 3-d-old etiolated seedlings were analyzed by immunoblotting of 25 μg of protein and probing with an antiserum against phyB. b, For No-0, 30 μg of protein and appropriate dilutions for ABO-1 and C7g were loaded onto the gel.
Seedling Growth in FR
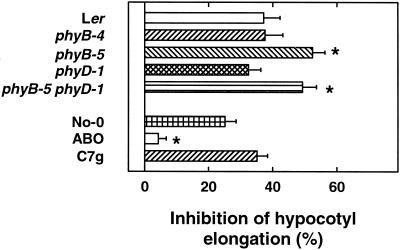
First we tested the effect of phyB on hypocotyl elongation in FR, a bona fide HIR. In FR of 20 μmol m−2 s−1 inhibition of hypocotyl growth was strong (90%) and very similar for all tested ecotypes and mutants (data not shown). Under non-saturating conditions of 0.6 μmol m−2 s−1, ecotype Landsberg erecta (Ler), phyB-4, and phyD-1 displayed an identical inhibition of approximately 40% (Fig. 2). Nonetheless, growth of seedlings of phyB-5 and phyB-5 phyD-1 was significantly stronger inhibited under these conditions. Moreover, No-0 and C7g behaved very similar in 0.6 μmol m−2 s−1, whereas ABO-1 showed only a very small inhibition of hypocotyl elongation.
Figure 2.
Inhibition of hypocotyl elongation in FR. After induction of germination by 24-h R, seeds were placed in 0.6 μmol m−2 s−1 FR. Hypocotyl lengths of at least 25 seedlings were determined after 3 d. Means of at least three replica experiments are given ±se. Asterisks mark significant differences according to Student's t test (P < 0.05).
Inhibition of Hypocotyl Elongation by R-FR Dichromatic Irradiation
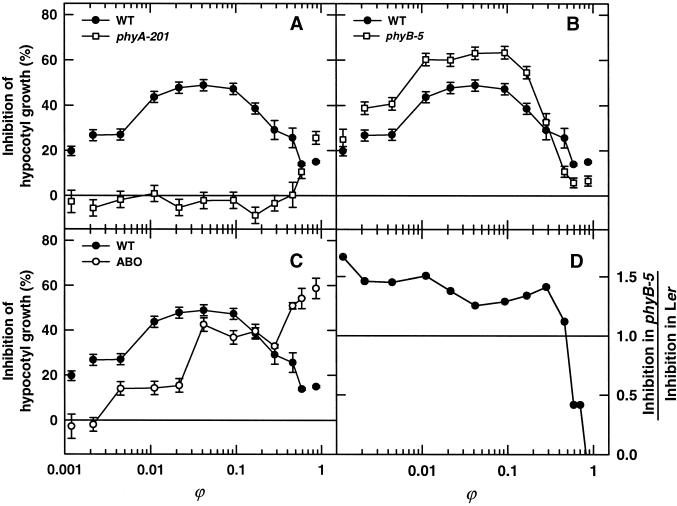
Since the seminal experiments of Hartmann (1966), dichromatic irradiation has been a useful tool for studying the HIR. By varying the relative fluences of R and FR different Pfr:Ptot ratios (ϕ) values can be obtained. Due to the high fluence rate of constant FR, total effective fluence rates do not vary significantly under these conditions (Fukshansky and Schäfer, 1983). We consequently used R-FR dichromatic irradiation to investigate the influence of ϕ on the antagonistic effect of phyB on phyA-mediated HIR in seedlings of Ler, phyA-201, phyB-5, and ABO. Figure 3 A shows that Pfr:Ptot ratios larger than 50% were required for inhibition of hypocotyl elongation in phyA-201 under our conditions. In contrast, ratios between 0.02 and 0.2 were very effective in Ler and phyB-5, representing the action of the HIR. The shape of the curves are identical for Ler and phyB-5; however, under all Pfr:Ptot ratios the inhibition was considerably stronger in the phyB mutant than in its wild type (Fig. 3B). In contrast, only minor growth inhibition was observable for ABO under low Pfr:Ptot ratios whereas at ϕ larger 0.3 the pronounced action of the overexpressed phyB became visible.
Figure 3.
Inhibition of hypocotyl elongation by R+FR dichromatic irradiation. Inhibition of growth of phyA-201 (A), phyB-5 (B), and ABO (C) seedlings was determined after 3 d under different fluence rates of R (660 nm) in addition to a constant irradiation with light of 756 nm (12.6 μmol m−2 s−1). The white symbols correspond to the R (11.0 μmol m−2 s−1) or FR irradiation alone. D, Relative inhibition of phyB-5 compared to wild type. Means of at least three replica experiments are given ±se.
Induction of Anthocyanin Synthesis in FR
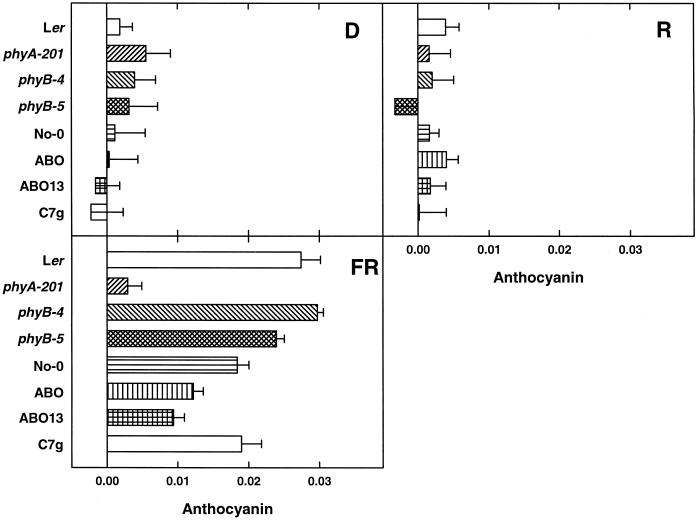
Beside inhibition of hypocotyl elongation, anthocyanin accumulation is a hallmark of phyA-mediated HIR. We tested anthocyanin accumulation in seedlings of Ler, phyA-201, phyB-4, phyB-5, phyD-1, No-0, ABO-1, ABO-13, and C7g in darkness, continuous R, or FR. In darkness and in R all tested lines contained only residual anthocyanin (Fig. 4, A and B). In FR anthocyanin accumulation was stronger in Ler than in No-0 (Fig. 4C). Nearly no anthocyanin was detectable in phyA-201 and significantly less in ABO-1 and ABO-13 compared with their background No-0. No alterations to the respective controls were detectable in the mutants phyB-4 and phyB-5 and the line C7g.
Figure 4.
Accumulation of anthocyanin in seedlings. After induction of germination by 24-h R, seeds were placed in darkness (D), 28 μmol m−2 s−1 R (R), or 20 μmol m−2 s−1 FR (FR). Anthocyanin contents were determined as described in “Materials and Methods.” Means of at least three replica experiments are given ±se.
Induction of Germination by FR
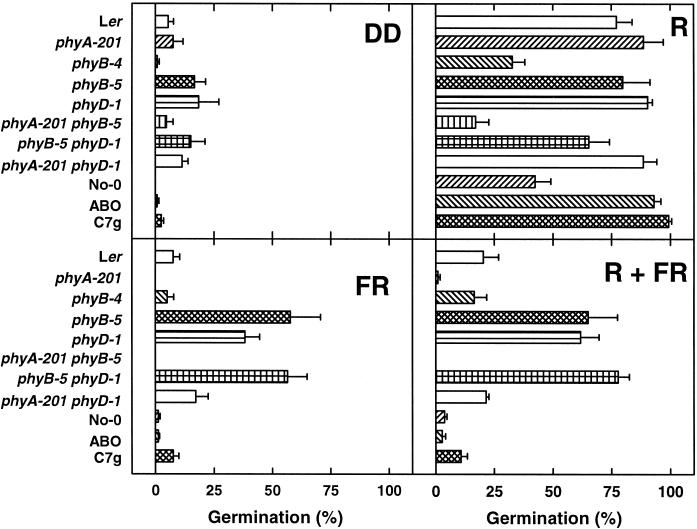
Light-dependent germination of Arabidopsis seeds was shown to be mainly under the control of phyA and phyB (Shinomura et al., 1996). Induction of germination by FR represents a phyA mediated VLFR (Botto et al., 1996). Figure 5 shows the germination of seeds after imbibition for 24 h at 4°C in the dark followed by either 3-h R, 3-h FR, or 3-h R + 3-h FR. A treatment with white light for 3 d lead to a germination rate larger than 80% in all mutants (data not shown). No light treatment at all, in contrast, resulted in very low germination frequencies (Fig. 5A). Irradiation with 3-h R caused efficient germination (60%–100%), except for the phyA-201 phyB-5 double mutant (less than 20%). No-0 and phyB-4 germinated only at approximately 40%. In contrast to R, 3-h FR led only to neglectable germination in wild type (Ler and No-0), phyB-4, phyA-201, phyA-201 phyB-5, ABO-1, and C7g. However, 40%–60% of seeds of phyB-5, phyD-1, and phyB-5 phyD-1 germinated. Moreover, 3-h R followed by 3-h FR caused germination frequencies similar to that after 3-h FR alone.
Figure 5.
Phytochrome control of germination. After sowing, seeds were incubated for 24 h at 4°C in darkness followed by the indicated light treatments (28 μmol m−2 s−1 R or 6 μmol m−2 s−1 FR). Germination frequencies were determined after further incubation for 6 d in darkness. Means of three to six replica experiments are given ±se.
DISCUSSION
The inhibitory action of phyB on control of hypocotyl elongation by phyA has been reported in several publications (McCormac et al., 1993; Wagner et al., 1996). However, their work on phyB overexpressor lines led to partly conflicting results. A study by Short resolved this conflict. It was shown that the presence of metabolizable carbohydrates (e.g. Suc, Glc) strongly enhanced the inhibitory action of phyB (Short, 1999). Crosstalk between sugars and light signaling has been studied extensively, for instance for the transcriptional suppression of some light responsive genes (Smeekens, 1998). However, excess of metabolizable sugars constitutes an extreme situation. Therefore, we wanted to test whether negative interaction of phyB and phyA occurs also in the absence of sugars. Furthermore, overexpression studies may not necessarily reflect endogenous mechanisms. Thus, we wanted to test any inhibitory effect of endogenous phyB amounts on phyA. We used null alleles of phyB and phyD (phyB-5 and phyD-1), a missense mutation of phyB (phyB-4), two phyB overexpressor lines (ABO-1 and ABO-13), and a line overexpressing a C357S mutation that cannot incorporate the chromophore (C7g). Overexpression in ABO-1 is approximately 10-fold and approximately 20-fold in ABO-13 as indicated by western blotting (Fig. 1). These results are in agreement with previous reports (Wagner et al., 1991, 1996). Expression levels in C7g seedlings were very similar to ABO-13.
phyB Holoprotein Inhibits the HIR Even in the Absence of Sugars
The presented results show that negative interference of phyB with phyA-dependent HIR occurs even in the absence of additional sugars (Fig. 2). While the presence of sugars strongly enhances the inhibitory effect (Short, 1999), supplementary sugars are not required. Thus, the negative interaction is a bona fide property of the light signaling pathways. It is interesting that the line C7g did not display a significantly decreased inhibition of hypocotyl elongation under our conditions. As this cannot be caused by lower expression levels (Fig. 1), an apparently lower inhibitory effect of phyB apoprotein escapes detection in the absence of sugars but becomes observable in their presence.
Not only does overexpressed phyB lead to decreased HIR-mediated effects but similarly the loss of endogenous phyB causes an increased HIR (Fig. 2). It is interesting that phyB-4, which carries a point mutation leading to considerable loss of function, does not display an increased HIR. Thus, the mutated phyB-4 is able to inhibit phyA. Likewise, phyD-1 does not show enhanced phyA action. Only phyB but not the very similar phyD, consequently, inhibits the phyA-mediated HIR in wild type. Moreover, phyB does not only interfere with inhibition of hypocotyl growth by phyA but also suppresses anthocyanin accumulation (Fig. 4). On the other hand, even strong overexpression of phyB does not confer the ability to induce anthocyanin synthesis, thus confirming previous conclusions of distinct signaling chains of phyA and phyB. Taken together, these results show that negative interference of phyA and phyB is not restricted to growth control and that the inhibition occurs probably early in the signaling chain of phyA.
phyB and phyD Inhibit the VLFR
In addition to growth and anthocyanin accumulation, we analyzed the effect of phyB and phyD on germination. It has been reported previously that phyA, phyB, and at least one additional phytochrome induce germination in Arabidopsis (Shinomura et al., 1996; Poppe and Schäfer, 1997). Furuya and coworkers showed that phyA controls induction of germination by FR via the VLFR only after a prolonged incubation at 25°C in darkness (Shinomura et al., 1996). A FR-HIR does not induce germination of Arabidopsis seeds; in many other species, prolonged FR even acts antagonistic toward induction of germination by the LFR (Casal and Sánchez, 1998). In contrast, phyB can mediate germination in R via an LFR immediately after stratification. Confirming these results, we observed efficient germination after 3 h R, except for No-0, phyB-4, and the phyA-201 phyB-5 double mutant (Fig. 5). Nevertheless, germination frequencies of phyA-201 phyB-5 in R are significantly higher than in darkness or FR. Immediately after stratification, 3 h FR do not induce germination in wild type, in phyA-201, and in phyB-4. In contrast, high germination frequencies were observed in phyB-5, phyD-1, and phyB-5 phyD-1. The low germination in phyA-201 phyB-5 and phyA-201 phyD-1 demonstrates that germination in FR in the absence of phyB or phyD requires phyA. A treatment of R followed by FR confirms these observations. Overexpression of phyB or C357S phyB does not alter the germination frequencies significantly.
Induction of germination by FR is a VLFR and not an HIR (Botto et al., 1996; Casal and Sánchez, 1998). phyB, consequently, inhibits not only the HIR but also the second response mode of phyA, the VLFR. In contrast, endogenous phyD inhibits only the VLFR but not the HIR. Nevertheless, it may be possible that in the special situation of a phyD overexpressor, also an inhibition of the HIR, could become detectable.
Mechanism of Negative Interaction of Phytochromes
The negative effects of phyB and phyD on phyA action could be caused by several potential mechan-isms. First both phyB and phyD could influence the amount of phyA. However, it was shown that neither total phyA levels in etiolated seedlings nor degradation kinetics in light are altered by overexpression of phyB (Short, 1999). Similarly, we did not observe significant differences in total spectroscopically detectable phytochrome levels between Ler and phyB-5 (data not shown). Identical amounts and degradation kinetics of phyA were observed in the Wassilewskija ecotype, a naturally occurring phyD mutant, and in ecotypes Ler or Columbia (Col) (Eichenberg et al., 2000).
Components of the activated signal transduction cascades of phyB and phyA could directly interact. Nevertheless, our results indicate that phyB does not need to activate its signaling pathway. The action spectrum of phyB responses has its peak around 660 nm; there is no physiological activity in the FR region (Shinomura et al., 1996, 1998; Furuya and Schäfer, 1996). In contrast, inhibition of phyA action occurs in FR. Moreover, dichromatic irradiation demonstrated that interference of phyB with phyA is largely independent of the photoequilibrium (Fig. 3). Under some conditions, even the C357S phyB apoprotein can inhibit phyA action (Short, 1999). Here, we showed that the mutant phyB-4, which is impaired in its signaling capabilities, nonetheless is able to inhibit the HIR. In summary, activation of the phyB-signaling cascade appears not to be required to inhibit phyA action.
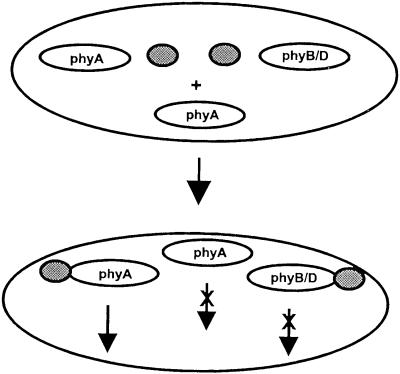
An attractive hypothesis explaining the experimental results is direct competition of phyB and phyA photoreceptors for a binding partner. The binding of phyB to a cognate partner protein of phyA without initiating a signaling event would be favored by the native structure of the phyB holoprotein. Clearly, phyB is able to evoke inhibitory effects on phyA in its native Pr as well as its Pfr form (Fig. 3B). In contrast, Pfr is strictly required for active physiological responses of phyB. Similar inhibitory effectiveness of Pfr and Pr fully accounts for the observed action under diverse photoequilibria. As the apoprotein appears to be similar but not identical to the Pr form of the holoprotein, the inhibitory effect of phyB holoprotein is expected to be larger than that of C357S apoprotein. The reported dependence of the inhibition of phyA action on the concentration of phyB and the dominant negative effects of overexpressed fragments of phyB (Sakamoto and Nagatani, 1996a; Wagner et al., 1996) strengthen this hypothesis. It is interesting that two of the known phytochrome interacting proteins, PIF3 and PKS, bind to both phyA and phyB, whereas they are discussed mainly in phyB signaling (Ni et al., 1998; Fankhauser et al., 1999). Consequently, phyA and phyB are sufficiently similar to bind to the same proteins. Figure 6 summarizes the proposed model of inhibitory interactions of phyB and phyD with phyA.
Figure 6.
Schematic representation of a molecular model for negative interactions of phyB and phyD with phyA function. Binding to an interaction partner, which is present in limiting amounts, is required for phyA function. The similar proteins phyB and phyD can bind to the partner of phyA; however, they cannot initiate effective signaling. Loss of phyB and/or phyD consequently frees the interaction partner to form functional complexes with phyA while overexpression titrates out even more partner molecules.
It remains unknown whether phyB and phyD act by an identical or via two independent mechanisms to inhibit phyA. The high sequence similarity and close evolutionary relation of both proteins favors a common mechanism. It appears possible that quantitative rather than qualitative differences between phyB and phyD give at least partly rise to the different observations. The loss of relatively low amounts of endogenous phyD may relief the VLFR but not the HIR.
It is important that both phyB and phyA are imported into the nucleus upon irradiation (Sakamoto and Nagatani, 1996b; Kircher et al., 1999; Yamaguchi et al., 1999). In tobacco, a phyB GFP fusion, which cannot incorporate the chromophore, does not accumulate in the nucleus (Kircher et al., 1999). Nuclear import of phyB, similarly, is not caused by FR (Gil et al., 2000). The inhibition of phyA action by phyB in FR, therefore, indicates that the negative interaction occurs in the cytoplasm. In the future, it will be highly interesting to analyze nuclear transport of phytochromes in the background of various photoreceptor mutants.
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Light Sources
The following ecotypes of Arabidopsis were used: Ler, Col, and No-0 (all obtained from Lehle Seeds, Tuscon, AZ). The mutants and their amino acid substitutions (specified in parentheses) used were phyA-201 (Q980STOP; Nagatani et al., 1993; Reed et al., 1994), phyB-4 (H283W; Reed et al., 1993), phyB-5 (W552STOP; Koornneef et al., 1980; Reed et al., 1993), and phyD-1 (chromosomal deletion, Aukerman et al., 1997). The double mutants phyA-201 phyB-5 and phyB-5 phyD-1 were generated by crossing (Devlin et al., 1999). The cDNA of Arabidopsis phyB was fused to the 35S promoter and transformed into No-0 yielding the ABO lines (Wagner et al., 1991, 1996). Use of a point mutated cDNA (C357S) alternatively gave rise to the line C7g; the overexpressed phyB in this line cannot incorporate the chromophore. All overexpressor lines were a kind gift of P. Quail (Albany, CA).
Seeds were plated on four layers of water-soaked filter papers, which were placed into clear plastic boxes. A 24-h dark treatment at 4°C was followed by induction of germination by white light for 24 h and further incubation of seedlings in the dark or under indicated light conditions at 25°C. Standard R (656 nm) or FR (730 nm) fields were used (Heim and Schäfer, 1982). For dichromatic R+FR irradiation Xenosol III projectors (Zeiss, Jena, Germany) were used (Beggs et al., 1981). Monochromatic light of different wavelengths was supplied using Prado light projectors (Leitz, Midland, Ontario) with appropriate interference filters (Schott, Mainz, Germany). The values of the photoconversion cross-sections were assumed to be close to those given by Mancinelli (1994) for oat phyA.
Protein Extraction and Immunoblotting
Seedlings were extracted with SDS-sample buffer (65 mm Tris-HCl, pH 7.8, 4 m urea, 10 mm dithioerythritol, 0.05% [w/v] bromphenol blue) by sonification (Sonifier Bandelin Sonopuls GM 70 MS 72) and heated to 95°C for several minutes. The crude extracts were clarified by centrifugation for 15 min at 20,000g (25°C).
SDS-PAGE, protein blotting, and immunodetection were performed as described by Harter et al. (1993). Monoclonal antibodies against phyB of Arabidopsis were a generous gift of P. Quail (Albany, CA) (Hirschfeld et al., 1998).
Determination of Hypocotyl Length and Germination Frequencies
Hypocotyl lengths were measured manually for at least 25 seedlings. The mean value of at least three independent experiments and the se of the mean are shown. Germination percentages of 80 to 120 seeds were determined by taking the protrusion of the radicle as the criterion of germination. The mean value of at least four independent experiments and the se of the mean are shown. At least two independent seed batches per line or mutant were used for germination experiments.
Anthocyanin Assay
Sixty seedlings of each line or mutant were harvested after appropriate light treatments. Extraction of anthocyanin and its spectroscopical determination was performed as described (Poppe et al., 1998). Seedlings were boiled in 0.75 mL of extraction buffer (18% [v/v] isopropanol, 1% [v/v] HCl) for 3 min and subsequently extracted by shaking in darkness at 4°C for 24 h. The samples were centrifugated at room temperature for 5 min and the absorbance of the supernatant at 535 and 650 nm was measured. Anthocyanin content was given as corrected A535 according to:
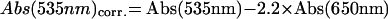 |
ACKNOWLEDGMENTS
We thank Peter H. Quail for the antiserum against phyB and seeds of phyB overexpressor lines. Seeds of phyD mutants were kindly provided by Garry C. Whitelam. Furthermore, we thank Rena Wiehe for excellent technical assistance.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft.
LITERATURE CITED
- Aukerman MJ, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino RM, Sharrock RA. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell. 1997;9:1317–1326. doi: 10.1105/tpc.9.8.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschauer A. Photoreceptors of higher plants. Planta. 1998;206:479–492. doi: 10.1007/s004250050425. [DOI] [PubMed] [Google Scholar]
- Beggs CJ, Geile W, Holmes MG, Jabben M, Jose AM, Schäfer E. High irradiance response promotion of a subsequent light induction response in Sinapis alba L. Planta. 1981;151:135–140. doi: 10.1007/BF00387814. [DOI] [PubMed] [Google Scholar]
- Botto JF, Sanchez RA, Whitelam GC, Casal JJ. Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 1996;110:439–444. doi: 10.1104/pp.110.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. Coupling of phytochrome B to the control of hypocotyl growth in Arabidopsis. Planta. 1995;196:23–29. doi: 10.1007/BF00193213. [DOI] [PubMed] [Google Scholar]
- Casal JJ. Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol. 2000;71:1–11. doi: 10.1562/0031-8655(2000)071<0001:pcppii>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Boccalandro H. Co-action between phytochrome B and HY4 in Arabidopsis thaliana. Planta. 1995;197:213–218. doi: 10.1007/BF00202639. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Mazzella MA. Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiol. 1998;118:19–25. doi: 10.1104/pp.118.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Sánchez RA. Phytochromes and seed germination. Seed Sci Res. 1998;8:317–329. [Google Scholar]
- Casal JJ, Sánchez RA, Botto JF. Modes of action of phytochromes. J Exp Bot. 1998;49:127–138. [Google Scholar]
- Cerdán PD, Yanovsky MJ, Reymundo FC, Nagatani A, Staneloni RJ, Whitelam GC, Casal JJ. Regulation of phytochrome B signaling by phytochrome A and FHY1 in Arabidopsis thaliana. Plant J. 1999;18:499–507. doi: 10.1046/j.1365-313x.1999.00475.x. [DOI] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Patel SR, Whitelam GC. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell. 1998;10:1479–1487. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Robson PRH, Patel SR, Goosey L, Sharrock R, Whitelam GC. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 1999;119:909–915. doi: 10.1104/pp.119.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenberg K, Hennig L, Martin A, Schäfer E. Variation in dynamics of phytochrome A in Arabidopsis ecotypes and mutants. Plant Cell Environ. 2000;23:311–319. [Google Scholar]
- Fankhauser C, Yeh KC, Lagarias JC, Zhang H, Elich TD, Chory J. PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science. 1999;284:1539–1541. doi: 10.1126/science.284.5419.1539. [DOI] [PubMed] [Google Scholar]
- Fukshansky L, Schäfer E. Models in photomorphogenesis. In: Shropshire W, Mohr H, editors. Encyclopedia of Plant Physiology New Series. Berlin: Springer-Verlag; 1983. pp. 69–95. [Google Scholar]
- Furuya M, Schäfer E. Photoperception and signalling of induction reactions by different phytochromes. Trends Plant Sci. 1996;1:301–307. [Google Scholar]
- Gil P, Kircher S, Adam E, Bury E, Kozma-Bognar L, Schäfer E, Nagy F. Photocontrol of subcellular partitioning of phytochrome-B:GFP fusion protein in tobacco seedlings. Plant J. 2000;22:135–145. doi: 10.1046/j.1365-313x.2000.00730.x. [DOI] [PubMed] [Google Scholar]
- Harter K, Talke-Messerer C, Barz W, Schäfer E. Light-dependent and sucrose-dependent gene expression in photomixotrophic cell suspension cultures and protoplasts of rape (Brassica napus L.) Plant J. 1993;4:507–516. [Google Scholar]
- Hartmann KM. A general hypothesis to interpret “high energy phenomena” of photomorphogenesis on the basis of phytochrome. Photochem Photobiol. 1966;5:349–366. [Google Scholar]
- Heim B, Schäfer E. Light-controlled inhibition of hypocotyl growth in Sinapis alba seedlings: fluence rate dependence of hourly light pulses and continuous irradiation. Planta. 1982;154:150–155. doi: 10.1007/BF00387909. [DOI] [PubMed] [Google Scholar]
- Hennig L, Funk M, Whitelam GC, Schäfer E. Functional interaction of cryptochrome 1 and phytochrome D. Plant J. 1999a;20:289–294. doi: 10.1046/j.1365-313x.1999.t01-1-00599.x. [DOI] [PubMed] [Google Scholar]
- Hennig L, Poppe C, Unger S, Schäfer E. Control of hypocotyl elongation in Arabidopsis thaliana by photoreceptor interaction. Planta. 1999b;208:257–263. doi: 10.1007/s004250050557. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Tepperman JM, Clack T, Quail PH, Sharrock RA. Coordination of phytochrome levels in phyB mutants of Arabidopsis as revealed by apoprotein-specific monoclonal antibodies. Genetics. 1998;149:523–535. doi: 10.1093/genetics/149.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S, Kozma-Bognar L, Kim L, Adam E, Harter K, Schäfer E, Nagy F. Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell. 1999;11:1445–1456. doi: 10.1105/tpc.11.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana. Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- Mancinelli AL. The physiology of phytochrome action. In: Kendrick RE, Kronenberg HHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 211–270. [Google Scholar]
- Mathews S, Sharrock RA. Phytochrome gene diversity. Plant Cell Environ. 1997;20:666–671. [Google Scholar]
- McCormac AC, Wagner D, Boylan MT, Quail PH, Smith H. Photoresponses of transgenic Arabidopsis seedlings expressing introduced phytochrome-B encoding cDNAs: evidence that phytochrome A and phytochrome B have distinct photoregulatory functions. Plant J. 1993;4:19–27. [Google Scholar]
- Mockler TC, Guo HW, Yang HY, Duong H, Lin CT. Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development. 1999;126:2073–2082. doi: 10.1242/dev.126.10.2073. [DOI] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Chory J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998;118:27–36. doi: 10.1104/pp.118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Fankhauser C, Chory J. Light: an indicator of time and place. Genes Dev. 2000;14:257–271. [PubMed] [Google Scholar]
- Ni M, Tepperman JM, Quail PH. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell. 1998;95:657–667. doi: 10.1016/s0092-8674(00)81636-0. [DOI] [PubMed] [Google Scholar]
- Poppe C, Schäfer E. Seed germination of Arabidopsis thaliana phyA/phyB double mutants is under phytochrome control. Plant Physiol. 1997;114:1487–1492. doi: 10.1104/pp.114.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Sweere U, Drumm-Herrel H, Schäfer E. The blue light receptor cryptochrome 1 can act independently of phytochrome A and B in Arabidopsis thaliana. Plant J. 1998;16:465–471. doi: 10.1046/j.1365-313x.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- Quail PH. An emerging molecular map of the phytochromes. Plant Cell Environ. 1997;20:657–665. [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red-far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Nagatani A. Over-expression of a c-terminal region of phytochrome B. Plant Mol Biol. 1996a;31:1079–1082. doi: 10.1007/BF00040726. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Nagatani A. Nuclear localization activity of phytochrome B. Plant J. 1996b;10:859–868. doi: 10.1046/j.1365-313x.1996.10050859.x. [DOI] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Hanzawa H, Schäfer E, Furuya M. Mode of phytochrome B action in the photoregulation of seed germination in Arabidopsis thaliana. Plant J. 1998;13:583–590. doi: 10.1046/j.1365-313x.1998.00049.x. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M. Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:8129–8133. doi: 10.1073/pnas.93.15.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short TW. Overexpression of Arabidopsis phytochrome B inhibits phytochrome A function in the presence of sucrose. Plant Physiol. 1999;119:1497–1505. doi: 10.1104/pp.119.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. Sugar regulation of gene expression in plants. Curr Opin Plant Biol. 1998;1:230–234. doi: 10.1016/s1369-5266(98)80109-x. [DOI] [PubMed] [Google Scholar]
- Wagner D, Koloszvari M, Quail PH. Two small spatially distinct regions of phytochrome B are required for efficient signaling rates. Plant Cell. 1996;8:859–871. doi: 10.1105/tpc.8.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Tepperman JM, Quail PH. Overexpression of phytochrome B induces a short hypocotyl phenotype in transgenic Arabidopsis. Plant Cell. 1991;3:1275–1288. doi: 10.1105/tpc.3.12.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Devlin PF. Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ. 1997;20:752–758. [Google Scholar]
- Yamaguchi R, Nakamura M, Mochizuki N, Kay SA, Nagatani A. Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J Cell Biol. 1999;145:437–445. doi: 10.1083/jcb.145.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky MJ, Casal JJ, Luppi JP. The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia, dissect two branches of phytochrome A signal transduction that correspond to very-low-fluence and high-irradiance responses. Plant J. 1997;12:659–667. doi: 10.1046/j.1365-313x.1997.00659.x. [DOI] [PubMed] [Google Scholar]