Abstract
Background
The definition of sepsis has evolved over time, along with the clinical and scientific knowledge behind it. For years, sepsis was defined as a systemic inflammatory response syndrome (SIRS) in the presence of a documented or suspected infection. At present, sepsis is defined as a life‐threatening organ dysfunction resulting from a dysregulated host response to infection. Even though sepsis is one of the leading causes of mortality in critically ill patients, and the World Health Organization (WHO) recognizes it as a healthcare priority, it still lacks an accurate diagnostic test. Determining the accuracy of interleukin‐6 (IL‐6) concentrations in plasma, which is proposed as a new biomarker for the diagnosis of sepsis, might be helpful to provide adequate and timely management of critically ill patients, and thus reduce the morbidity and mortality associated with this condition.
Objectives
To determine the diagnostic accuracy of plasma interleukin‐6 (IL‐6) concentration for the diagnosis of bacterial sepsis in critically ill adults.
Search methods
We searched CENTRAL, MEDLINE, Embase, LILACS, and Web of Science on 25 January 2019. We screened references in the included studies to identify additional studies. We did not apply any language restriction to the electronic searches.
Selection criteria
We included diagnostic accuracy studies enrolling critically ill adults aged 18 years or older under suspicion of sepsis during their hospitalization, where IL‐6 concentrations were evaluated by serological measurement.
Data collection and analysis
Two review authors independently screened the references to identify relevant studies and extracted data. We assessed the methodological quality of studies using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool. We estimated a summary receiver operating characteristic (SROC) curve by fitting a hierarchical summary ROC (HSROC) non‐linear mixed model. We explored sources of heterogeneity using the HSROC model parameters. We conducted all analyses in the SAS statistical software package and R software.
Main results
We included 23 studies (n = 4192) assessing the accuracy of IL‐6 for the diagnosis of sepsis in critically ill adults. Twenty studies that were available as conference proceedings only are awaiting classification. The included participants were heterogeneous in terms of their distribution of age, gender, main diagnosis, setting, country, positivity threshold, sepsis criteria, year of publication, and origin of infection, among other factors. Prevalence of sepsis greatly varied across studies, ranging from 12% to 78%. We considered all studies to be at high risk of bias due to issues related to the index test domain in QUADAS‐2. The SROC curve showed a great dispersion in individual studies accuracy estimates (21 studies, 3650 adult patients), therefore the considerable heterogeneity in the collected data prevented us from calculating formal accuracy estimates. Using a fixed prevalence of sepsis of 50% and a fixed specificity of 74%, we found a sensitivity of 66% (95% confidence interval 60 to 72). If we test a cohort 1000 adult patients under suspicion of sepsis with IL‐6, we will find that 330 patients would receive appropriate and timely antibiotic therapy, while 130 patients would be wrongly considered to have sepsis. In addition, 370 out of 1000 patients would avoid unnecessary antibiotic therapy, and 170 patients would have been undiagnosed of sepsis. This numerical approach should be interpreted with caution due to the limitations described above.
Authors' conclusions
Our evidence assessment of plasma interleukin‐6 concentrations for the diagnosis of sepsis in critically ill adults reveals several limitations. High heterogeneity of collected evidence regarding the main diagnosis, setting, country, positivity threshold, sepsis criteria, year of publication, and the origin of infection, among other factors, along with the potential number of misclassifications, remain significant constraints for its implementation. The 20 conference proceedings assessed as studies awaiting classification may alter the conclusions of the review once they are fully published and evaluated. Further studies about the accuracy of interleukin‐6 for the diagnosis of sepsis in adults that apply rigorous methodology for conducting diagnostic test accuracy studies are needed. The conclusions of the review will likely change once the 20 studies pending publication are fully published and included.
Plain language summary
Levels of interleukin‐6 in identifying severely ill adult patients with sepsis
Review question
We evaluated the evidence on the ability of interleukin‐6 (IL‐6) levels in plasma to identify adult patients with sepsis. Interleukin‐6 is a cytokine (a broad and loose category of small proteins) secreted by immune cells that mediates a wide range of biological activities.
Background
Sepsis is a potentially life‐threatening response by the immune system to an infection that can result in tissue damage, organ failure, and even death, and should be considered as a medical emergency. About 288 septic cases by 100,000 person‐years occur in hospital settings, and 17% of those patients could die. Early identification of patients having sepsis is the first step for immediate medical management, which is essential to avoid further complications and death. Treatment consists mainly of the use of antibiotics (a drug that inhibits the growth of dangerous micro‐organisms). Several tools have been proposed for sepsis diagnosis, as well as the physical examination of blood cultures (the assessment of blood samples to identify micro‐organisms causing the infection). Interleukin‐6 is a molecule that helps in the communication of cells during the body's response to an infection. It has been suggested that the measurement of levels of IL‐6 in the plasma from blood samples during the onset of sepsis can be helpful in identifying sepsis patients early and initiating adequate treatment.
Study characteristics
We performed a thorough literature search for studies reporting the use of IL‐6 levels for detection of sepsis up to January 2019. We found 23 studies enrolling 4192 severely ill adults.
Key results
Our assessment of the evidence reveals the complexity of the research topic, represented in the high variability of information reported by the studies. We found the characteristics of assessed patients to vary considerably between studies in terms of age, gender, setting, initial diagnosis, indicative value for sepsis, and source of infection, among other factors. This variability in the collected data prevented a formal numerical synthesis of the findings. Using the available data to perform an approximated estimation of the consequences, we found that 700 out of 1000 patients under suspicion of sepsis might be correctly classified, but 130 out of 1000 patients would be wrongly considered as having sepsis, while 170 out of 1000 patients might be incorrectly considered as not having sepsis. These errors would result in a serious increase in the risk of further morbidity and death due to delays of adequate treatment. This information should be interpreted with caution due to limitations in the collected data.
Quality of the evidence
We judged the included studies to have important limitations in their validity, hence they are at high risk of providing distorted results (i.e. to be at high risk of bias).
Summary of findings
Summary of findings'. 'Plasma interleukin‐6 concentration for diagnosis of sepsis in critically ill adults.
| Population | Critically ill adults under suspicion of sepsis (i.e. with SIRS symptoms) | |||||||
| Prior testing | Physical examination and history | |||||||
| Index test | Plasma interleukin‐6, measured before antibiotic treatment. Cut‐off for positivity ranged from 40 to 200,000 pg/mL. | |||||||
| Role of the test | Triage: First test for patients with SIRS symptoms and waiting for further results (i.e. culture) | |||||||
| Setting | Intensive care units, emergency departments, institutional setting (no details provided) | |||||||
| Reference standard | 3 sepsis definitions derived from expert consensus were considered valid reference standards:
Sepsis criteria were applied by critical care, emergency, or internal medicine clinicians. |
|||||||
| Index test | Prevalence | Number of studies | Number of participants | Sensitivity (under fixed specificity)1 | Specificity (fixed)2 | Number of false positives out of 1000 patients | Number of false negatives out of 1000 patients | Comments |
| Plasma interleukin‐6 concentration | 12% | 21 | 3650 | 66% (95% confidence interval 60 to 72) | 74% | 229 | 41 | High heterogeneity of collected evidence remains a significant constraint for plasma interleukin‐6 implementation. |
| 50% | 130 | 170 | ||||||
| 78% | 57 | 265 | ||||||
1HSROC (hierarchical summary receiver operating characteristic) parameters were used to illustrate sensitivity for a fixed specificity. 2Median specificity estimated from included studies.
Abbreviations: AB: antibiotic; ACCP: American College of Chest Physicians; ATS: American Thoracic Society; ESICM: European Society of Intensive Care Medicine; QUADAS: Quality Assessment of Diagnostic Accuracy Studies; SCCM: Society of Critical Care Medicine; SIRS: systemic inflammatory response syndrome; SIS: Surgical Infection Society.
Background
The diagnosis of sepsis in critically ill patients with non‐specific findings of an acute inflammatory process can be challenging (Harbarth 2001). In a significant number of cases, the diagnosis of sepsis becomes clear after completing the patient medical history and physical examination. However, in other circumstances, including comatose, elderly, or pregnant patients, the diagnosis of sepsis remains difficult (Abraham 2000). Currently, the diagnosis of sepsis is based on clinical findings and the presence of organ dysfunction (Singer 2016). Several new biological indicators (biomarkers) have been proposed for the diagnosis of sepsis, but no single one of them has gained unanimous acceptance (Rello 2017; van Engelen 2018).
Target condition being diagnosed
The clinical understanding of sepsis has evolved over the years. For several years, sepsis was defined as a systemic inflammatory response syndrome (SIRS) in the presence of a documented or suspected infection (Dellinger 2013; Levy 2003; Shankar‐Hari 2015). In 2001, the Society of Critical Care Medicine (SCCM), the European Society of Intensive Care Medicine (ESICM), the American College of Chest Physicians (ACCP), the American Thoracic Society (ATS), and the Surgical Infection Society (SIS) stated that SIRS involves changes, by unknown causes, of clinical baseline parameters such as body temperature, hypothermia, heart rate, respiratory rate, and white blood cell count, among others (Levy 2003; Rangel‐Frausto 1995). Under these criteria, in patients with symptoms of sepsis, the attending physician used the term 'clinically suspected infection' to indicate the suspicion of an ongoing infection, followed by the prescription of immediate initiation of antimicrobial therapy and submission of a request for a complete set of tests to determine the presence or absence of an infection (Rangel‐Frausto 1995).
In 2015, a consensus task force of the ESICM and the SCCM updated the definition of sepsis and septic shock, which is currently in use (Singer 2016). At present, sepsis is defined as a life‐threatening organ dysfunction resulting from a dysregulated host response to infection (Singer 2016). The new definition withdraws the terms 'SIRS' and 'severe sepsis' and instead prioritizes organ dysfunction. Organ dysfunction can be identified as an acute change in the total Sequential Organ Failure Assessment (SOFA) score ≥ 2 points due to infection. A SOFA score ≥ 2 reflects an overall mortality risk of approximately 10% in a general hospital population with suspected infection (Singer 2016). In addition, septic shock is defined as a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to increase mortality (Singer 2016). Patients with septic shock can be identified from a clinical construct of sepsis with persisting hypotension requiring vasopressors to maintain blood pressure and hyperlactataemia despite adequate volume resuscitation. Hospital mortality in patients with septic shock has been estimated to be higher than 40% (Singer 2016).
The worldwide burden of sepsis is difficult to estimate due to the variability of settings, designs, and sepsis criteria found in the various studies (Fleischmann 2016). Based on information from high‐income countries only, Fleischmann and colleagues estimated a population incidence rate of 288 hospital‐treated sepsis cases per 100,000 person‐years, with an increase to 437 cases per 100,000 person‐years in the last 13 years. In addition, an extrapolation of information from the last decade suggests that a total annual number of 31.5 million sepsis and 19.4 million severe sepsis cases are treated worldwide each year, with a case fatality rate of 5.3 million deaths (Fleischmann 2016). A retrospective cohort study in seven states in the USA identified 192,980 cases of severe sepsis, with an estimated incidence of sepsis of 3 cases per 1000 persons at the population level, and 2.26 cases per 100 hospital discharges; the authors projected an increase in severe sepsis of 1.5% per year (Angus 2001). Finfer 2004 reported that 11.8 per 100 patients admitted to an intensive care unit (ICU) between 1999 and 2000 were diagnosed with severe sepsis, with an incidence of 0.77 (95% confidence interval (CI) 0.76 to 0.79) per 1000 adult patients. According to Kumar 2011, the mortality rate for severe sepsis decreased from 39% to 27% between 2000 and 2007. However, the rates of mortality were higher in people with more organ systems failing. In 2011, the average cost for the treatment of severe sepsis was USD 22,100 per case, with potentially higher expenses depending on patient age, the need for surgical procedures, the presence of organ failure, and variation in costs charged by ICUs (Angus 2001).
Sepsis originates as an infection caused by bacteria, fungus, virus, or parasites (Dellinger 2013). One half (52%) of sepsis cases in hospitals in the USA originate from gram‐positive bacteria (Finfer 2004). For bacteria to cause infections, they must evade the immune system of the host, either at the site of infection or in the bloodstream. Innate immune cells recognize pathogenic micro‐organisms by sensing common microbial structures known as pathogen‐associated molecular patterns, such as lipoteichoic acid, lipopeptides, lipopolysaccharides, and nucleic acids (Christaki 2014). The first barriers against pathogen invasion are the skin and mucosal surfaces. Neutrophils are the primary and most important cells that defend the host against invading pathogens. Other mechanisms of defence include monocytes and macrophages, cytokines storm, and complement activation. The pathogenesis presents unique features, as they are under the influence of the genetic makeup of the host. (Christaki 2014).
A deficient immune system is a risk factor for the development of sepsis, which can be caused by functional asplenia, an infectious disease, or haematologic malignancy (Dellinger 2013). Moreover, malignancy has been associated with an increase in the incidence of sepsis, with a risk ratio of 9.77 (95% CI 9.67 to 9.88) as compared to non‐cancer patients (Danai 2006). Complications associated with the onset of sepsis include acute renal failure, polyneuropathy, cardiomyopathy, and multiple organ dysfunction (Latronico 2011; Puthucheary 2013; Romero‐Bermejo 2011). Survivors of sepsis report persistent problems that can last for years after hospital discharge. About 50% to 70% of sepsis survivors report physical alterations (weakness and dyspnoea), psychological problems (post‐traumatic stress syndrome and depression), and cognitive (poor concentration and memory loss) and social issues (delayed return to work and loss of earnings) (Dowdy 2005). Management of septic stages remains a daily challenge for clinicians. Early administration of effective intravenous antimicrobials is highly recommended due to their association with reduced mortality (Castellanos‐Ortega 2010; Ferrer 2009).
Index test(s)
Interleukin‐6 (IL‐6) is a cytokine secreted by immune cells, such as activated monocytes and macrophages, adipocytes, and endothelial cells, and it mediates a wide range of biological activities (Tanaka 2014; Thompson 2012). Some studies have shown that cytokines such as IL‐1 and tumour necrosis factor (TNF) induce a state of shock with haemodynamic and haematologic alterations, which are classic characteristics of septic stages (Carson 1999; Dinarello 1997; Hauptmann 1991; van der Poll 1990). Both IL‐6 and IL‐1 play a role in the stimulation of the synthesis of the adrenocorticotropic hormone in the pituitary gland. They induce the synthesis of neuronal growth factor and regulate the growth and development of haematopoietic cells and embryonic stem cells (Song 2005). In addition, IL‐6 is an endogenous pyrogen that plays a role in systemic changes associated with infection, tissue injury and in the stimulation of hepatic protein synthesis during acute‐phase responses (Kishimoto 1995). Interleukin‐6 concentrations can be measured in blood samples at different times during hospitalization (Thompson 2012); however, IL‐6 measurement in other biological fluids has been suggested as potentially useful for diverse pathological conditions, Heney 1995, including cerebrospinal (Takahashi 2014), pleural (Thomas 2016), and peritoneal fluids (Cheong 2002). In healthy adults, IL‐6 plasma concentrations range from 0.2 to 7.8 pg/mL, while IL‐6 concentration in adults with sepsis can exceed 1600 pg/mL (Thompson 2012). Clinical response and the severity of infection affect the values of IL‐6 in adults, but this relationship is not clear in children (Aneja 2011). On the contrary, IL‐6 concentrations in newborns have been estimated at between 18 to 26 pg/mL, with a significant decrease during the first few years of life without the presence of infection (Song 2005). In addition, some authors have reported elevated concentrations of IL‐6 in paediatric burn patients without sepsis (Finnerty 2007).
Clinical pathway
Since the 2015 sepsis consensus, the underlying organ dysfunction is identified as an acute change in total SOFA score ≥ 2 points due to the infection, and immediate treatment is highly recommended (Singer 2016). Management of patients with suspected sepsis includes volume resuscitation, a collection of samples for microbiological diagnosis, early antimicrobial therapy, and infection source control (Dellinger 2013; Rhodes 2017; Singer 2016). Current clinical practice guidelines recommend administration of empiric antimicrobial therapy, including one or more drugs that have activity against most pathogens (Dellinger 2013; Green 2008; Levy 2018; Reinhart 2010; Rhodes 2017). However, one consequence of this strategy is the overtreatment of patients with non‐infectious diseases, which can induce antimicrobial resistance and increased economic costs. The use of biomarkers for early diagnosis and to guide empiric antimicrobial agents has only been suggested as supplementary data to clinical assessment, in cases of difficult‐to‐culture pathogens, or in clinical situations where the suspected infection is unclear (Dellinger 2013; Rhodes 2017).
Prior test(s)
No prior tests for the diagnosis of sepsis have been proposed. The basis of future tests, including blood tests and microbiological cultures, lies in the identification of signs of inflammation and/or end‐organ hypoperfusion by a clinical assessment (Dellinger 2013; Rizoli 2002).
Role of index test(s)
At present, sepsis is defined as a suspected or documented infection accompanied by an acute increase of the quick Sequential Organ Failure Assessment (qSOFA) scores (Singer 2016). For years, cultures have been essential to document the presence of an infection, though their results can take 24 to 48 hours (Levy 2003). In addition, the results of the cultures may be undeterminable due to the use of an empiric antimicrobial before the sampling or difficult‐to‐culture pathogens (Dellinger 2013; Rhodes 2017). Biomarkers used for the diagnosis of sepsis may provide faster results in comparison with microbiology tests, resulting in a quicker initiation of treatment (Boucher 1999). Interleukin‐6 appears to be a mediator of sepsis, and its secretion is rapidly induced in the course of acute inflammatory reactions (Song 2005). Most patients with sepsis have increased plasma levels of IL‐6 at their admission to the ICU (Waage 1989). High IL‐6 levels have been directly associated with risk of death, especially death caused by intra‐abdominal sepsis (Patel 1994). Likewise, an association between mean plasma IL‐6 concentration over time and mortality rate has been shown. Persistent elevation of IL‐6 appears to be more important than that of the initial or peak levels in terms of outcome (Pinsky 1993). Interleukin‐6 could be considered as a potential triage test of sepsis, in order to ensure quick initiation of empirical antibiotic management in critically ill patients who are waiting for culture results. In addition, if the detection of IL‐6 levels demonstrates high specificity and sensitivity towards sepsis, it might play an important role in replacing other diagnostic tools, thus reducing unnecessary patient exposure to antibiotics (Gentile 2013).
Alternative test(s)
Currently, several biomarkers that may have the ability to improve early recognition and decrease the severity of sepsis have been evaluated. For example, the use of C‐reactive protein concentrations has been proposed as an acute‐phase reactant for the diagnosis of bacterial infections, as well as a factor that can lead to a reduction in the mortality rate of septic patients (Andriolo 2017; Onyenekwu 2017; Simon 2004). A C‐reactive protein level that exceeds 0.8 mg/L is abnormal and may indicate the presence of an inflammatory process. Likewise, the diagnostic value of procalcitonin has been evaluated in several systematic reviews, with contradictory results (Simon 2004; Tang 2007; Wacker 2013). Other biomarkers, such as IL‐ 8, Livaditi 2006, and soluble triggering receptor expressed on myeloid cells 1 (sTREM‐1), Gamez‐Diaz 2011, have also been evaluated, without conclusive results (de Montmollin 2014).
Rationale
Currently, the World Health Organization (WHO) recognizes sepsis as a healthcare priority, and it urges the Member States to include and reinforce the prevention, diagnosis, and treatment of this condition in national health systems (WHO 2017). Despite the fact that sepsis is one of the leading causes of mortality in critically ill patients, it lacks an accurate diagnostic test (Bloos 2014). The differentiation of sepsis from other syndromes is essential in order to avoid unnecessary administration of antibiotics and to start appropriate therapy sooner. Some authors have reported higher levels of IL‐6 in patients with sepsis and multiple organ dysfunction, but not in other conditions, such as trauma or cardiac arrest (Bloos 2014; Song 2005). The detection of higher IL‐6 levels could therefore be potentially useful in early diagnosis (Jekarl 2015). Determining the accuracy of the detection of IL‐6 levels as a biomarker for the diagnosis of sepsis might help to provide adequate and timely management of critically ill patients. This could reduce the morbidity and mortality associated with sepsis. Furthermore, an accurate measurement tool may also limit hospitalization costs and potential antimicrobial resistance. This current review focused on one biomarker (IL‐6) only, and did not include comparisons of diagnostic accuracy with other biomarkers, as there is a Cochrane Review in process assessing the roles of C‐reactive protein, procalcitonin, and presepsin as biomarkers for sepsis (Onyenekwu 2017).
Objectives
To determine the diagnostic accuracy of plasma interleukin‐6 (IL‐6) concentration for the diagnosis of bacterial sepsis in critically ill adults.
Secondary objectives
To explore the effects of different thresholds in the accuracy of IL‐6 for the diagnosis of sepsis.
To determine whether the pathological source of sepsis (i.e. pneumonia, bacteraemia, urinary infections, among others) or other prespecified sources affect the accuracy of plasma IL‐6 concentration as a diagnostic tool.
Methods
Criteria for considering studies for this review
Types of studies
We considered diagnostic test accuracy studies that included patients aged 18 years or older with suspicion of sepsis during their hospitalization, and where IL‐6 levels were evaluated by serological measurement, as well as sepsis confirmation by means of clinical diagnosis and/or identification of microbiological pathogens in cultures. Studies should have provided information about the specificity and sensitivity of the results. We considered abstracts and conference proceedings in the initial selection of references. However, due to these selected references not providing enough information for the assessment of the methodological quality, they were classified as studies awaiting classification. We excluded before‐after studies, case‐control studies (see Differences between protocol and review), and case reports.
Participants
We included studies evaluating critically ill adults aged 18 years or older under suspicion of sepsis (i.e. fulfilling SIRS criteria). These studies included participants from different clinical settings, such as emergency departments, hospital wards, and ICUs. We excluded studies of neonatal or paediatric patients with suspicion of sepsis.
Index tests
We included articles with a description of the index test for the measurement of IL‐6 in plasma as a sign of systemic inflammatory, metabolic, and physiologic activity. Measurement of IL‐6 concentrations should have been performed before initiation of empirical antibiotic treatment. We excluded measurements of IL‐6 other than serum (i.e. pleural effusion, peritoneal fluid, or cerebrospinal fluid).
Target conditions
As we mentioned earlier in the Background section of this report, for years sepsis was considered to be a systemic inflammatory response syndrome of the host due to an infection (Appendix 1) (Levy 2003). Recently, a consensus task force of the European Society of Intensive Care Medicine (ESICM) and the Society of Critical Care Medicine (SCCM) updated the definition of sepsis and septic shock to be defined as a life‐threatening organ dysfunction resulting from a dysregulated host response to inflammation (Singer 2016).
Reference standards
The criteria used for the diagnosis of sepsis have been modified from the initial proposals by Bone and colleagues in 1991 (Bone 1992), which were endorsed by the American College of Chest Physicians (ACCP) and the SCCM, to those developed in 2015 by the SCCM and the ESICM (Singer 2016). A full description of the mentioned definitions and clinical criteria can be found in Appendix 1, Appendix 2, and Appendix 3. Briefly, the following three sets of criteria have been used over the years.
1991 ACCP/SCCM criteria (Bone 1992): these criteria were the first attempt to provide a conceptual framework to diagnose, monitor, and treat sepsis. In addition, this consensus was the first to introduce the term 'systemic inflammatory response syndrome' (SIRS), broadly defined as all findings associated with systemic activation of the innate immune response. Sepsis is defined under Bone 1992 as SIRS plus signs of infection, while severe sepsis is considered to be any sepsis associated with organ dysfunction, hypoperfusion, or hypotension.
2001 SCCM/ESICM/ACCP/ATS/SIS criteria (Levy 2003): the 2001 consensus conference reviewed the strengths and weaknesses of the 1992 criteria, and developed modified criteria incorporating the latest clinical understanding of sepsis, as well as findings of clinical trials. These criteria highlighted the role of systemic inflammation in response to an infection, broadly defined as a pathological process induced by a micro‐organism (Levy 2003). Sepsis was then defined as a systemic inflammatory response syndrome of the host due to an infection (Levy 2003).
2015 SCCM/ESICM criteria (Singer 2016): sepsis is defined under Singer 2016 as a life‐threatening organ dysfunction caused by a dysregulated host response to infection. For clinical cases, organ dysfunction can be defined by an increase in the Sequential Organ Failure Assessment (SOFA) score of 2 points or more. In addition, septic shock is considered as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality. Under Singer 2016, terms such as SIRS and severe sepsis are no longer recommended in the management of this condition.
A common element to all these criteria is the requisition of cultures to document the suspected infection, however positive findings are not a requirement for antibiotic management (Bone 1992; Levy 2003; Singer 2016). We considered all these criteria as valid reference standards for sepsis, recognizing that the knowledge in this field is still evolving.
Search methods for identification of studies
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 1, Appendix 4);
MEDLINE via Ovid SP (1956 to 25 January 2019, Appendix 5);
Embase via Ovid SP (1982 to 25 January 2019, Appendix 6);
LILACS (Latin American and Caribbean Health Science Information database) via BIREME (1982 to 25 January 2019, Appendix 7);
Web of Science Indexes (Science Citation Index Expanded (SCI‐EXPANDED), Social Sciences Citation Index (SSCI), Arts & Humanities Citation Index (A&HCI), Emerging Sources Citation Index (ESCI); from inception to 25 January 2019, Appendix 8).
We designed structured search strategies using controlled search terms appropriate for each database as well as free‐text search terms as outlined in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Deeks 2013). We did not use search filters (collections of terms aimed at reducing the number needed to screen) as an overall limit because in those reviews that have used them they have not proved to be sensitive enough (Whiting 2011a). We did not apply any language restriction to the electronic searches.
Searching other resources
We screened the reference lists of all relevant papers for additional studies and searched for similar articles related to the final included studies. We contacted relevant authors for further details about studies, but we did not receive replies from the contacted authors at the time of review publication (see Results of the search). We did not perform handsearching, as there is little published evidence of the benefits of handsearching for reports of diagnostic test accuracy studies (Glanville 2012).
Data collection and analysis
Selection of studies
Two review authors (IAR, DMF) independently identified potentially eligible studies based on title and abstract. Any disagreements were resolved by discussing the paper(s) in question with a third review author (MR). We retrieved the full‐text copy of each study assessed as potentially eligible, and two review authors independently evaluated the full texts for inclusion or exclusion according to the selection criteria. We documented the study selection process in a PRISMA flow diagram.
Data extraction and management
Four review authors (DMF, IAR, XN, NM) extracted the study characteristics from each included study, including data on quality assessment and investigation of heterogeneity, and transferred this information into a study‐specific format, as described in Appendix 9. Any disagreements were resolved by discussion with a third review author (JZ or MR). We cross‐tabulated the numerical information from the index test results (positive or negative) in 2 x 2 tables against the target disorder (positive or negative), and presented the results in tables (Appendix 10).
Assessment of methodological quality
Four review authors (NM, JZ, MR, IAR) independently and in duplicate assessed the methodological quality using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool (Whiting 2011b), as recommended by the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Deeks 2013). This tool consists of four domains: patient selection, index test, reference standard, and patient flow. We assessed each domain in terms of risk of bias, and further considered the first three domains in terms of applicability. We reported the QUADAS‐2 methodological assessment of studies using bespoke tables. Operational definitions describing the use of QUADAS‐2 are presented in Appendix 11. This format was piloted against 10 primary diagnostic studies in order to standardize this assessment and to identify any possible disagreement between review authors. Any discrepancies were resolved by discussion.
Statistical analysis and data synthesis
For all included studies we extracted data from the 2 x 2 tables (numbers of true positives, false positives, true negatives, and false negatives) showing the cross‐classification between binary test results and the binary reference standard. For each study, we calculated sensitivities, specificities and their 95% confidence intervals (CIs) (Appendix 10). We presented results graphically by plotting estimates of sensitivities and specificities (both with 95% CIs) in a forest plot and in a receiver operating characteristic (ROC) space in order to visually assess the between‐study variability. We considered these findings in light of the methodological quality of individual studies. We used the Cochrane statistical software Review Manager 5 to document these analyses (Review Manager 2014).
We planned to obtain summary sensitivity and summary specificity estimates using the bivariate model (Reitsma 2005), analysing information of the most common thresholds when data with more than one positive threshold was reported within the same study (Molano Franco 2015). However, we were unable to perform this analysis because we observed high heterogeneity in the data that prevented the estimation of summary accuracy estimates. Instead, we estimated a summary ROC (SROC) curve by fitting a hierarchical summary ROC (HSROC) non‐linear mixed model (Rutter 2001). Using HSROC parameter estimates, we derived sensitivity at the median value of specificity along with corresponding 95% CIs calculated using the delta method as implemented in R package. We calculated the potential numerical consequences given a positive and negative IL‐6 test result, using different prevalences. All analyses were conducted in the SAS statistical software package, SAS 2014, and R software (R Development Core Team 2008). This is a diversion from the protocol that is explained in the Differences between protocol and review section.
Investigations of heterogeneity
We initially investigated heterogeneity by visual examination of forest plots of sensitivities and specificities and through visual examination of individual study results in the ROC space. Anticipated sources of heterogeneity included the year of publication, country/geographical area, setting (emergency, ICUs, hospitalization ward, or other), baseline diagnosis, the origin of infection (pneumonia, urinary infection, meningitis), type of sepsis (severe, septic shock), and type of reference standard. As mentioned above, we estimated an SROC curve by fitting an HSROC (Rutter 2001), and explored the effect of predefined sources of heterogeneity on model parameters. When possible, we investigated the effect of covariates by including each potential source of heterogeneity, one at each time, in the original HSROC model. We initially explored whether there was a significant difference in the shape of the SROC curve. If we ruled out a significant effect of the covariate on the shape of the curve, we then analysed whether the covariates affected both accuracy and threshold parameters of the model, or either. Conversely, if the shape varies with the covariate, no further simplifications of the model can be performed. We used likelihood ratio tests to compare models with and without the corresponding covariates effects on shape, accuracy and threshold. We conducted all these analyses using the SAS statistical software package (SAS 2014). This is a diversion from the protocol that is explained in the Differences between protocol and review section.
Sensitivity analyses
We planned to examine the robustness of the meta‐analyses by conducting sensitivity analyses. Our primary analysis included all studies; sensitivity analysis would exclude studies at high risk of bias or studies for which there were important concerns about potential applicability. However, since we judged all included studies as at high risk of bias due to index test issues, this analysis could not be performed. This is a diversion from the protocol that is explained in the Differences between protocol and review section.
Assessment of reporting bias
Quantitative methods for exploring reporting bias are not well established for diagnostic test accuracy studies. We did not perform a formal assessment of publication bias using methods such as funnel plots or regression tests because such techniques have not been useful for diagnostic test accuracy studies (Deeks 2013). This is a diversion from the protocol that is explained in the Differences between protocol and review section.
Results
Results of the search
The details of our search and selection process are shown in Figure 1. The electronic database searches yielded 41,588 references from selected databases after removal of duplicates. We searched for primary studies through other resources but did not find additional potentially eligible studies. Our initial screening of titles and abstracts identified 108 references to assess in full text. We excluded 65 of the 108 full‐text studies for the following reasons: a) no reporting of accuracy data for IL‐6; b) focus was on the prediction of sepsis; c) case‐control studies; d) other reasons (see Characteristics of excluded studies). We classified 20 conference proceedings that contained insufficient information to apply all of the selection criteria, as well as to perform a full data extraction, as studies awaiting classification (see Characteristics of studies awaiting classification). We included 23 studies in the qualitative synthesis (Aalto 2004; Anand 2015; Du 2003; Endo 2012; Fu 2013; Gao 2018; Gomez 2010; Harbarth 2001; Hou 2016; Jekarl 2013; Jiang 2015; Li 2013; Liu 2005; Llewelyn 2013; Mat‐Nor 2016; Meynaar 2011; Moscovitz 1994; Ramirez 2009; Sakr 2008; Tromp 2012; Tsalik 2012; Tsantes 2013; Zhao 2014). However, because we were unable to rebuild the 2 x 2 table in two studies (we contacted the authors of Gao 2018 and Hou 2016 by email, but at the time of publication of this review had not received replies), we only included 21 studies in the main quantitative analysis (Aalto 2004; Anand 2015; Du 2003; Endo 2012; Fu 2013; Gomez 2010; Harbarth 2001; Jekarl 2013; Jiang 2015; Li 2013; Liu 2005; Llewelyn 2013; Mat‐Nor 2016; Meynaar 2011; Moscovitz 1994; Ramirez 2009; Sakr 2008; Tromp 2012; Tsalik 2012; Tsantes 2013; Zhao 2014). In addition, we contacted the main author of Harbarth 2001 by email to confirm the use of threshold to define sepsis, but at the time of the analysis had not received a reply. We included this study with the reported cut‐off transformed to pg/mL (Harbarth 2001; 200,000 pg/mL).
1.
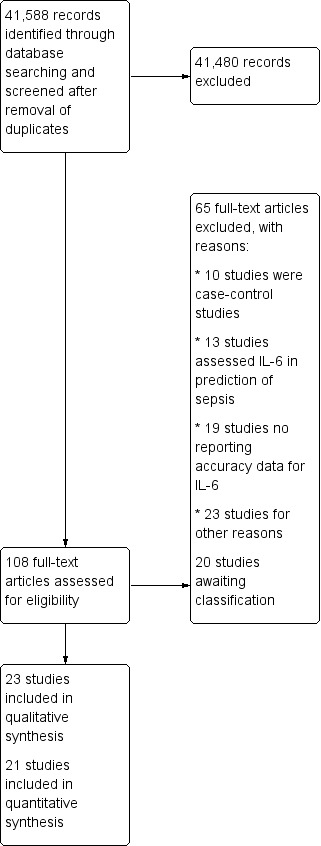
Study flow diagram.
Characteristics of included studies
Details of the population, index test, target condition, and reference standard for the 23 included studies are provided in the Characteristics of included studies table. The main characteristics of the included studies are summarized in Table 2.
1. Characteristics of included studies.
| ID | Year of publication | Country | Age (as reported) | # Male (%) | Target condition | Baseline diagnosis | APACHE (as reported) | Origin of infection | Reference standard | Setting | IL‐6 measurement brand | Use of empirical antibiotics | Funding |
| Aalto 2004 | 2004 | Finland | Mean: 52 | 44 (47.8) | Community‐acquired bloodstream infection with positive blood cultures | Mix/unclear | Not reported | Pneumonia, urinary tract infection, encephalitis and meningitis, abscesses, sinusitis, malaria, erysipelas | SIRS criteria + microbiological evidence of local infection | Emergency | Chemiluminescent immunoassay system (Immulite; Diagnostic Products, Los Angeles, CA, USA) | Yes (after sampling) | Not stated |
| Anand 2015 | 2015 | India | Mean: 42 (culture‐negative sepsis) vs 53.7 (culture‐positive sepsis) vs 54 (SIRS) | 122 (58.6) | Culture‐negative/positive bacterial sepsis | Mix/unclear | Mean APACHE‐II: 15.9 (SIRS) vs 22.9 (culture‐negative sepsis) vs 25.8 (culture‐positive groups) | Intra‐abdominal infection, bacterial pneumonia, urosepsis, cellulitis, puerperal sepsis | 2001 SCCM/ESICM/ACCP/ATS/ SIS sepsis definition conference ‐ applied for clinicians (critical care and emergency medicine) | ICU | Chemiluminescent Access Immunoassay System (Beckman Coulter Inc, Brea, CA, USA) | Not stated | Academic/ governmental/ health agency |
| Du 2003 | 2003 | China | Mean: 64.7 | 31 (60.7) | Sepsis (general) | Mix/unclear | Mean APACHE‐II: 19.9 (sepsis) vs 15.9 (SIRS) | Lower respiratory tract infection, intra‐abdominal infection, bloodstream infection, and others | 1992 ACCP/SCCM Consensus Conference Committee ‐ no further details provided | ICU | IL‐6 EASIA test kit (Medgenics Diagnostics SA, Fleurus, Belgium) | Not stated | Not stated |
| Endo 2012 | 2012 | Japan | Median: 66 to 76 | 122 (58.9) | Sepsis (general) | Mix/unclear | Not reported | Systemic and localized infection (general) | SIRS criteria + microbiological evidence of local infection | Emergency | Immulyze 2000 assay system (Siemens Healthcare Diagnostics, Japan) | Not stated | Not stated |
| Fu 2013 | 2013 | China | Mean: 43 to 46 | 152 (51.1) | Bacteraemia in haematologic malignancies (neutropenia febrile) | Haematological malignancy with febrile neutropenia | Not reported | Bacteraemia (general) | SIRS criteria + microbiological evidence of local infection | Unclear | Roche Diagnostics Inc | Not stated | Not stated |
| Gao 2018 | 2018 | China | Mean: 56.7 vs 56.2 | 116 (59.7) | Sepsis | Mix/unclear | Sepsis group only = APACHE II > 25: 26.9% | Head/neck, thorax, abdomen, pelvic cavity, arms and legs, blood, others | 1992 ACCP/SCCM Consensus Conference Committee ‐ no further details provided | Unclear | Electrochemical luminescence on a Roche COBAS‐e601 | Not stated | Academic/ governmental/ health agency |
| Gomez 2010 | 2010 | Spain | Mean: 62 | 115 (60.2) | Sepsis (general) | Mix/unclear | Not reported | Unclear | 2001 SCCM/ESICM/ACCP/ATS/ SIS sepsis definition conference ‐ applied for clinicians (unclear) | ICU | IMMULITE 1.000 System (Siemens) | Not stated | Industry |
| Harbarth 2001 | 2001 | Switzerland | Mean range: 51 to 59 | 57 (73.1) | Sepsis (general) | Mix/unclear | Not reported | Respiratory tract, intra‐abdominal space, bloodstream, others | 1992 ACCP/SCCM Consensus Conference Committee ‐ applied for clinicians (unclear) | ICU | (ImmuliteOne; DPC Biermann, Bad Nauheim, Germany) | Yes (after sampling) | Academic/ governmental/ health agency |
| Hou 2016 | 2016 | China | Mean: 58.3 (sepsis) vs 55.4 (SIRS) | 41 (61.1) | Sepsis (general) | Mix/unclear | Mean APACHE‐II: 18.2 (sepsis) | Abdomen, thorax, and blood sources | SIRS criteria + microbiological evidence of local infection | ICU | Electrochemical luminescence on a Roche COBAS‐e601 | Yes (after sampling) | Academic/ governmental/ health agency |
| Jekarl 2013 | 2013 | South Korea | Mean: 51.5 | 88 (49.7) | Sepsis (general) | Mix/unclear | Not reported | Acute pyelonephritis, pneumonia, digestive tract infection, others | 1992 ACCP/SCCM Consensus Conference Committee ‐ no further details provided | Emergency | Chemiluminescence method using the Elecsys IL‐6 kit (Roche) | Yes (after sampling) | Academic/ governmental/ health agency |
| Jiang 2015 | 2015 | China | Mean range: 45.1 to 65.3 | 61 (58.6) | Gram‐negative bacterial sepsis | Biliary and intra‐abdominal infections | Mean APACHE‐II = 8.4 (SIRS) vs 9.3 (sepsis) | Biliary infection | 1992 ACCP/SCCM Consensus Conference Committee ‐ no further details provided | Unclear | BioLegend | Yes (after sampling) | Academic/ governmental/ health agency |
| Li 2013 | 2013 | China | Mean: 59.47 (sepsis) vs 45.1 (SIRS) | 40 (76.9) | Sepsis (general) | Mix/unclear | Mean APACHE‐II = 13.64 (SIRS) vs 19.37 (sepsis) | Not stated | 1992 ACCP/SCCM Consensus Conference Committee ‐ applied for clinicians (critical care) | ICU | EK0410; Boster Biological Technology | Yes (after sampling) | No funding |
| Liu 2005 | 2005 | China | Mean: 60.6 (sepsis) vs 51.7 (SIRS) | 21 (70.1) | Sepsis (general) | Mix/unclear | Mean APACHE‐II = 15.4 (sepsis) vs 7.9 (SIRS) | Intraperitoneal infection, lower respiratory tract infection, lower respiratory tract infection complicated with haematogenous infection, intraperitoneal infection complicated with haematogenous infection, haematogenous infection and biliary tract infection | 1992 ACCP/SCCM Consensus Conference Committee ‐ no further details provided | ICU | Genzyme | Yes (after sampling) | Not stated |
| Llewelyn 2013 | 2013 | United Kingdom | Median: 65.9 | 126 (77.7) | Sepsis (general) | Mix/unclear | Not reported | Respiratory tract, abdomen, others | 2001 SCCM/ESICM/ACCP/ATS/ SIS sepsis definition conference ‐ applied for clinicians (unclear) | ICU | Luminex LX200 using Invitrogen’s Human Inflammatory 5‐Plex panel (Invitrogen/Life Technologies, Darmstadt, Germany) | Not stated | Industry |
| Mat‐Nor 2016 | 2016 | Malaysia | Mean: 47 | 167 (69.8) | Sepsis (general) | Mix/unclear | Not reported | Respiratory, others | 2001 SCCM/ESICM/ACCP/ATS/ SIS sepsis definition conference ‐ applied for clinicians (critical care) | ICU | Quantikine enzyme‐linked immunosorbent assay kit from R&D Systems (Minnesota, USA) | Yes (after sampling) | Academic/ governmental/ health agency |
| Meynaar 2011 | 2011 | Netherlands | Mean: 66 | Unclear | Sepsis (general) | Mix/unclear | Median APACHE‐IV = 57 | Gastrointestinal, pulmonary, others | 1992 ACCP/SCCM Consensus Conference Committee ‐ no further details provided | ICU | IMMULITE 2000; Siemens Healthcare, the Netherlands | Not stated | Industry |
| Moscovitz 1994 | 1994 | USA | Median: 51 | 37 (37) | Bacteraemia | Mix/unclear | Mean: 12.1 | Bloodstream, other sites | SIRS criteria + microbiological evidence of local infection | Emergency | ELISA kits (Genzyme, Cambridge, MA, USA) | Yes (after sampling) | Academic/ governmental/ health agency |
| Ramirez 2009 | 2009 | Spain | Range: 61 to 66 | 27 (61.3) | Ventilator‐associated pneumonia | Patients mechanically ventilated | Median APACHE‐II: 18 (suspected VAP) vs 20 (confirmed VAP) | Pneumonia | VAP clinical criteria + BAL positive cultures | ICU | Commercial enzimoimmunoassay technique (BioSource, Nivelles, Belgium) | Yes (1 to 2 days at the day of diagnosis) | Academic/ governmental/ health agency |
| Sakr 2008 | 2008 | Germany | Mean: 63 | 207 (63.3) | Sepsis/severe sepsis | Mix/unclear | Mean APACHE‐II: 15.1 | Respiratory system, others | 1992 ACCP/SCCM Consensus Conference Committee ‐ applied for clinicians (critical care) | ICU | Immulite (DPC Biermann) | Not stated | Academic/ governmental/ health agency |
| Tromp 2012 | 2012 | Netherlands | Median: 59 | 193 (56.4) | Bacteraemia | Mix/unclear | Not reported | Pneumonia, others | SIRS criteria + microbiological evidence of local infection | Emergency | Immulite 2500 (Siemens Healthcare Diagnostics, Deerfield, IL, USA) | Yes (no details) | No funding |
| Tsalik 2012 | 2012 | USA | Median: 52 | 173 (51.4) | Sepsis (general) | Mix/unclear | Median APACHE‐II: 8 | Lung, urinary tract, skin, others | 1992 ACCP/SCCM Consensus Conference Committee ‐ applied for clinicians (emergency medicine or internal medicine) | Emergency | Roche Elecsys 2010 analyser (Roche Diagnostics, Laval, Canada) by electro chemiluminescent immunoassay | Not stated | Academic/ governmental/ health agency |
| Tsantes 2013 | 2013 | Greece | Mean: 59 (septic ARDS) vs 62.2 (non‐septic ARDS) | 60 (60) | Sepsis in acute respiratory distress syndrome | ALI/ARDS | Mean APACHE‐II: 24.3 (septic) vs 19.2 (non‐septic) | Lung, bloodstream, skin‐soft tissue, others | 2001 SCCM/ESICM/ACCP/ATS/ SIS sepsis definition conference ‐ no further details provided | ICU | Elecsys IL‐6 immunoassay (Roche Diagnostics GmbH, Mannheim, Germany) | Not stated | No funding |
| Zhao 2014 | 2014 | China | Mean: 69 vs 73 | 357 (54.7) | Sepsis (general) | Mix/unclear | Not reported | Not stated | 2001 SCCM/ESICM/ACCP/ATS/ SIS sepsis definition conference ‐ no further details provided | Emergency | ELISA (RapidBio Systems) | Not stated | Not stated |
ACCP: American College of Chest Physicians;ALI: acute lung injury; APACHE score: Acute Physiology And Chronic Health Evaluation score; ARDS: acute respiratory distress syndrome; ATS: American Thoracic Society; BAL: bronchoalveolar lavage; EASIA: enzyme‐amplified sensitivity immunoassay; ED: emergency department; EDTA: ethylenediaminetetraacetic acid;ELISA: enzyme‐linked immunosorbent assay; ESICM: European Society of Intensive Care Medicine; IL‐6: interleukin‐6;IL‐8: interleukin‐8; ICU: intensive care unit IQR: interquartile range; PCT: procalcitonin; SAPS: Simplified Acute Physiology Score; SCCM: Society of Critical Care Medicine; SIRS: systemic inflammatory response syndrome; SIS: Surgical Infection Society; VAP: ventilator‐associated pneumonia.
General characteristics
We included a total of 23 studies and 4192 critically ill adults in the qualitative analysis. The sample size ranged from 20, in Ramirez 2009, to 652 ill adults, in Zhao 2014. Mean sample size was 167.68 participants (interquartile range (IQR): 66 to 231). The included studies were published between 1994 and 2018. Most studies were published after the sepsis consensus of 2001, but before the release of the 2015 consensus criteria (16 studies; 69.5%; Anand 2015; Endo 2012; Fu 2013; Gomez 2010; Harbarth 2001; Jekarl 2013; Jiang 2015; Li 2013; Llewelyn 2013; Meynaar 2011; Ramirez 2009; Sakr 2008; Tromp 2012; Tsalik 2012; Tsantes 2013; Zhao 2014). The three studies published after 2015 did not apply the updated definition (Gao 2018; Hou 2016; Mat‐Nor 2016). A considerable number of studies were performed in Asia (12 studies; 52.1%), with China being the country with the most studies (8 studies; Du 2003; Fu 2013; Gao 2018; Hou 2016; Jiang 2015; Li 2013; Liu 2005; Zhao 2014). Nine studies were performed in Europe, and only two studies were conducted in the USA (Moscovitz 1994; Tsalik 2012).
All studies provided information about participants with sepsis versus patients under suspicious of sepsis (i.e. SIRS adult patients). In addition, some studies provided data from healthy controls, but we did not include this information in the review. Four studies using the 2001 criteria provided additional information about sepsis versus SIRS plus organ dysfunction (Llewelyn 2013), severe sepsis versus SIRS (Sakr 2008), severe sepsis and shock versus SIRS (Jekarl 2013), and septic shock versus SIRS (Gomez 2010). Most of the studies were funded by academic, governmental, or institutional sources (11 studies; 47.8%). Three studies were funded by medical device companies (Gomez 2010; Llewelyn 2013; Meynaar 2011), and three other studies stated that no funds were received for their development (Li 2013; Tromp 2012; Tsantes 2013).
Population
The age of the participants was heterogeneously reported; seven studies reported mean age for all enrolled participants, ranging from 45.1 to 66 years (Aalto 2004; Du 2003; Jekarl 2013; Jiang 2015; Mat‐Nor 2016; Meynaar 2011; Sakr 2008). The percentage of included men ranged from 37%, in Moscovitz 1994, to 77%, in Llewelyn 2013. The included studies reported multiple baseline diagnoses, with most studies only referring to their participants as ill patients, ICU patients, or patients with suspected sepsis (19 studies; 82.6%). Four studies were developed in specific populations, such as patients with acute respiratory distress syndrome (ARDS) (Tsantes 2013), biliary and intra‐abdominal infections (Jiang 2015), haematological malignancies (Fu 2013), or mechanically ventilated patients (Ramirez 2009). Thirteen studies (56.5%) reported basal measurements of the Acute Physiology And Chronic Health Evaluation (APACHE‐II in most cases), with mean APACHE in septic groups ranging from 9.3 to 25.8 units.
The origin of the infection was heterogeneous in the enrolled samples, and included acute pyelonephritis, pneumonia, digestive tract infection, intra‐abdominal infection, urosepsis, cellulitis, lower respiratory tract infections, urinary tract infections, and bloodstream infections, among others. The included studies did not provide subgroup information by source of infection. Four studies assessed bacteraemia (Aalto 2004; Fu 2013; Moscovitz 1994; Tromp 2012). One study focused its main analysis on the comparison between sepsis by culture findings (positive or negative) versus SIRS (Anand 2015).
The most common setting was ICUs (mixed and surgical) (13 studies; Anand 2015; Du 2003; Gomez 2010; Harbarth 2001; Hou 2016; Li 2013; Liu 2005; Llewelyn 2013; Mat‐Nor 2016; Meynaar 2011; Ramirez 2009; Sakr 2008; Tsantes 2013). The remaining studies were set in emergency departments, with the exception of three studies for which the setting was unclear (Fu 2013; Gao 2018; Jiang 2015). The use of empirical antibiotics was not explicitly stated or was unclear in 13 studies; in nine studies antibiotics were administered after blood sampling (Aalto 2004; Harbarth 2001; Hou 2016; Jekarl 2013; Jiang 2015; Li 2013; Liu 2005; Moscovitz 1994; Ramirez 2009). In one study, participants were excluded if they had received antimicrobial treatment for more than 24 hours before blood sampling (Mat‐Nor 2016).
Index test
All but five of the included studies, Endo 2012; Gao 2018; Hou 2016; Li 2013; Llewelyn 2013, reported IL‐6 as the main test assessed. Fourteen studies used automated immunoassay analysers, and the remaining studies used enzyme‐linked immunosorbent assay (ELISA) kits (Du 2003; Jiang 2015; Li 2013; Liu 2005; Llewelyn 2013; Mat‐Nor 2016; Moscovitz 1994; Ramirez 2009; Zhao 2014). The methods/techniques used to read the results were absorbance/optical density (6 cases; Du 2003; Jiang 2015; Li 2013; Mat‐Nor 2016; Ramirez 2009; Zhao 2014); chemiluminescence (8 cases; Aalto 2004; Anand 2015; Endo 2012; Gomez 2010; Harbarth 2001; Meynaar 2011; Sakr 2008; Tromp 2012); and electrochemiluminescence (6 cases; Fu 2013; Gao 2018; Hou 2016; Jekarl 2013; Tsalik 2012; Tsantes 2013).
The threshold of positivity for sepsis ranged from 40 pg/mL, in Tsalik 2012, to 200,000 pg/mL, in Harbarth 2001. Only one value (100 pg/mL) was reported by more than one study (Endo 2012; Tsalik 2012). Llewelyn 2013 analysed the threshold of 200 pg/mL for sepsis versus SIRS and for sepsis versus SIRS with organ dysfunction. In addition, Tsalik 2012 provided information about three different cut‐offs comparing sepsis versus SIRS patients (40, 100, and 500 pg/mL). No threshold was prespecified before statistical analysis (see Characteristics of included studies). Llewelyn 2013 also reported undetermined results for assessed groups; no additional studies reported these results.
Reference standard
Ten studies used the 1991 ACCP/SCCM Consensus Conference Committee to define sepsis (Bone 1992; 40.9%; Du 2003; Gao 2018; Harbarth 2001; Jekarl 2013; Jiang 2015; Li 2013; Liu 2005; Meynaar 2011; Sakr 2008; Tsalik 2012). In addition, six studies used the 2001 SCCM/ESICM/ACCP/ATS/SIS sepsis definition conference (27%; Anand 2015; Gomez 2010; Llewelyn 2013; Mat‐Nor 2016; Tsantes 2013; Zhao 2014). Sepsis was diagnosed by critical care, emergency, or internal medicine clinicians in five studies (Anand 2015; Li 2013; Mat‐Nor 2016; Sakr 2008; Tsalik 2012), while this information was either unclear or not stated in the remaining studies.
Sepsis prevalence ranged from 12.2%, in Aalto 2004, to 77.9%, in Gomez 2010. In 12 studies the prevalence of sepsis was greater than 50% (Anand 2015; Endo 2012; Gomez 2010; Harbarth 2001; Jekarl 2013; Jiang 2015; Li 2013; Llewelyn 2013; Mat‐Nor 2016; Tsalik 2012; Tsantes 2013; Zhao 2014). The median estimated prevalence was 47% (IQR: 37 to 69).
Methodological quality of included studies
We appraised the quality of primary diagnostic accuracy studies using the QUADAS‐2 tool. The overall risk of bias and applicability concerns of the studies are summarized in Figure 2. Quality assessment results for individual studies are presented in the Characteristics of included studies tables and in Figure 3.
2.
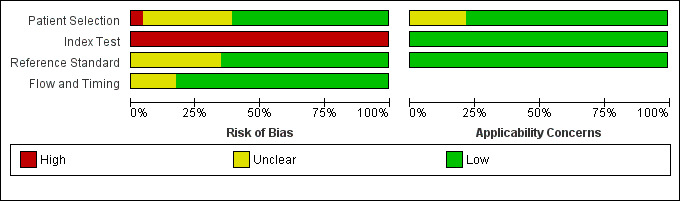
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
3.
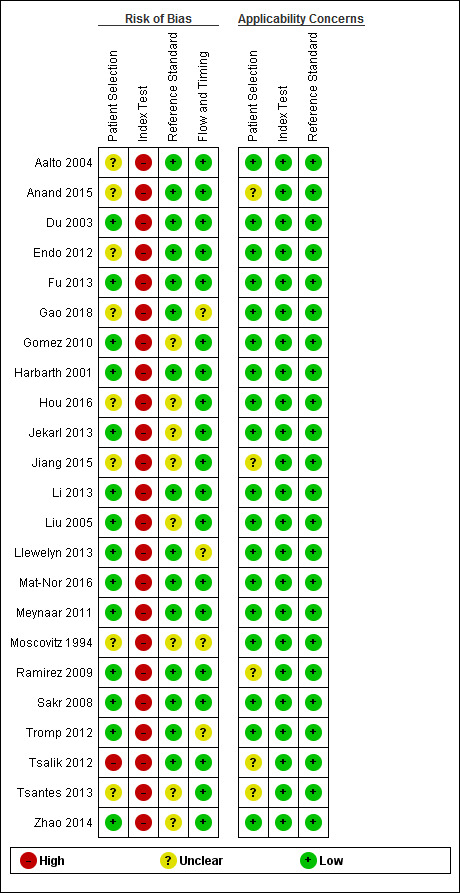
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
Due to concerns related to the source of participants, we judged the risk of bias of patient selection (QUADAS‐2, domain 1) to be high in one study (Tsalik 2012). We considered eight trials to have an unclear risk of bias for this domain, mostly due to insufficient information related to the patient sampling (consecutive or random), as well as insufficient information about exclusions (Aalto 2004; Anand 2015; Endo 2012; Gao 2018; Hou 2016; Jiang 2015; Moscovitz 1994; Tsantes 2013). We considered the remaining 14 studies to be at low risk of bias. We had few concerns about applicability in most of the included trials. However, we had concerns about five studies that focused on specific populations, that is culture‐negative septic patients (Anand 2015), intra‐abdominal infections (Jiang 2015), ventilator‐associated pneumonia patients (Ramirez 2009), a mix of cohorts of previous studies (Tsalik 2012), or ARDS patients (Tsantes 2013).
Regarding the index test assessment (QUADAS‐2, domain 2), we considered all studies to be at high risk of bias because none of them used a prespecified cut‐off to estimate sensitivity/specificity of IL‐6 (Figure 3; Figure 2). Likewise, most of the studies did not provide sufficient information to enable us to determine if index test results were interpreted without prior knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and were thus not affected by additional information. We had minor concerns about the applicability of index tests in all of the included trials.
We judged risk of bias due to conduct or interpretation of the reference standard(s) (QUADAS‐2, domain 3) to be unclear in eight studies (Gomez 2010; Hou 2016; Jekarl 2013; Jiang 2015; Liu 2005; Moscovitz 1994; Tsantes 2013; Zhao 2014). This was due to a lack of information about whether the reference standard results were interpreted with, or without, prior knowledge of the results of the IL‐6 measurements. We considered the risk to be low for the remaining 15 studies. We considered applicability of this domain to be low in all cases.
Finally, with regard to the flow and timing assessment (QUADAS‐2, domain 4), we considered four studies to have an unclear risk of bias because there was insufficient information about the interval between IL‐6 measurement and the application of the reference standard, as well as the exclusion of participants from the final analysis of accuracy (Gao 2018; Llewelyn 2013; Moscovitz 1994; Tromp 2012).
Findings
We included 21 studies collecting information from 1894 septic cases and 1756 non‐septic cases in the main analysis (Figure 4). For this analysis, we excluded data for other comparisons provided by studies, including severe sepsis versus septic shock; severe sepsis versus SIRS; and shock versus SIRS. In addition, we selected one of the groups/thresholds reported by Anand 2015 (positive‐culture group) and by Tsalik 2012 (threshold of 100 pg/mL), since these groups were also found in other included studies. The range of sensitivities and specificities estimated by study, as well as the different thresholds proposed to define IL‐6 positive findings, are shown in Figure 4. The SROC curve under the HSROC model, representing the accuracy of plasma IL‐6 thresholds used across studies, as well as individual study accuracy estimates, are shown in Figure 5. Given the considerable variation in collected data shown by Figure 5, and considering the heterogeneity of data described in the Results of the search section, we refrained from calculating a formal sensitivity and specificity summary.
4.
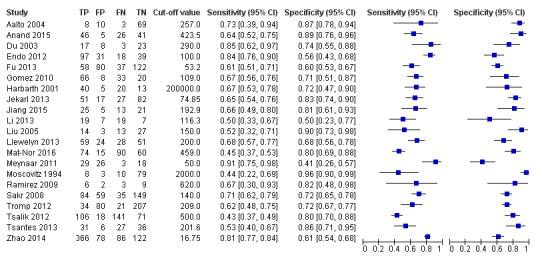
Forest plot of 1 Plasma interleukin‐6 concentrations.
5.
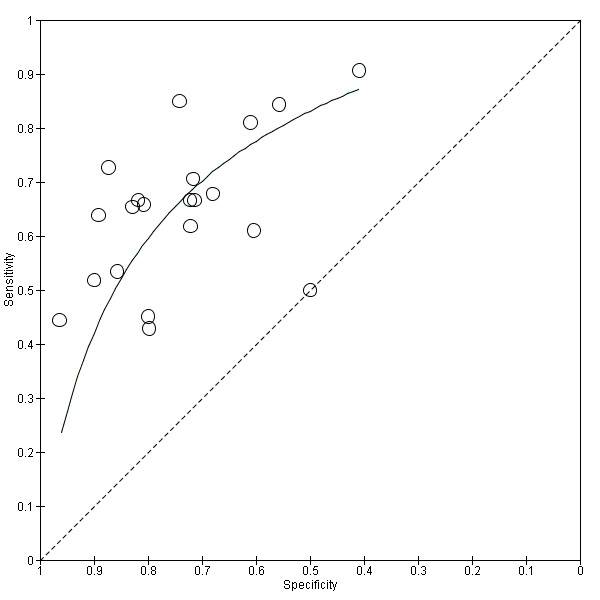
Summary receiver operating characteristic (SROC) plot of plasma interleukin‐6 concentrations, using hierarchical SROC parameters (estimated with SAS statistical software package).
We used the HSROC parameters to illustrate variations in sensitivity under a fixed specificity. Using a prevalence of sepsis of 50% and a fixed specificity of 74%, the estimated sensitivity was 66% (95% confidence interval (CI) 60 to 72). We noticed that 10 out of 21 individual studies included in this analysis reported sensitivities inferior or equal to this estimation. In terms of the possible consequences in a population of 1000 adult patients under suspicion of sepsis, this would translate into:
330 out of 1000 patients would receive appropriate and timely antibiotic therapy;
130 out of 1000 patients would be wrongly considered to have sepsis, and would receive unnecessary antibiotic therapy, with the added risk of resistance in a hospital setting, and may suffer delays in receiving appropriate treatment for their situation;
370 out of 1000 patients would avoid unnecessary antibiotic therapy;
170 out of 1000 patients would have undiagnosed sepsis and would be at serious risk of further morbidity and death due to delays of immediate antibiotic treatment.
Additional estimations with prevalences of 12% and 78% (minimum and maximum values from included studies) show an increase in the number of false positives or false negative, respectively (Table 1).
Investigation of heterogeneity
Due to the considerable variability of collected data, which prevented us from performing subgroup analysis (i.e. baseline diagnosis or origin of infection; see Differences between protocol and review), we were not able to evaluate the potential effect of several sources of heterogeneity as initially planned. We were able to assess the effect of three covariates: year of publication (studies published in or after the year 2011; see Differences between protocol and review), country/geographical area (studies conducted in Asia versus other settings) and setting (emergency versus ICU). We did not find statistical evidence of a difference in shape, test accuracy, or threshold parameters for models including country and setting as covariables. We found differences regarding shape and accuracy parameters according to publication year (Likelihood ratio test = 7.91; df = 2; P = 0.019). Approximately half of the studies published after 2011 showed specificities lower than 70%, while none of the studies published before 2011 showed specificities lower than 70% (Figure 6).
6.
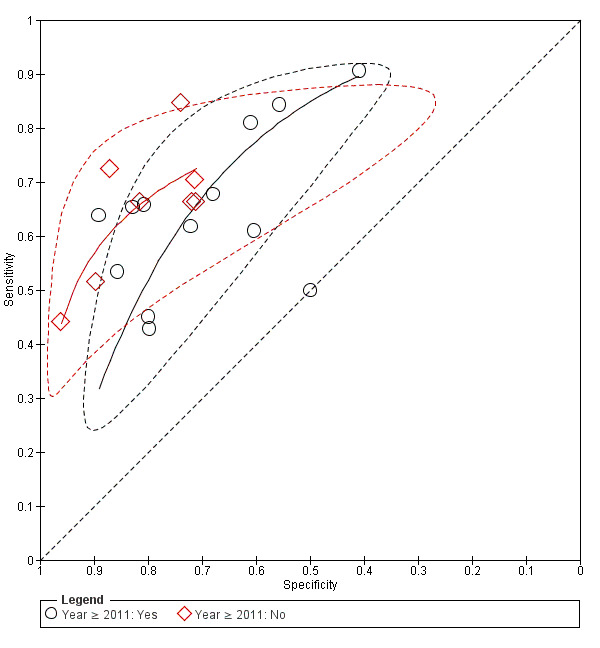
Investigation of heterogeneity: year of publication (≥ 2011).
Discussion
Summary of main results
We included 23 studies (4192 critically ill adult patients) assessing the accuracy of plasma IL‐6 concentrations for the diagnosis of sepsis. The included studies enrolled patients under suspicion of sepsis (i.e. SIRS patients), with further confirmation of sepsis, severe sepsis, septic shock, or no infection. The samples were heterogeneous in terms of their distribution of age, gender, main diagnosis, setting, country, positivity threshold, sepsis criteria, year of publication, and origin of infection, among other factors. In addition, the prevalence of sepsis reported in the included studies varied widely (from 12% to 78%; median: 47.3%).
The main results of plasma IL‐6 concentration as a biomarker are provided in the Table 1. This biomarker was assessed using a considerable number of thresholds that were not prespecified (from 40 to 200,000 pg/mL). We considered all studies to be at high risk of bias (QUADAS‐2/index test domain). The SROC curve showed a great dispersion in individual studies accuracy estimates (21 studies, 3650 adult participants), therefore the considerable heterogeneity in the collected data prevented us from calculating formal accuracy estimates. Using a fixed prevalence of sepsis of 50% and a fixed specificity of 74%, we found a sensitivity of 66% (95% CI 60 to 72). If we test a cohort of 1000 adult patients under suspicion of sepsis with IL‐6, we will find that 330 patients would receive appropriate and timely antibiotic therapy, while 130 patients would be wrongly considered to have sepsis. In addition, 370 out of 1000 patients would avoid unnecessary antibiotic therapy, and 170 patients would have been undiagnosed of sepsis. This numerical approach should be interpreted with caution due to the limitations described above. Due to the severe consequences of sepsis regarding its associated morbidity and mortality, we consider that the number of patients without adequate treatment, as a consequence of IL‐6 results, can be considered as unacceptable and deleterious for daily clinical management of this target condition (Table 1). Heterogeneity of data was not fully explained for any of the covariates investigated within this review.
Strengths and weaknesses of the review
The strengths of this review include a comprehensive literature search performed to identify all relevant studies, a rigorous assessment of the risk of bias of included studies using the QUADAS‐2 tool, as well as duplicate data extraction. We did not impose restrictions on population characteristics such as age, site of infection, or setting. As a result, we found a highly heterogeneous body of evidence, with the included studies presenting differences in baseline diagnosis, origin of infection, reference standard applied, and prevalence of sepsis in their participants, among other factors. We tried to explore and quantify the possible sources of heterogeneity, but most of the assessed factors failed to explain the observed variability. Only one covariate (year of publication ≥ 2011) affected the accuracy and shape parameters of the HSROC model. We analysed this variable following the suggestions of the peer reviewers of the review, in order to assess if the most recent evidence reflects a better IL‐6 performance (Differences between protocol and review). Approximately half of the studies published after 2011 showed specificities lower than 70%, while none of the studies published before 2011 showed specificities lower than 70%. This fact has an impact while evaluating differences in the shape of the curve by publication year. We cannot exclude that other factors, such as prevalence or sepsis criteria, are involved in this modification of effect. In addition, the sensitivity analyses planned in our protocol, Molano Franco 2015, based on the 'Risk of bias' assessments were made redundant by the serious risk of bias introduced by the threshold issues in the index test domain (See Differences between protocol and review). Due to this high variability that was not fully explained, we refrained from calculating a summary sensitivity and specificity.
Finally, we attempted to conduct a comprehensive search for studies, but the fact that a significant number of potentially eligible studies were only retrieved as conference proceedings may be considered as a source of potential bias. We expect that these studies will be fully published when our review is updated.
Agreements and disagreements with other studies or reviews
We identified three reviews assessing the accuracy of IL‐6 in the diagnosis of sepsis (Hou 2015; Liu 2016; Ma 2016). Ma and colleagues collected and pooled the results of 20 studies assessing IL‐6 in sepsis (Ma 2016), with cut‐off values of IL‐6 ranging between 18 and 423.5 pg/mL. The authors estimated a pooled sensitivity of 68% (95% CI 65% to 70%) and specificity of 73% (95% CI 71% to 76%) by means of a univariate model calculated by Meta‐DiSc 1.4 (Zamora 2006). Likewise, Hou and colleagues included six studies in their review, three of which were conducted in a paediatric population (Hou 2015). Analysing studies in an adult population under a random‐effects model (Littenberg and Moses method; Littenberg 1993; Moses 1993), the estimated pooled sensitivity was 85% (95% CI 80% to 88%), and the estimated specificity was 62% (95% CI 55% to 68%). Cut‐off values in this review ranged between 40 and 145 pg/mL, and risk of bias was not assessed. Finally, Liu and colleagues identified 22 studies addressing the accuracy of IL‐6 with a median cut‐off of 138 pg/mL (ranging from 75 to 220 pg/mL) (Liu 2016). Using a bivariate mixed‐effects regression model, the authors estimated a pooled sensitivity of 72% (95% CI 63% to 80%) and specificity of 73% (95% CI 67% to 79%). Risk of bias assessed with QUADAS‐1 was affected for issues related to blinding of test and reference standard results, as well as uninterpretable results.
Compared with our review, these studies used a different set of criteria to select eligible references, such as case‐control studies, paediatric populations, and data about prediction of sepsis, as well as alternative statistical approaches to analyse the collected information. In addition, two out of the three reviews mentioned used QUADAS‐1 or no assessment of the methodological quality for eligible studies. In our review, we refrained from calculating a summary sensitivity and specificity due to the considerable variation in the collected data. Due to this variability, especially that generated by threshold variations, only an HSROC approach is recommendable to analyse the gathered data (Deeks 2013).
We also noticed that the three identified reviews warn about significant heterogeneity among included studies, mostly guided by information provided by I2 statistics, but only Liu 2016 and Ma 2016 investigated their effects through a formal statistical method (meta‐regression analysis; no further details provided). Liu and colleagues analysed six potential sources of heterogeneity, including publication year, age of patients, prevalence, and methodological quality (Liu 2016). Likewise, Ma and colleagues analysed the effect of seven factors (Ma 2016), finding that admission category, setting, and reference standard make a significant contribution in explaining data variability. In our review, using an HSROC approach in the analysis of sources of heterogeneity, we were unable to fully explain the heterogeneity of data using a number of prespecified covariates.
Applicability of findings to the review question
Our findings show the complexity of the study of sepsis in critically ill patients, as well as the multiplicity of factors involved in the adequate diagnosis of this life‐threatening dysfunction. Due to its close relationship with inflammatory processes, plasma interleukin‐6 has been proposed as a potentially useful test to identify critical patients while microbiological confirmation is achieved. However, we found several limitations in translating these results into clinical practice.
One of the major issues that limits the applicability of findings to the review question is the considerable heterogeneity in the gathered evidence, which was a major constraint in our statistical analysis. The effect of some critical covariates, such as baseline diagnosis, cut‐off value for positivity, origin of infection, and reference standard, cannot be fully analysed by the methods stated in our protocol. This substantial variability in sepsis research was previously noticed by Singer and colleagues (Singer 2016). The authors of the Sepsis‐3 consensus stated that the lack of a validated standard clinical criterion in this field led to important discrepancies in the estimation of sepsis incidence and related mortality (Singer 2016). Currently, there are agreements about the need to redefine or to abandon some terms (i.e. severe sepsis), as well as the lack of clinical significance of extended‐use criteria for the diagnosis of sepsis (i.e. two or more SIRS criteria). None of the novel biomarkers suggested for the diagnosis of sepsis were formally included in the Sepsis‐3 criteria, due to the fact that they “require broader validation before they can be incorporated into the clinical criteria describing sepsis” (Singer 2016).
In the critical care setting, the use of biomarkers has other well‐known limitations such as the associated costs, limited availability in low/middle‐income settings, and the lack of experience of clinicians in its use. Our findings do not suggest that the accuracy of this biomarker is sufficient enough to assure the role of this test in the current clinical sepsis pathway. At present, the role of IL‐6, as well as other biomarkers, is being assessed in the prediction of severe outcomes (i.e. organ failure and mortality), under a prognostic approach (McGuire 2014; Pallas 2016; Rios‐Toro 2017; Stoppelkamp 2015; Wong 2015).
Authors' conclusions
Implications for practice.
Our evidence assessment of plasma interleukin‐6 concentrations for the diagnosis of sepsis in critically ill adults reveals several limitations. High heterogeneity of the collected evidence regarding the main diagnosis, setting, country, positivity threshold, sepsis criteria, year of publication, and origin of infection, among other factors, along with the potential number of misclassifications, remain significant constraints for its implementation. The 20 conference proceedings assessed as studies awaiting classification may alter the conclusions of the review once they are fully published and evaluated.
Implications for research.
Further studies about the accuracy of interleukin‐6 for diagnosis of sepsis in adult patients are needed. These studies should follow well‐known methodologies for the performance of diagnostic test accuracy (DTA) studies, including:
the predefinition of a range of thresholds to test positivity of this biomarker;
adherence to recognized guidelines for reporting of DTA studies, such as the Standards for Reporting of Diagnostic Accuracy Studies (STARD) initiative (equator‐network.org/reporting‐guidelines/stard/);
exploration of the diagnostic value of interleukin‐6 concentrations added to other biomarkers, or as a part of a diagnostic algorithm;
in addition, we suggest that future studies adopt the current definitions and clinical criteria recommended by Singer and colleagues in order to improve the accuracy of research in this field (Singer 2016).
History
Protocol first published: Issue 7, 2015 Review first published: Issue 4, 2019
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Anna Lee (content editor), Janne Vendt (Information Specialist), Djillali Annane, Benjamin G Chousterman (peer reviewers), Brian Stafford (consumer referee), the Cochrane Diagnostic Test Accuracy Working Group, and Harald Herkner (Co‐ordinating Editor) for their help and editorial advice during the preparation of this systematic review. We would also like to thank Aidan Tan (Via Task exchange) for his help with papers written in Chinese.
Appendices
Appendix 1. 2001 criteria for diagnosis of sepsis (Levy 2003)
| Infection: Diagnosis of an infection on the basis of documented or suspected and some of the following: |
|
Criteria 1. General parameters 1.1 Fever (core temperature > 38.3 °C) 1.2 Hypothermia (core temperature < 36 °C) 1.3 Heart rate > 90 bpm or > 2 SD above the normal value for age 1.4 Tachypnoea > 30 bpm 1.5 Altered mental status 1.6 Significant oedema or positive fluid balance (> 20 mL/kg over 24 h) 1.7 Hyperglycaemia (plasma glucose > 100 mg/dL or 7.7 mm/L) in the absence of diabetes |
|
Criteria 2. Systemic inflammatory host response (at least 2 criteria) 2.1 Fever (> 38 °C) or hypothermia (< 36 °C) confirmed by rectal, intravascular, or intravesical assessment 2.2 Tachycardia: heart rate > 90 bpm 2.3 Tachypnoea (frequency > 20/min) or hyperventilation (PCO2 < 4.3 kPa/ < 33 mmHg) 2.4 Leukocytosis (> 12,000/mm3) or leukopenia (< 4000/mm3) or > 10% immature neutrophils in blood cell count |
|
Criteria 3. Acute organ dysfunction (at least 1 criterion) 3.1 Acute encephalopathy: reduced alertness, disorientation, agitation, delirium 3.2 Relative or absolute thrombocytopenia: decrease in platelet count by more 30% or count of less 100,000/mm3 3.3 Coagulation abnormalities (international normalized ratio > 1.5 or active partial thromboplastin time > 60 s) 3.4 Arterial hypoxaemia: PaO2 < 10 kPa (< 75 mmHg) while breathing ambient air or PaO2/FiO2 < 300 3.5 Renal impairment: diuresis < 0.5 mL/kg/h for at least 2 h, creatinine increase > 0.5 mg/dL 3.6 Metabolic acidosis: base excess < 0.5 mmol/L or lactate concentration > 1.5 upper limit of normal 3.7 Hyperbilirubinaemia > 4 mg/dL or 70 mmol/L |
|
Criteria 4. Haemodynamic parameters 4.1 Arterial hypotension (systolic blood pressure < 90 mmHg, mean arterial pressure < 70, or systolic blood pressure decrease > 40 mmHg in adults or < 2 SD below normal for age) 4.2 Mixed venous oxygen saturation > 70% 4.3 Cardiac index > 3.5 L/min |
|
Criteria 5. Tissue perfusion parameters 5.1 Hyperlactataemia ( > 3 mmol/L) 5.2 Decreased capillary refill or mottling |
|
Severe sepsis: Sepsis complicated by organ dysfunction Criteria:
|
Footnotes
bpm: contractions (beats) of the heart per minute; PaO2/FiO2: The ratio of partial pressure arterial oxygen and fraction of inspired oxygen; PCO2:Partial pressure of carbon dioxide; SD: standard deviation.
Appendix 2. 1991 criteria for diagnosis of sepsis (Bone 1992)
| Definitions |
| Infection: microbial phenomenon characterized by an inflammatory response to the presence of micro‐organisms or the invasion of normally sterile host tissue by those organisms |
| Bacteraemia: the presence of viable bacteria in the blood |
Systemic inflammatory response syndrome (SIRS): the systemic inflammatory response to a variety of severe clinical insults. The response is manifested by 2 or more of the following conditions:
|
Sepsis: the systemic response to infection, manifested by 2 or more of the following conditions as a result of infection:
|
| Severe sepsis: sepsis associated with organ dysfunction, hypoperfusion, or hypotension. Hypoperfusion and perfusion abnormalities may include, but are not limited to, lactic acidosis, oliguria, or an acute alteration in mental status. |
| Septic shock: sepsis‐induced hypotension despite adequate fluid resuscitation along with the presence of perfusion abnormalities that may include, but are not limited to, lactic acidosis, oliguria, or an acute alteration in mental status. Patients who are receiving inotropic or vasopressor agents may not be hypotensive at the time that perfusion abnormalities are measured. |
| Sepsis‐induced hypotension: a systolic blood pressure < 90 mmHg or a reduction of ≥ 40 mmHg from baseline in the absence of other causes of hypotension |
| Multiple organ dysfunction syndrome (MODS): presence of altered organ function in an acutely ill patient such that homeostasis cannot be maintained without intervention |
Footnotes
PaCO2:Partial pressure of carbon dioxide in arterial blood.
Appendix 3. 2015 criteria for diagnosis of sepsis (Singer 2016)
| Definitions |
Screening for sepsis: assessment is based on qSOFA (quick sequential organ failure assessment) scoring system; an increase of 2 or more of the following points in the qSOFA score is indicative of sepsis and organ dysfunction.
If 2 out of 3 criteria are positive, the qSOFA is considered as positive. |
| Sepsis: life‐threatening organ dysfunction caused by a dysregulated host response to infection. It can be defined as a suspected or documented infection plus an acute increase of ≥ 2 SOFA points. |
| Organ dysfunction: it can be defined as an acute change in total SOFA score of 2 points consequent to the infection. |
| Septic shock: a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are related to a significant increase of mortality. Patients with septic shock can be identified with a clinical construct of sepsis with persisting hypotension requiring vasopressors to maintain MAP ≥ 65 mmHg and having a serum lactate level > 2 mmol/L (18 mg/dL) despite adequate volume resuscitation. |
Footnotes
GCS: Glaswog Coma Score;MAP: mitogen‐activated protein.
Appendix 4. Cochrane Library search strategy
1 MeSH descriptor: [Interleukin‐6] explode all trees 2 MeSH descriptor: [Receptors, Interleukin‐6] explode all trees 3 MeSH descriptor: [Interleukin‐6 Receptor alpha Subunit] explode all trees 4 (interleukin* or IL6* or IL 6* or Interleukin 6) (Word variations have been searched) 5 (diagnostic NEAR/3 marker*) (Word variations have been searched) 6 (procalcitonin or cytokin*) (Word variations have been searched) 7 #1 or #2 or #3 or #4 or #5 or #6 (Word variations have been searched) 8 MeSH descriptor: [Hemorrhagic Septicemia] explode all trees 9 MeSH descriptor: [Sepsis] explode all trees 10 MeSH descriptor: [Shock, Septic] explode all trees 11 MeSH descriptor: [Critical Illness] explode all trees 12 MeSH descriptor: [Bacteremia] explode all trees 13 Flavimonas oryzihabitans Bacteremia or Systemic Inflammatory Response Syndrome (Word variations have been searched) 14 sepsis or septic* or bacterem* or bacteraem* (Word variations have been searched) 15 (critical* NEAR/3 ill*) (Word variations have been searched) 16 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 (Word variations have been searched) 17 #7 and #16 (Word variations have been searched) 18 #17 in Cochrane Reviews, Cochrane Protocols, Trials, Clinical Answers (Word variations have been searched)
Appendix 5. MEDLINE (Ovid SP) search strategy
1 exp Interleukin‐6/ 2 exp Receptors, Interleukin‐6/ 3 Interleukin‐6 Receptor alpha Subunit/ 4 (interleukin* or IL?6* or IL 6*).mp. 5 (procalcitonin or cytokin*).ti,ab. 6 (diagnostic adj3 marker*).ti,ab. 7 1 or 2 or 3 or 4 or 5 or 6 8 Hemorrhagic Septicemia/ 9 Sepsis/ 10 Shock, Septic/ 11 Critical Illness/ 12 Bacteremia/ 13 (Flavimonas oryzihabitans Bacteremia or Systemic Inflammatory Response Syndrome).mp. 14 (sepsis or septic* or bacter?em*).mp. 15 (critical* adj3 ill*).ti,ab. 16 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17 7 and 16 18 ((child* or neonat*) not adult*).af. 19 17 not 18 20 (animal* not human*).sh. 21 19 not 20
Appendix 6. Embase (Ovid SP) search strategy
1 interleukin 6/ 2 interleukin 6 receptor/ 3 interleukin 6 receptor alpha/ 4 (interleukin* or IL?6* or IL 6*).mp. 5 (procalcitonin or cytokin*).ti,ab. 6 (diagnostic adj3 marker*).ti,ab. 7 1 or 2 or 3 or 4 or 5 or 6 8 hemorrhagic septicemia/ 9 exp sepsis/ 10 exp bacteremia/ 11 septic shock/ 12 critical illness/ 13 (Flavimonas oryzihabitans Bacteremia or Systemic Inflammatory Response Syndrome).ti,ab. 14 (sepsis or septic* or bacter?em*).ti,ab. 15 (critical* adj3 ill*).ti,ab. 16 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17 7 and 16 18 ((child* or neonat*) not adult*).af. 19 17 not 18 20 (animal* not human*).sh. 21 19 not 20
Appendix 7. LILACS search strategy
(interluquina‐6 OR il‐6 OR citoquina OR receptores interluquina OR interleucina OR citocinas inflamatorias OR citocinas OR interleucina‐6) AND (sepsis OR septicemia OR síndrome de respuesta inflamatoria sistémica OR bacteremia OR bacteriemia OR shock septico)
Appendix 8. Web of Science search strategy
Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=All years
1 TS=(interleukin* or IL6 or “IL 6”) 2 TS=(diagnostic NEAR/3 marker*) 3 TS=(procalcitonin or cytokin*) 4 #3 OR #2 OR #1 5 TS=("Flavimonas oryzihabitans Bacteremia" or "Systemic Inflammatory Response Syndrome" ) 6 TS=(sepsis or septic* or bacterem* or bacteraem*) 7 TS= (critical* NEAR/3 ill*) 8 #7 OR #6 OR #5 9 #4 and #8 10 TS=(child* or neonat* or pediat* or paediat* or newborn* or infant*) 11 TS=(adult* or aged or elderly or "middle age") 12 #10 not #11 13 #9 not #12 14 TS=(animal*) 15 TS=(human*) 16 #14 not #15 17 #13 not #16
Appendix 9. Data extraction form
| Study name / date | Authors, publication date and number. |
| Setting |
|
| Participants | Sample size. Characteristics if reported:
Origin of infection: pneumonia, urinary infection, meningitis, bacteraemia, abdominal sepsis. Use of antibiotics (empiric management). |
| Study design | Sampling strategy. Duration of follow‐up. |
| Target condition | Proportion of people with sepsis in sample. Subtype of sepsis (severe, septic shock), if available. |
| Reference standard | Clinical diagnosis. Type of culture. Culture and clinical diagnosis. Time between IL‐6 assessment and reference test. Relationship between IL‐6 value and initial empirical antibiotics. Blinding of operator to IL‐6 levels. Was any subset subject to a different reference test? Positive cultures: micro‐organism isolated. Clinical diagnosis: composition of expert panel, training. |
| Index test | Kit name – commercial name, batch number. Who did the test? Training provided to operator. Thresholds used to define positive and negative levels for sepsis. |
| Index and reference standard test results | Missing results for index and reference. Uninterpretable results for index and reference. Borderline results for index and reference. True and false positives. True and false negatives. Sensitivity and specificity of index tests. |
Appendix 10. Results of the 2 x 2 tables cross‐relating index test results of the reference standards
| Index test information | Reference standard information | |
| Sepsis present | Sepsis absent | |
| Index test positive | IL‐6 positive and sepsis (true positives) at baseline | IL‐6 positive and no sepsis (false positives) at baseline |
| Index test negative | IL‐6 negative and sepsis (false negatives) at baseline | IL‐6 negative and no sepsis (true negatives) at baseline |
Appendix 11. Anchoring statements for quality assessment of IL‐6 for diagnosis of sepsis
| Patient selection | |
| Was a consecutive or random sample of patients enrolled? | 'Yes' if it is well described in the paper (e.g. consecutive or a random sample from consecutive patients under suspicion of sepsis). 'No' if the sample was non‐random or patients under suspicion of sepsis were not consecutively recruited. 'Unclear' if there is insufficient information to make a judgement on the selection of patients. |
| Was a case‐control design avoided? | Self explanatory |
| Did the study avoid inappropriate exclusions? | 'Yes' if inclusion and exclusion criteria are clearly described and appropriate. 'No' if inclusion and exclusion criteria are clear but excluded inappropriate participants. 'Unclear' if there is insufficient information to make a judgement on the inclusion/exclusion of participants. |
| Could the selection of patients have introduced bias? | 'Yes' if it is clear that bias is introduced through, for example, non‐random selection. 'No' if the selection of participants is clearly described and does not introduce bias. 'Unclear' if there is insufficient information to make a judgement on the impact of selection on bias. |
| Are there concerns that included patients do not match the review question? | 'Yes' if included participants are inherently different from the group of patients who would be expected to receive IL‐6 for diagnosis of sepsis. 'No' if there are no such concerns. 'Unclear' if patient characteristics are not sufficiently explained to make a judgement on patient inclusion. |
| Index test | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | 'Yes' if the paper states that plasma IL‐6 findings were interpreted by individual(s) who did not know the results of the reference test(s) (i.e. 1991/2001/2015 consensus criteria). 'No' if the plasma IL‐6 results were known by the individuals performing the reference test, or if the same individual was involved in both processes. 'Unclear' if there is insufficient information to make a judgement about test result interpretation. |
| If a threshold was used, was it prespecified? | 'Yes' if the paper states that plasma IL‐6 cut‐off for positivity was defined in advance of data collection. 'No' if the plasma IL‐6 optimal cut‐off was estimated from collected data (according to sensitivity/specificity estimations). 'Unclear' if there is insufficient information to make a judgement about test result interpretation. |
| Could the conduct or interpretation of the index test have introduced bias? | 'Yes' if a subset of plasma IL‐6 measurements were conducted or interpreted in a different manner, or under different conditions, or by people with differing levels of training. 'No' if it is clear that the conduct and interpretation of plasma IL‐6 measurements was appropriate and could not have introduced bias. 'Unclear' if information is insufficient to assess the potential of conduct and interpretation of the index test to introduce bias. |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | 'Yes' if the index test is not plasma IL‐6 analysis for sepsis or if the conduct of test or its interpretation is not applicable to the review question. 'No' if there are no concerns based on the information presented. |
| Reference standard | |
| Is the reference standard likely to correctly classify the target condition? | 'Yes' if the reference standard used in the paper matches the 1991, 2001, or 2015 criteria for diagnosis of sepsis. 'No' if the above criteria are not met. 'Unclear' if insufficient information is presented. |
| Were the reference standard results interpreted without knowledge of the results of the index test? | 'Yes' if the paper states that the plasma IL‐6 findings were not included as supplementary information in the consensus criteria used. 'No' if the result(s) of the plasma IL‐6 analysis were known to the individual(s) applying the consensus criteria. |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | 'Yes' if a subset of consensus criteria were conducted or interpreted in a different manner, or under different conditions, or by people with differing levels of training. 'No' if it is clear that the application and interpretation of all consensus criteria were appropriate and could not have introduced bias. 'Unclear' if information is insufficient to assess the potential of conduct and interpretation of the consensus criteria to introduce bias. |
| Are there concerns that the target condition as defined by the reference standard does not match the review question? | 'Yes' if the assessed target condition is not sepsis or it is not clearly stated. 'No' if it is clearly stated that the target condition is sepsis. |
| Flow and timing | |
| Was there an appropriate interval between index test(s) and reference standard? | 'Yes' if the time between plasma IL‐6 results and application of consensus criteria was less than 48 hours. 'No' if the time is longer than 48 hours for a significant proportion of patients. |
| Did all patients receive a reference standard? | 'Yes' if all patients who received plasma IL‐6 measurement were also assessed under a sepsis consensus criteria. 'No' if not all the patients who received measurement of plasma IL‐6 were also assessed under a sepsis consensus criteria, or if a non‐random sample was selected. 'Unclear' if this cannot be determined from the information presented in the paper. |
| Did all patients receive the same reference standard? | 'Yes' if the same consensus criteria were used for all patients. 'No' if different criteria were used in a subgroup of patients. 'Unclear' if this cannot be determined from the information presented in the paper. |
| Were all patients included in the analysis? | 'Yes' if there were no withdrawals or exclusions, or if the reasons for withdrawals or exclusions are adequately explained with a flow chart. 'No' if withdrawals or exclusions are not explained or accounted for. 'Unclear' if withdrawals or exclusions cannot be determined or if there is insufficient information on which to base a judgement. |
| Could the patient flow have introduced bias? | 'Yes' if subsets of patients or samples were treated, included, or excluded in systematic ways that could have introduced bias. 'No' if patient flow is reported clearly and does not have the potential to introduce significant bias. |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 Plasma interleukin‐6 concentrations | 21 | 3650 |
1. Test.
Plasma interleukin‐6 concentrations.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aalto 2004.
| Study characteristics | |||
| Patient sampling | Authors included 121 acutely ill participants admitted to the emergency department between September 1997 and November 1997. Participants suspected of having systemic infection, as determined by the treating clinician's request for a blood culture within 24 hours of admission. Unclear if participants were enrolled in a consecutive or random manner. People with active haematological malignancies, who had undergone surgery within the previous 6 weeks, and those in systemic immunosuppressive treatment at the time of blood sampling were excluded. |
||
| Patient characteristics and setting | Authors divided the participants into 3 groups according to clinical features on admission: a) participants with infectious focus; b) participants with acute‐onset fever without focus; c) participants with neither fever nor focus. 13 participants with positive blood cultures were considered to have a diagnosis of bacteraemia. | ||
| Index tests | IL‐6 concentrations were measured by a chemiluminescent immunoassay system (Immulite; Diagnostic Products, Los Angeles, CA, USA) with detection limits of 5 ng/L. On admission, an additional blood sample was collected into an evacuated tube (Venoject; Terumo Europe, Leuven, Belgium) containing citrate as an anticoagulant. Each tube was immediately pressed into thawing ice (0 °C) to minimize phagocyte activation ex vivo. Plasma was separated by centrifugation at 4 °C and stored in aliquots at −70 °C until use. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors presented best cut‐off value for accuracy data. |
||
| Target condition and reference standard(s) | Target condition: community‐acquired bloodstream infection/bacteraemia Reference standard: SIRS criteria + microbiological evidence of local infection Blood culture was requested within 24 hours of admission. No further details provided. Unclear if reference standard results were interpreted without knowledge of the results of the index test. |
||
| Flow and timing | Blood cultures and IL‐6 samples were collected simultaneously. All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: not stated | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Anand 2015.
| Study characteristics | |||
| Patient sampling | Authors included 208 participants from a single centre in New Delhi, India, from January 2013 to May 2014. Informed consent was taken from each participant or their next of kin if participant was unconscious or not in a fit state to give consent. Unclear if participants were enrolled in a consecutive or random manner. Exclusion criteria were people who had received prior antibiotics, transferred from other ICUs; having conditions that were considered lethal in the next 24 hours; postoperative; immunocompromised; and with malignancy. People with bilateral pneumonia (suspected viral infection) and diagnosed tropical diseases such as malaria, dengue, Leptospira, and rickettsiae were also excluded from the study. |
||
| Patient characteristics and setting | Authors enrolled adult participants admitted from the community to the ICU and diagnosed with non‐infectious SIRS, sepsis, severe sepsis, or septic shock. Participants were divided into 3 groups: a) non‐infectious SIRS; b) culture‐negative sepsis; c) culture‐positive sepsis. Analyses were performed according to findings in the cultures (positive or negative). | ||
| Index tests | Blood samples were assayed for microbiological culture and biomarkers on the day of ICU admission. They were obtained in serum evacuated separator tubes and centrifuged for the separation of serum and processed on the same day. IL‐6 estimation was done by solid‐phase Chemiluminescent Access Immunoassay System (Beckman Coulter Inc, Brea, CA, USA). Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors presented best cut‐off value for accuracy data. |
||
| Target condition and reference standard(s) | Target condition: culture positive/negative bacterial sepsis Reference standard: presence of 2 or more signs of SIRS with/without positive cultures, defined by 4 clinicians at the time of enrolment Culture‐negative and culture‐positive groups were defined once the microbiological results were available. Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Participant demographics, principal diagnosis, and all clinical parameters were recorded at the time of enrolment. All participants receive the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: Indian Council of Medical Research | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Du 2003.
| Study characteristics | |||
| Patient sampling | Authors included all people admitted to ICU with an expected ICU stay of more than 72 h between October 2001 and March 2002. 51 participants with diagnosis of SIRS, sepsis, severe sepsis, or septic shock were included for analysis. Exclusion criteria not clearly stated. | ||
| Patient characteristics and setting | 51 ICU participants (31 males and 20 females) were evaluated. The mean age of these participants was 64.7 ± 16.3 years; mean APACHE II score at admission was 17.4 ± 7.6. A total of 13 participants (25%) died during hospitalization. | ||
| Index tests | Blood samples were taken by venipuncture (within 24 h of study inclusion) and centrifuged at 4000 rpm for 10 min before serum was frozen at −20 °C for IL‐6 measurements (analysed as batch analyses at the end of the study). IL‐6 concentrations were determined with a commercially available IL‐6 EASIA test kit. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors presented best cut‐off value for accuracy data. |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: ACCP/SCCM criteria. Clinical investigation and classification were carried out without knowledge of the test results of IL‐6. |
||
| Flow and timing | Routine septic work‐up (including all blood samples) was performed within 24 h of study inclusion. IL‐6 samples were analysed at the end of the study. All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: not stated | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Endo 2012.
| Study characteristics | |||
| Patient sampling | Authors included blood samples from 207 participants with suspicion of sepsis admitted to the emergency room or the ICU between June 2010 and June 2011. Unclear if participants were enrolled in a consecutive or random manner. People with non‐bacterial infection or suspected bacterial infection with negative cultures were excluded. | ||
| Patient characteristics and setting | Participants fulfilled at least 1 of the following diagnostic criteria for SIRS:
Participants were classified into subgroups according to blood culture results. |
||
| Index tests | IL‐6 concentrations were measured using the Immulyze 2000 assay system (Siemens Healthcare Diagnostics, Japan) using EDTA plasma as a sample. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically, then were not affected by additional information. Authors reported 2 cut‐off values for accuracy data (not prespecified in the methods). |
||
| Target condition and reference standard(s) | No further details were provided. Target condition: sepsis/bacterial infectious disease Reference standard: positive cultures and diagnosis of bacterial infection by the physician Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples were collected at the same time, but this is not clearly stated) All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: not stated | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Fu 2013.
| Study characteristics | |||
| Patient sampling | Authors revised information from 1253 cases of hospitalized patients from Sichuan University, West China Hospital, Department of Hematology in a retrospective manner (March 2011 to October 2012). 297 participants with haematologic malignancy combined with neutropenia febrile were analysed. People with other organ malignancies and other pathogenic micro‐organisms (viruses, fungi, parasites, etc.) were excluded. | ||
| Patient characteristics and setting | Authors included participants 18 years of age or older; with diagnosis of blood cancer by the West China Hospital blood medicine; and presence of fever and granulocyte reduction. Participants were divided into bacteraemia group and non‐bacteraemia group according to the results of the blood culture. | ||
| Index tests | PCT, IL‐6 reagents were purchased from Roche Diagnostics Inc; the test instrument was Roche E170 (electrochemical luminescence quantitative method). Samples for IL‐6 measurement were collected before antibiotic application. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically, then were not affected by additional information. Authors presented best cut‐off value for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: bacteraemia in haematologic malignancies (neutropenia febrile) Reference standard: SIRS criteria + microbiological evidence of local infection Blood culture using the French biological Meridian automatic blood culture detector and supporting reagents Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples were collected at the same time, but this is not clearly stated) All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: not stated | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Gao 2018.
| Study characteristics | |||
| Patient sampling | Plasma from 50 normal adults, 60 patients with SIRS, and 134 patients with sepsis was collected from December 2012 to November 2013 when the patients were admitted in the first affiliated hospital of Fujian Medical University of China. Unclear if participants were recruited in a consecutive or random manner; authors stated that a follow‐up cohort was performed (we contacted authors by email for clarification of the study design, but at the time of review publication a reply had not been received) No exclusion criteria are reported. |
||
| Patient characteristics and setting | Plasma from 50 normal adults, 60 patients with SIRS, and 134 patients with sepsis was collected from December 2012 to November 2013 when the patients were admitted in the hospital of Fujian Medical University of China. All patients with SIRS or sepsis were hospitalized due to complications of major surgery; trauma; heart, lung, liver, pancreas, kidney, or gastrointestinal diseases; or systemic infection. The diagnosis of SIRS and sepsis was based on the 1992 ACCP/SCCM consensus classification. SIRS and septic patients were classified according to blood cultures. Patients were 59% male and 41% female. Normal adult samples were obtained from volunteers via blood bank donations. The normal group was not included in this review. |
||
| Index tests | IL‐6 was measured with a COBAS E601 Analyzer (Roche, Mannheim, Germany). First plasma samples were collected 1 to 2 days prior to or on the day of blood culture. Plasma was divided into 0.5 mL/tube and stored at −80 °C until analysis. | ||
| Target condition and reference standard(s) | Target condition: sepsis, based on the ACCP/SCCM consensus classification, based on clinical symptoms and blood culture Reference standard: positive blood cultures, either gram‐negative, gram‐positive bacteria or fungi in 12 to 72 hours after culture Blood culture was requested within 24 hours of admission. No further details provided. Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | First plasma samples were collected 1 to 2 days prior to or on the day of blood culture. All participants received the same reference standard. Unclear if participants were excluded from the analysis |
||
| Comparative | Not stated | ||
| Notes | Funding: State Science and Technology funds (2015DFA31770); Fujian Development and Reform Commission (FGW2014); Fujian Science and Technology Foundation (2014Y4002); Fujian Medical University (0‐0000‐081919); Fujian Education Ministry (2013‐58); and Fujian Association for international exchange of personnel funds (W13350000137) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Gomez 2010.
| Study characteristics | |||
| Patient sampling | Authors enrolled between February 2007 and September 2007, in a prospective manner, all medical and surgical patients older than 14 years who entered the institutional emergency room or the ICU, as well as hospitalized patients presenting with SIRS, with suspicion of infection or septic shock criteria. Exclusion criteria included people aged younger than 14 years of age; those who had had a pre‐hospital cardiac arrest; those with severe peripheral vascular disease defined by the presence of diagnosed intermittent claudication or the need for revascularization surgery; those with orders of limitation of the therapeutic effort or a diagnosis other than sepsis. | ||
| Patient characteristics and setting | Authors analysed information from 191 participants with SIRS (115 men and 76 women, mean age: 62 ± 19): 28 (15%) with SIRS without infection, 99 (52%) with sepsis, 37 (19%) with septic shock, and 27 (14%) death. The studied population consisted of 32% ICU participants and 68% hospitalized participants. | ||
| Index tests | IL‐6 was determined with IMMULITE 1.000 system (Siemens) by immunoquiluminiscence in solid stage, with a sensitivity of 0.2 μg/mL. The analysis of biomarkers (PCT, IL‐6, and LBP) was performed blindly (without knowing patient evolution data, only identified by the sample identification number) in 2 series of approximately 100 samples each. Authors reported best cut‐off value for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: SCCM/ESICM/ACCP/ATS/SIS 2001 criteria Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples and criteria were collected at the same time, but this is not clearly stated) All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: BRAHMS AG and Siemens Healthcare Diagnostics (provision of kits) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Harbarth 2001.
| Study characteristics | |||
| Patient sampling | Authors enrolled all consecutive participants admitted with a suspected diagnosis of infection and hospitalized in the medical and surgical ICUs of the University of Geneva Hospitals over a 7‐month period. Patients were not enrolled in case of early discharge or death, withholding of life support, or complete absence of antimicrobial treatment. At time of inclusion, participants had to be newly admitted to 1 of the ICUs; had to have a clinically suspected infection; and had to fulfil at least 2 criteria of SIRS. | ||
| Patient characteristics and setting | Authors enrolled 78 adult ICU participants meeting the criteria for SIRS (n = 18), sepsis (n = 14), severe sepsis (n = 21), or septic shock (n = 25). Infections were microbiologically proven in 44 of 60 infected participants (73%) with 53% gram‐negative, 38% gram‐positive bacteria, and 9% mixed infections. Leading sources of infection were the respiratory tract (n = 38) and the intra‐abdominal space (n = 10). 23 infected participants had a documented bloodstream infection. | ||
| Index tests | Within 12 h after admission and study entry, whole heparinized blood was drawn via an arterial line for cytokine measurements and daily thereafter at 6:00 A.M. during the entire ICU stay until ICU discharge or death. Blood was put on ice and plasma was collected by centrifugation at 4 °C and stored at 70 °C until the day of assay. IL‐6 was determined batch wise using commercial assays. IL‐6 measurements were performed by trained laboratory technicians blinded to the participants' clinical course. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: consensus definition (Bone 1992) Case ascertainment was done retrospectively by 2 independent investigators without knowledge of plasma PCT, IL‐6, and IL‐8 values, on the basis of the review of the complete patient charts and results of microbiologic cultures, chest radiographs, and, when available, postmortem examination reports. |
||
| Flow and timing | Case ascertainment done retrospectively on the basis of the review of the complete patient charts. All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: Swiss National Science Foundation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Hou 2016.
| Study characteristics | |||
| Patient sampling | Authors analysed information from 67 consecutive participants who were admitted to the Medical ICU of SongJiang Central Hospital (Shanghai, China) over a 1‐year period. Inclusion and exclusion criteria are not clearly stated. | ||
| Patient characteristics and setting | 24 septic participants admitted to ICU were included. The leading causes of sepsis were major surgery, intestinal obstruction, intestinal perforation, and multiple traumas. Abdomen, thorax, and blood were the primary sites of infection for both gram‐negative and gram‐positive infections. 43 SIRS participants had a mean age of 55.4 ± 10.6 years. Authors recruited 73 healthy volunteers (45 males and 28 females) with a mean age of 52.6 ± 12.9 years (not included in this review). | ||
| Index tests | Blood samples were collected into clot activation additive‐containing tubules, centrifuged at 1600 g for 10 minutes, followed by 16,000 g for 1 minute. Serum was collected into a clear tube and stored at −80 °C until use. IL‐6 concentrations were evaluated by electrochemical luminescence on a Roche COBAS‐e601. This is an automated heterogeneous sandwich immunoassay, with a total assay time of 18 minutes. An IL‐6 concentration higher than 7 pg/mL is considered positive. Authors reported optimal cut‐off value for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: for the SIRS group, patients had to fulfil at least 2 criteria of SIRS but have no evidence of organ dysfunction or sepsis. For the sepsis group, patients had to fulfil both the criteria for SIRS and have microbiological evidence of local infection. Bacteria, fungi, or parasites were cultured from the blood of all sepsis participants. SIRS participants had no symptoms of local infection, and no micro‐organisms were cultured from their blood. No additional details provided. Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples and criteria were collected at the same time, but this is not clearly stated) All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: scientific foundation of Shangai scientific and technologic bureau | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Jekarl 2013.
| Study characteristics | |||
| Patient sampling | Authors included 177 participants (≥ 18 years of age) with diagnosis of SIRS from an emergency department in South Korea in a prospective fashion. Patients were excluded from the study if they showed evidence of an immunocompromised state (e.g. malignancy, HIV infection), had visited the hospital or were discharged from the hospital within 14 days before visiting the emergency department, or had been administered antibiotics before admission to the emergency department. | ||
| Patient characteristics and setting | Of the 177 enrolled cases, 99 were classified as SIRS, 62 as sepsis, and 16 as severe sepsis/septic shock. Among the 78 sepsis and severe sepsis/septic shock participants, 70 showed infection confirmed by bacterial growth and 8 showed suspected bacterial infections. | ||
| Index tests | Blood samples for IL‐6 levels were drawn immediately after admission to the emergency department and were analysed in a central laboratory within 2 h. IL‐6 was measured by a chemiluminescence method using the Elecsys IL‐6 kit (Roche). Authors reported optimal cut‐off value for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: sepsis was defined as SIRS manifestation with microbial infection. Severe sepsis was defined as sepsis associated with organ dysfunction, hypoperfusion, or hypotension. Septic shock was defined as sepsis induced with hypotension despite adequate fluid resuscitation, along with the presence of hypoperfusion abnormalities or organ dysfunction (Bone 1992). No further information was available. Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples and criteria were collected at the same time, but this is not clearly stated) All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: Yeouido St. Mary's Hospital Clinical Research Center, the Catholic University of Korea. Sa‐Gang Lab Tech Co Ltd donated IL & kits. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Jiang 2015.
| Study characteristics | |||
| Patient sampling | Authors collected a total of 104 serum samples from participants treated at the Department of General Surgery, Wuhan General Hospital of Guangzhou Military Command, Wuhan, China from May 2014 to October 2014. Unclear if the samples were collected in a consecutive or random fashion. Exclusion criteria were not stated. | ||
| Patient characteristics and setting | 53 participants were diagnosed with biliary infection (including cholangitis and cholecystitis) and 10 other intra‐abdominal infections, as well as 26 non‐infected participants diagnosed with SIRS. An additional control group consisted of 15 healthy volunteers with no history of autoimmune, inflammatory, or tumour disease (not included in the present review). | ||
| Index tests | Blood samples were obtained from each participant at the time of admission when they were suspected to have intra‐abdominal bacterial gram‐negative infection or SIRS before any treatment. Peripheral venous blood samples were collected in tubes without additive and allowed to clot at room temperature for 40 minutes. Serum was separated by centrifugation. All serum samples were stored at −80 °C until tested. Serum concentrations of IL‐6 were measured by ELISA (BioLegend). Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: gram‐negative bacterial sepsis Reference standard: sepsis criteria (Bone 1992; Dellinger 2013) Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples and criteria were collected at the same time, but this is not clearly stated) All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: National Natural Science Foundation of China | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Li 2013.
| Study characteristics | |||
| Patient sampling | Authors included information from 52 consecutive adult participants (aged between 18 and 80 years) who were newly hospitalized in the 12‐bed surgical ICU of the First Hospital of Sun Yat‐sen University in Guangzhou between January 2006 and October 2006. All participants had clinically suspected infection and fulfilled at least 2 criteria of SIRS. Patients who were immunocompromised due to treatment with corticosteroids, receipt of bone marrow or organ transplants, leukopenia (leukocyte count <1.0 x 109 cells/L) or neutropenia (polymorphonuclear granulocyte count <0.5 x 109 cells/L), a haematologic malignant condition, or AIDS were excluded. Patients who died or were discharged early (within 12 hours after admission) were also excluded. | ||
| Patient characteristics and setting | Authors reported information from 52 participants: 14 participants with SIRS without infection and 38 participants with sepsis. Compared with the SIRS group, the sepsis group tended to be older (60.0 versus 44.0 years) and comprised more males (86.8% versus 50.0%). The sepsis group had higher APACHE II (20.0 versus 12.0), SOFA (8.0 versus 6.0), and SAPS II (39.5 versus 27.5) scores than the SIRS group. Participants with sepsis were hospitalized for a longer time (8.0 versus 3.0 days) and required the use of mechanical ventilation for a longer period (5.5 versus 0.3 days). In the sepsis group, bacterial infection was found in 23 participants (60.5%); fungal infection in 2 participants (5.3%); and the remaining 11 (28.9%) participants were infected with both bacteria and fungus. Among 34 participants infected by bacteria, 14 (26.8%) of them were infected with Bacillus and 20 (52.6%) were infected with cocci. | ||
| Index tests | Within 12 hours after admission, 10 mL of whole heparinized blood was drawn via an arterial line for biomarker measurements. The blood was put on ice, and plasma was collected by centrifugation at 4 °C, separated into aliquots and stored at −80 °C until use. IL‐6 (EK0410; Boster Biological Technology) was quantified using ELISA tests. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: 2 intensivists retrospectively reviewed all medical records pertaining to each patient and independently classified the diagnosis as SIRS (no infection), sepsis, severe sepsis, or septic shock at the time of ICU admission, according to established consensus definitions (Bone 1992). Both intensivists were blinded to the plasma measurements. |
||
| Flow and timing | Biomarkers samples were taken within 12 hours after admission. 2 intensivists retrospectively reviewed all medical records pertaining to each patient and independently classified the diagnosis as SIRS (no infection), sepsis, severe sepsis, or septic shock at the time of ICU admission. All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: no financial | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Liu 2005.
| Study characteristics | |||
| Patient sampling | Authors enrolled all critically ill patients admitted to the ICU from April 2002 to October 2002. Diagnosis of SIRS systemic infection, severe systemic infection, and septic shock were based on diagnostic criteria of the American Association of Chest Physicians and Critical Care Medicine Consensus Conference. Exclusion criteria included thyroid myeloid cell carcinoma or small cell lung cancer patients. | ||
| Patient characteristics and setting | Authors reported information from 57 participants: 30 cases of non‐infectious SIRS participants and 27 cases of systemic infection. The average APACHE II score was 11.5 ± 7.8, mean age was 55.9 ± 21.0, and 70.2% of participants were male. Mean SOFA score was 4.9 ± 4.5. A total of 12 participants died. | ||
| Index tests | Serum samples were collected after 3 hours of blood collection. Serum IL‐6 levels were measured by immunofluorescence assay with a sensitivity of 0.1 ug/L; serum IL‐6 levels were measured by ELISA kit. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: diagnostic criteria of the American Association of Chest Physicians and Critical Care Medicine Consensus Conference. No further details provided. Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples and criteria were collected at the same time, but this is not clearly stated) All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: not stated | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Llewelyn 2013.
| Study characteristics | |||
| Patient sampling | Authors enrolled all admissions to the general ICU (17 beds) and HDU (8 beds) at Brighton and Sussex Hospitals NHS Trust between August 2010 and January 2011, who had study blood samples within 6 hours of admission and met 2 or more of the SIRS criteria in the first 24 hours of admission to ICU. Patients were excluded if they were under 18 years of age or where it was not possible to obtain patient consent or consultant approval to enrol the participant within 6 hours of admission. | ||
| Patient characteristics and setting | Between August 2010 and January 2011, 198 participants met 2 or more of the SIRS criteria. In 36 participants it was not possible to determine whether infection was present or not. Of the remaining participants, 87 participants with SIRS (43.9%) were deemed to have sepsis, while 75 (37.9%) were deemed not to have infection and were thus classified as having non‐infective SIRS. | ||
| Index tests | Blood was collected from participants within 6 hours of their admission to the unit. Samples were taken into sodium citrate tubes, centrifuged, and plasma was stored at −80 °C until the end of the study, when all samples were analysed for each marker as a single batch. IL‐6 levels were measured on a Luminex LX200 using Invitrogen’s Human Inflammatory 5‐Plex panel (Invitrogen/Life Technologies, Darmstadt, Germany) and Millipore filter plates (VWR, Darmstadt, Germany) as per the manufacturer's instructions. All biomarker analyses were conducted blind to the clinical data. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: the 2001 International Sepsis Definitions Conference definitions of SIRS and sepsis were used. Sepsis was defined as SIRS plus either proven infection (on the basis of microbiological sampling or radiology) or probable infection (considering the patient’s clinical presentation, white cell count, CRP, radiology), and non‐infective SIRS was defined as SIRS associated with an established underlying non‐infective diagnosis and no reason to suspect any ongoing infection. Categorization of participants was made independently by 2 members of the study team (ML and SD) blind to the biomarker results, with any disagreements resolved by discussion. |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples and criteria were collected at the same time, but this is not clearly stated) All participants received the same reference standard. In 36 participants (18.2%), it was not possible to determine with certainty whether infection was present or not. This group included 4 participants with pancreatitis. |
||
| Comparative | |||
| Notes | Funding: Abbott GmbH & Co. KG, Wiesbaden, Germany | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Mat‐Nor 2016.
| Study characteristics | |||
| Patient sampling | Authors enrolled consecutive adult participants aged older than 18 years who fulfilled the SIRS definition. This prospective cohort was conducted over 3 years (from July 2011 to June 2014) in a 12‐bed ICU of a major tertiary hospital in Pahang, Malaysia. Patients who had received antimicrobial treatment for more than 24 hours before the first blood samples for biomarker analysis were taken were excluded from the study. If a participant had more than 1 ICU admission, only the first episode was included in the study. |
||
| Patient characteristics and setting | 239 consecutive participants diagnosed with SIRS were recruited, of whom 164 (69%) were diagnosed with sepsis. Participants with sepsis were more severely ill with significantly higher SAPS II and SOFA scores on admission as compared to those with non‐infectious SIRS. Most participants in the sepsis group had respiratory as the primary diagnostic class, whereas most non‐infectious SIRS were classified as trauma. | ||
| Index tests | Daily serum concentrations of IL‐6 were measured during the first 3 days. The samples were centrifuged and stored at −80 °C for later analysis. IL‐6 was determined using Quantikine ELISA kit from R&D Systems (Minnesota, USA). The assay uses quantitative sandwich enzyme immunoassay technique and normal values corresponding to less than 9.7 pg/mL. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: ACCP criteria (Bone 1992; Levy 2003). 2 intensive care doctors completed a validated questionnaire for each participant on day 1 and day 3 to define clinical suspicion of sepsis. Participants were grouped into sepsis if there was clinical suspicion of infection with or without positive culture; otherwise, they were grouped into non‐infectious SIRS. The treating ICU physicians were blinded to the IL‐6 concentrations when caring for participants. |
||
| Flow and timing | No participants were excluded from the analysis. Samples were taken concurrently with the diagnosis. Unclear time interval between IL‐6 and reference standard (high probability that samples and criteria were collected at the same time, but this is not clearly stated) All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: International Islamic University Malaysia Endowment B research grant | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Meynaar 2011.
| Study characteristics | |||
| Patient sampling | Authors enrolled all consecutive participants admitted to the ICU between February 2009 and April 2009 if they were expected to be treated in the ICU for more than 24 hours. Exclusion criteria included patients who had neither SIRS nor sepsis; if patient had more than 1 ICU episode during the study period, only the first episode was included in the study. | ||
| Patient characteristics and setting | A total of 76 participants were included in the study, 32 with sepsis and 44 with SIRS. Participants with sepsis had significantly higher illness severity scores on admission as compared to participants with SIRS. Participants with sepsis were more often ventilated and put on renal replacement therapy. | ||
| Index tests | Blood samples for measuring IL‐6 were taken on admission and subsequently at 6 A.M. every morning until ICU discharge. IL‐6 levels were measured using a solid‐phase, enzyme‐labelled chemiluminescent immunometric assay (IMMULITE 2000; Siemens Healthcare, the Netherlands). A cut‐off level of 50 mg/L is commonly used in clinical practice. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Unclear if reported optimal cut‐off values for accuracy data were prespecified in the methods |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: signs of SIRS + bacterial infection (Bone 1992). The final diagnosis of sepsis or SIRS was made at a later stage from participant records and blinded to IL‐6 results. |
||
| Flow and timing | IL‐6 values were taken during 24 hours of admission. Categorization was made at the later stage. All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: IL‐6 kits were supplied free by Siemens Healthcare | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Moscovitz 1994.
| Study characteristics | |||
| Patient sampling | Authors enrolled participants admitted through the emergency department of the Hospital of the University of Pennsylvania. Patients were enrolled if they had: a) the presumptive diagnosis of bacteraemia as defined by the decision of the emergency physician to perform blood cultures; and b) findings of infection. Patients were excluded if they were known to have neoplastic disease or AIDS, if they were pregnant, or if they were currently taking immunosuppressive therapy, NSAID, or antibiotics. Unclear if participants were enrolled in a consecutive or random fashion |
||
| Patient characteristics and setting | 100 participants were enrolled in the study, 63 females and 37 males, with a median age of 51 years. 18 participants were bacteraemic at entry. A total of 32 participants who were not bacteraemic had a positive bacterial culture at 1 or more culture sites. | ||
| Index tests | Blood samples for cytokine determinations were obtained from participants in the emergency department before the administration of antibiotics. IL‐6 concentrations were measured using ELISA kits (Genzyme, Cambridge, MA, USA). Plasma samples for IL‐6 determinations were diluted 3‐fold before assay with diluent (Genzyme). The limits of detection were 160 pg/mL for IL‐6. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: bacteraemia Reference standard: SIRS criteria + microbiological evidence of local infection Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples were collected at the same time, but this is not clearly stated) All participants received the same reference standard. Unclear data (there were apparently no data for IL‐6 for 7 participants). No further data available. |
||
| Comparative | |||
| Notes | Funding: Ethel B. Foerderer Fund for Excellence of the Children's Hospital of Philadelphia | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Ramirez 2009.
| Study characteristics | |||
| Patient sampling | Authors included all participants expected to remain in mechanical ventilation for more than 48 hours and admitted to a 21‐bed medical ICU in an 18‐month period, without any active infection. Patients developing nosocomial infection other than VAP during hospitalization were excluded on diagnosis of these infections. | ||
| Patient characteristics and setting | 44 participants receiving mechanical ventilation were screened during the study period and included. 20 participants were suspected of having VAP throughout their ICU stay. Microbiological analysis of bronchoalveolar lavage confirmed the presence of VAP in 9 cases; the causative micro‐organisms were Staphylococcus aureus (3 cases), Acinetobacter baumannii (3 cases), Klebsiella pneumoniae (2 cases), and Streptococcus pneumoniae (1 case). The non‐microbiologically confirmed cases were not considered as having VAP. | ||
| Index tests | Authors determined IL‐6 at the day of study inclusion and every 96 hours. The blood samples taken for determination of inflammatory markers were centrifuged (1500 rpm, 10 min), and the supernatant was frozen at −80 °C. The determination of IL‐6 was performed with a commercial enzimoimmunoassay technique (BioSource, Nivelles, Belgium). Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: ventilator‐associated pneumonia Reference standard: VAP was suspected if participants met either: a) clinical criteria (new or progressive radiologic pulmonary infiltrate together with 2 of the following: temperature >38 °C, leukocytosis >12,000/mm3 or leukopenia <4000/mm3, or purulent respiratory secretions); or b) a simplified Clinical Pulmonary Infection Score ≥5 points. VAP was confirmed if the quantitative culture of BAL yielded 104 colony‐forming units per millilitre. Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Cytokines values were available at the day of VAP suspicion. All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: CibeRes and IDIBAPS | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Sakr 2008.
| Study characteristics | |||
| Patient sampling | Authors included all consecutive participants admitted to a surgical ICU between January 2001 and November 2001 with an estimated ICU length of stay of more than 48 hours. Exclusion criteria included patients younger than 18 years of age, patients with advanced malignancies or other conditions with shortened life expectancy (4 weeks), pregnancy, previous inclusion in the study, and patients for whom decisions to withhold or withdraw life‐sustaining treatments were established within the first 24 hours of ICU admission. | ||
| Patient characteristics and setting | 327 participants were included (207 males; mean age 63 years). 74 postoperative participants were referred from other facilities and did not undergo any surgical procedure in the 48 hours preceding ICU admission because of respiratory failure (n = 24), severe sepsis (n = 9), deterioration in the level of consciousness (n = 14), trauma (n = 6), successful cardiopulmonary resuscitation (n = 4), acute renal failure (n = 5), congestive heart failure or myocardial ischaemia (n = 5), gastrointestinal bleeding (n = 4), seizures (n = 2), and arrhythmia (n = 1). The median ICU length of stay was 6 days (25% to 75% IQR: 4 to 13 days), and the overall ICU mortality rate was 15% (n = 49). The SAPS II score varied considerably among the subgroups. | ||
| Index tests | Blood samples were collected daily during the first week after admission to the ICU. IL‐6 concentrations were measured with a commercially available immunoassay system (Immulite; DPC Biermann). The values of markers recorded on the first day of the diagnosis of sepsis syndromes or those recorded on the ICU admission day in patients without sepsis were used for this analysis. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: sepsis, severe sepsis, and septic shock were defined according to the ACCP/SCCM consensus conference criteria by the attending senior intensivist. Participants were categorized according to the worst grade of sepsis syndrome in the ICU. Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | In this study, values of markers recorded on the first day of the diagnosis of sepsis syndromes or those recorded on the ICU admission day in participants without sepsis were used. All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: Thuringian Ministry of Science and unrestricted grant of DPC Biermann GmbH, Bad Nauheim, Germany | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Tromp 2012.
| Study characteristics | |||
| Patient sampling | Authors enrolled participants in a prospective manner from an ED in the Netherlands during an 8‐month period. Inclusion criteria were: patients (16 years old) visiting the ED because of a suspected infection, who had at least 2 of the following clinical signs of sepsis: temperature > 38.3 °C or < 36 °C, heart rate > 90/min, respiratory rate > 20/min, chills, altered mental status, systolic blood pressure < 90 mmHg, MAP < 65 mmHg, and hyperglycaemia in the absence of diabetes mellitus. Exclusion criteria were not clearly stated. | ||
| Patient characteristics and setting | Authors reported information from 342 participants, of whom 55 (16%) had proven bacteraemia (positive blood culture). The most common causative agents were Escherichia coli (29%) and Streptococcus pneumoniae (23%). 37 participants with proven bacteraemia received antibiotics in the ED (67%). There was no significant difference in the administration of antibiotics between the patient group that was found to have positive blood cultures and the patient group with negative blood cultures. | ||
| Index tests | An additional blood sample was taken for measurement of IL‐6. Blood was collected in 3‐millilitre lithium heparin‐coated tubes. Plasma was obtained by centrifugation of the blood at 4 °C and 2200 g for 10 min. Plasma for the measurement of IL‐6 was frozen at −80 °C. IL‐6 was measured using the Immulite 2500 (Siemens Healthcare Diagnostics, Deerfield, IL, USA) with a detection limit of 2.00 pg/mL. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: bacteraemia Reference standard: bacteraemia was defined as growth of any pathogen in 1 or both blood culture sets. The isolation of coagulase‐negative staphylococci was considered as contamination and therefore not defined as bacteraemia. The final confirmed diagnosis at discharge was based on a combination of clinical signs and symptoms of sepsis, the presence/absence of an infiltrate on chest X‐ray, laboratory parameters, and culture results (e.g. blood, urine, sputum, and wound) obtained during the first 24 h following ED admission. No further details provided. Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Samples were taken concurrently with suspicion of sepsis. All participants received the same reference standard. Unclear information for 52 participants not included in final analysis (394 participants admitted to ED, 342 participants analysed) |
||
| Comparative | |||
| Notes | Funding: no source of funding received. Kits for PCT, IL‐6, and LBP donated by Brahms and Siemens. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Tsalik 2012.
| Study characteristics | |||
| Patient sampling | Authors included information from 2 sources: the Community Acquired Pneumonia and Sepsis Outcome Diagnostics study (CAPSOD; ClinicalTrials.gov NCT00258869) and the Duke Febrile Illness Cohort (DFIC; Grant/Cooperative Agreement Number U38/CCU423095). Eligible participants were identified in the Duke University Medical Center ED (annual census 70,000) and the Durham VAMC (annual census 40,000) over 2 periods: July 2003 to December 2003 and December 2006 to December 2007. Inclusion and exclusion criteria were the same for both studies and consist of the following: known or suspected infection at the time of screening with 2 or more SIRS criteria. Patients were excluded if they were < 18 years old, had an imminently terminal comorbid condition, had HIV/AIDS with CD4 count < 50 cells/mL, or were receiving antibiotics for an unrelated condition. |
||
| Patient characteristics and setting | Authors reported information from 336 participants with suspected sepsis in the ED. 89 participants (26.5%) had non‐infectious aetiologies at the time of initial presentation. Of the remaining 247 participants, 202 (81.8%) had uncomplicated sepsis, 28 (11.3%) had severe sepsis, and 17 (6.9%) had septic shock. Staphylococcus aureus (n = 36) and Escherichia coli (n = 24) together accounted for 53.1% of identified aetiologies. Lung, urinary tract, and skin together accounted for 60.6% of identified sites. Blood cultures were true‐positive in 55 of 259 (21.2%) participants. | ||
| Index tests | Samples for biomarker determination were frozen after their collection. They were later thawed at room temperature, gently mixed, and analysed within 8 hours. Measurements of IL‐6 are unaffected by a single freeze‐thaw cycle (24 to 26). IL‐6 measured on a Roche Elecsys 2010 analyser (Roche Diagnostics, Laval, Canada) by electrochemiluminescent immunoassay. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors reported 3 optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: infection/sepsis Reference standard: 1 of 2 study physicians with board certification in emergency medicine or internal medicine reviewed study data and the medical record excluding biomarker data. Determinations were made regarding likelihood of infection, site of infection, and causative organisms (SIRS criteria). Authors modified a previously published scale to define the likelihood of infection. |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples and criteria were collected at the same time, but this is not clearly stated) All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: NIH grant, National Institute of Allergy and Infectious Diseases grant, Roche Molecular Sciences grant | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Tsantes 2013.
| Study characteristics | |||
| Patient sampling | Authors reported information form 100 patients with ALI/ARDS treated in the mixed medical/surgical ICU of the Attikon University Hospital of Athens during a 3‐year period (June 2007 to June 2010). Participants were classified as severe sepsis or septic shock, or both according to ACCP/SCCM criteria. Participants with ARDS who fulfilled sepsis criteria and had positive cultures and/or compatible history/physical examination/laboratory were labelled as suffering from septic ARDS, while those with negative cultures, no other evidence of infection, and a clearly associated with the development of ARDS inciting factor were registered as having non‐septic ARDS. Unclear if participants were enrolled in a consecutive or random manner Exclusion criteria not clearly stated. |
||
| Patient characteristics and setting | 100 participants (79 ARDS and 21 ALI) were included, among which 58% were suffering from septic ALI/ARDS. 78 participants were classified as medical and 22 as surgical. The admission diagnosis can be grouped as follows: 57 participants with respiratory failure, 19 with septic shock, 11 with multiple trauma, 7 requiring postoperative monitoring and care, and 6 with altered mental status. The median (IQR) time of ARDS diagnosis following admission was 6 (2 to 12) days. The sites of infection in septic ARDS participants (n = 58) were determined as follows: lung 28, bloodstream 18, skin‐soft tissue 2, central nervous system 2, abdomen 7, and urinary tract (acute pyelonephritis) 1, while the following causes of non‐septic ARDS (n = 42) were identified: aspiration 21, trauma 11, multiple transfusions 3, pancreatitis 3, and drug overdose 4. | ||
| Index tests | Blood samples were drawn using EDTA blood collection tubes on study enrolment and prior to BAL procedure, and were immediately centrifuged at 1500 g for 30 min at 4 °C (or placed οn ice and centrifuged within 1 hour). Plasma was snap‐frozen in small portions and stored at −80 °C until the assays were performed. IL‐6 levels were assessed by the Elecsys IL‐6 immunoassay. The measuring IL‐6 detection range was between 1.5 and 5000 pg/mL. Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: ACCP/SCCM criteria. No details about administration were provided. Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples and criteria were collected at the same time, but this is not clearly stated) All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: not stated | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Zhao 2014.
| Study characteristics | |||
| Patient sampling | Authors enrolled in a prospective fashion SIRS participants treated in the emergency department of Beijing Chaoyang Hospital from March 2010 to March 2013. Inclusion criteria included age ≥ 18 years; non‐surgical trauma participants; survival time in emergency rescue room ≥ 24 hours. Exclusion criteria: patients with haematological or immunodeficiency diseases; patients who are using anticoagulants or hormones. | ||
| Patient characteristics and setting | Authors reported information from 652 cases, 357 males and 295 females; ages ranged from 18 to 96 years; 452 cases have infection and 200 cases non‐septic conditions. Severe sepsis was diagnosed in 160 cases, and 30 cases have septic shock. | ||
| Index tests | Blood samples taken at arrival to ED. Authors used ELISA for IL‐6 detection (RapidBio kit). Insufficient information to determine if index test results were interpreted without knowledge of the results of the reference standard. However, IL‐6 results were probably performed automatically and then were not affected by additional information. Authors reported optimal cut‐off values for accuracy data (not prespecified in methods). |
||
| Target condition and reference standard(s) | Target condition: sepsis Reference standard: the definition of SIRS, sepsis, severe sepsis, and septic shock was consistent with the diagnostic criteria established at the 2001 Washington International Septic Deficiency Conference. All participants were divided into sepsis group and non‐sepsis group according to the diagnosis of sepsis. No further details available. Unclear if reference standard results were interpreted without knowledge of the results of the index test |
||
| Flow and timing | Unclear time interval between IL‐6 and reference standard (high probability that samples and criteria were collected at the same time, but this is not clearly stated) All participants received the same reference standard. No participants were excluded from the analysis. |
||
| Comparative | |||
| Notes | Funding: not stated | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
ACCP: American College of Chest Physicians; ALI: acute lung injury; APACHE score: Acute Physiology and Chronic Health Evaluation score; ARDS: acute respiratory distress syndrome; BAL: bronchoalveolar lavage; CAPSOD: Community Acquired Pneumonia and Sepsis Outcome Diagnostics study; CD4: quadruple differentiation 4; CRP: C‐reactive Protein; EASIA: enzyme amplified sensitivity immunoassay; ESICM: European Society of Intensive Medicine; ED: emergency department ; EDTA: ethylenediaminetetraacetic acid; ELISA: enzyme‐linked immunosorbent assay; g: unit for centrifugation steps; HDU: high‐dependency unit; ICU: intensive care unit; IL‐6: interleukin‐6; IL‐8: interleukin‐8; IQR: interquartile range; LBP: lipopolysaccharide binding protein; MAP: mitogen‐activated protein; NSAID: Non‐steroidal Anti‐inflammatory Drugs; NHS: UK National Health Service; NIH: US National Institutes of Health; PaCO2:Partial pressure of carbon dioxide in arterial blood; PCT: procalcitonin; rpm: revolutions per minute; SAPS: Simplified Acute Physiology Score; SCCM: Society of Critical Care Medicine; SIRS: systemic inflammatory response syndrome; SOFA: Sequential Organ Failure Assessment; VAMC: Veterans Affairs Medical Center; VAP: ventilator‐associated pneumonia.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aikawa 2005 | No critically ill patients involved (unclear source). |
| Angeletti 2015 | Case‐control study |
| Aznar‐Oroval 2010 | No critically ill patients enrolled. |
| BalcI 2003 | Analysis was based on events of sepsis instead of patients with sepsis. |
| Behnes 2014 | No sensitivity/specificity data for IL‐6 |
| Byl 1997 | All patients with sepsis criteria at admission |
| Carlyn 2015 | AUROC analysis was used to evaluate the usefulness of the detectable biomarkers in determining the severity of the septic state. |
| Chalupa 2011 | No critically ill patients enrolled. |
| Chen 2018 | No sensitivity/specificity data for IL‐6 |
| de Bont 1999 | Insufficient data about accuracy of IL‐6; children included in the sample (no subgroup analysis) |
| de Mendonca‐Filho 2005 | Comparison focused on positive versus negative cultures; all patients with sepsis. |
| Durila 2012 | Prediction of sepsis during postoperative follow‐up |
| Endo 2008 | No sensitivity/specificity data for IL‐6 |
| Feng 2016 | Case‐control study |
| Fink‐Neuboeck 2016 | Prediction of postoperative SIRS |
| Gaini 2006 | Patients from department of internal medicine (no critically ill patients) |
| Gaini 2007 | Patients from department of internal medicine (no critically ill patients) |
| Giamarellos 2008 | No sensitivity/specificity data for IL‐6 |
| Gille‐Johnson 2012 | Not focused on diagnosis of sepsis (prediction of infections requiring antibiotics) |
| Groeneveld 2001 | Prediction of bloodstream infection at 7 days after inclusion |
| Grover 2014 | No sensitivity/specificity data for IL‐6 |
| Gurlich 1998 | No sensitivity/specificity data for IL‐6 |
| Hamed 2017 | Case‐control study |
| Hoenigl 2014 | No sensitivity/specificity data for IL‐6 |
| Huang 2016 | No sensitivity/specificity data for IL‐6 |
| Iapichino 2010 | Analysis based on patient days, not on participants. |
| Kweon 2014 | Case‐control study |
| Lin 2015 | Prediction of sepsis during hospitalization |
| Linder 2009 | Diagnosis of severity of sepsis (septic shock) |
| Lukaszewski 2008 | Prediction of sepsis |
| Mandi 2000 | No critically ill patients |
| Mardi 2010 | Case‐control study |
| Marie 2018 | No accuracy data for IL‐6 |
| Maruna 2002 | No sensitivity/specificity data for IL‐6 |
| Maruna 2011 | Prediction of infection (1 to 3 days postoperative) |
| Mickiewicz 2015 | No sensitivity/specificity data for IL‐6 |
| Mokart 2005 | Prediction of postoperative sepsis after major surgical procedures |
| Muller 2000 | Unclear data about groups compared |
| Nierhaus 2012 | Unclear data about groups compared |
| Parenica 2014 | Diagnosis of cardiogenic shock |
| Pauly 2016 | No sensitivity/specificity data for IL‐6 |
| Prat 2008 | No sensitivity/specificity data for IL‐6 |
| Qu 2015 | No critically ill patients enrolled (admissions from infectious disease department) |
| Rau 2010 | Diagnosis of rheumatological disease |
| Ravishankaran 2011 | Prediction of sepsis after surgery |
| Reichsoellner 2014 | Case‐control study |
| Rettig 2016 | Insufficient report of IL‐6 data about accuracy (composite endpoint = complications) |
| Sander 2006 | Prediction of sepsis after surgery |
| Schefold 2008 | Analysis based on serum samples instead of patients. |
| Shozushima 2011 | No sensitivity/specificity data for IL‐6 |
| Takahashi 2014 | IL‐6 measured in cerebrospinal fluid. |
| Takahashi 2016 | Case‐control study |
| Talebi‐Taher 2014 | Case‐control study |
| Tasabehji 2008 | No sensitivity/specificity data for IL‐6 |
| Uusitalo‐Seppala 2011 | No sensitivity/specificity data for IL‐6 |
| Uusitalo‐Seppälä 2012 | No sensitivity/specificity data for IL‐6 |
| Vanska 2012 | Prediction of complicated course in febrile neutropenia |
| von Dossow 2005 | Progression to septic shock |
| von Lilienfeld‐Toal 2004 | Febrile episodes instead of patients |
| Wang 2010 | Case‐control study |
| Weiss 2006 | All patients with severe sepsis at admission |
| Wutzler 2009 | No sensitivity/specificity data for IL‐6 |
| Yang 2016 | Prediction of sepsis (infection during ICU stay; blood samples taken within 12 hours after ICU admission) |
| Yousef 2010 | No sensitivity/specificity data for IL‐6 |
| Zhao 2018 | Case‐control study |
AUROC: area under the receiver operating characteristic curve; ICU: intensive care unit; IL‐6: interleukin‐6; SIRS: systemic inflammatory response syndrome.
Characteristics of studies awaiting classification [ordered by study ID]
Balci 2017.
| Study characteristics | |||
| Patient sampling | Not stated | ||
| Patient characteristics and setting | ICU patients with suspected infection admitted to the Health Sciences University, Kocaeli Derince Training Hospital's ICU | ||
| Index tests | Heparin‐binding protein, PCT, TNF‐alpha, IL‐6, and C‐reactive protein levels | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | Blood samples were taken on the 1st and 2nd day of hospitalization, and the 7th day, on day of discharge, or on the day of death. | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding. Objective (quote): "The present study was conducted to determine the heparin‐binding protein, procalcitonin level at early diagnosis in patients with sepsis, in comparison with C‐reactive protein, IL‐6 and TNF‐alfa." Page 1. Authors state that this study is ongoing. |
||
Blouin 2018.
| Study characteristics | |||
| Patient sampling | Not stated | ||
| Patient characteristics and setting | Cancer patients admitted with sepsis to the Urgent Care Center and ICU | ||
| Index tests | TNF‐alpha, IL‐10, IL‐1b, and IL‐6 | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | 2880 serum PCT results from 1944 patients were evaluated from December 2015 to June 2017. | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "We aimed to evaluate the performance of PCT and 4 citokines (TNF alpha, IL‐10, IL‐1b and IL‐6) for the diagnosis of bacterial bloodstream infections in critically ill cancer patients." Page 698 |
||
Cate 2013.
| Study characteristics | |||
| Patient sampling | Not stated | ||
| Patient characteristics and setting | Emergency department patients with SIRS and/or another sepsis risk factor (hypotension (SBP < 100), altered mental status, immunodeficiency, advanced age, or hyperglycaemia without diabetes) who were admitted to the medical ICU | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | Residual plasma specimens collected at ED admission were utilized. | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "to identify sepsis prediction models for ED patients with systemic inflammatory response syndrome (SIRS) and/or other sepsis risk factors" Page A197 |
||
Cebreiros‐Lopez 2017.
| Study characteristics | |||
| Patient sampling | 50 patients presenting at the ED with suspected sepsis were included. | ||
| Patient characteristics and setting | Blood samples were collected at first medical evaluation, and IL‐6 was analysed. After diagnosis, the patients were divided into 2 groups: group A (non‐infectious aetiology, localized infection, or SIRS) and group B (sepsis, severe sepsis, or septic shock). | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "the aim of this study was to investigate the diagnostic value of IL6 compared to PCT" Page S147 |
||
Das 2012.
| Study characteristics | |||
| Patient sampling | Not stated | ||
| Patient characteristics and setting | 3 patient groups, age > 18 years: group 1 (n = 41), proven infection; group 2 (n = 29), clinically suspected infection but negative culture; group 3 (n = 29), patients with SIRS | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis (microbiological cultures) | ||
| Flow and timing | Blood was collected at the time of admission for microbiological culture and estimation of IL‐6. | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "the aim of this study was to evaluate the diagnostic role of PCT and IL‐6 in differentiating sepsis (both culture positive and culture negative) from SIRS" Page 1 |
||
Fukui 2012.
| Study characteristics | |||
| Patient sampling | 18 patients operated at Kochi Health Sciences Center between April 2010 and March 2011 | ||
| Patient characteristics and setting | 9 patients with acute abdomen; 5 with oesophageal cancer; 2 with cerebral bleeding; and 1 each with Fournier's gangrene and trauma | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis. Sepsis was diagnosed according to ACCP/SCCM criteria. | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "in this study, we evaluated the specificity, clinical effectiveness of soluble sCD14st in patients with surgical sepsis" Page S2 |
||
Hakobyan 2012.
| Study characteristics | |||
| Patient sampling | 1589 patients observed between 2004 and 2011 | ||
| Patient characteristics and setting | 63 (16.6%) patients (76.3% (n = 200) male and 23.7% (n = 63) female) had sepsis. Average age was 16 to 84 years (43.8 ± 17.1). Days of hospitalization were 18.1 ± 18.8 (2 to 116 days); mortality 33.8%; consciousness by Glasgow Coma Scale 11.8 ± 7.6 points. | ||
| Index tests | Cytokines (unclear if IL‐6 was included) | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "the aim of study was to analyse informativity of current markers of sepsis (procalcitonin, glucose, C‐reactive protein, microbiological methods). The purpose was to improve current diagnosing of sepsis" Page Sii85 |
||
Kawano 2016.
| Study characteristics | |||
| Patient sampling | Single‐centre retrospective cohort study of patients who were admitted to the emergency ICU of the Fukuoka University Hospital, Japan, from May 2013 to April 2015 | ||
| Patient characteristics and setting | 232 patients were enrolled into this analysis. | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis and septic shock | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "the purpose of this study was to evaluate the clinical usefulness of sepsis biomarkers according to the new definitions of sepsis" Page 412 |
||
Kriplani 2017.
| Study characteristics | |||
| Patient sampling | Not stated | ||
| Patient characteristics and setting | 100 patients admitted to the Breach Candy Hospital Trust | ||
| Index tests | IL‐6 and procalcitonin | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "to identify alterations in haematological parameters like immature granulocytes, eosinopenia, immature platelet fraction, changes in morphology. 2. To correlate the haematological parameters with clinical findings and biomarkers of sepsis like IL‐6 and procalcitonin. 3. To establish haematological parameters as reliable initial markers of infections bacterial and viral" Page S67 |
||
Mendu 2015.
| Study characteristics | |||
| Patient sampling | Observational quality improvement study evaluating 25 patients who were admitted to the ICU with suspected severe sepsis or septic shock over a 6‐month period | ||
| Patient characteristics and setting | Setting: adult oncology medical‐surgical ICU at a tertiary cancer centre. Participants: 24 patients (10 with suspected severe sepsis or septic shock and 14 control participants) | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | IL‐6 levels were measured within 24 hours (Day 1) of ICU admission. The samples were batched, and the cytokine results were not available for clinical use. | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "our objective was to evaluate the diagnostic and prognostic value of procalcitonin (PCT) and 5 select cytokines (TNF‐α, IL‐1β, IL‐6, IL‐8, interferon‐gamma (IFN‐γ) in cancer patients admitted to the ICU with suspected severe sepsis and septic shock" Page S11 |
||
Mikaszewska‐Sokolewicz 2010.
| Study characteristics | |||
| Patient sampling | Not stated | ||
| Patient characteristics and setting | 92 adult patients (45 males and 47 females aged 18 to 93 years) with symptoms of inflammatory reaction and multiple organ failure, admitted to general ICU | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "clinical biomarkers of sepsis can be easily detected by bed site diagnostic tools. This process can add useful message to decision making process. The study was performed to determine if additional bed site tests can accelerate implementation of septic bundles and influence duration of treatment in the ICU." Page S314 |
||
Murai 2012.
| Study characteristics | |||
| Patient sampling | Observational cohort study in critically ill patients in the ICU of a tertiary care hospital | ||
| Patient characteristics and setting | 49 participants were enrolled. | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "we performed an observational cohort study in critically ill patients in the ICU of a tertiary care hospital. We investigated the correlation between endotoxin activity levels and blood concentration of endotoxin measured by the chromogenic limulus amoebocyte lysate (LAL) assay, causative microorganism identified in laboratory culture, procalcitonin (PCT), soluble CD14 subtype (named presepsin), IL‐6, antithrombin, protein C, thrombomodulin, lactate, disseminated intravascular coagulation scores in both the Japanese Ministry of Health and Welfare and the Japanese Association for Acute Medicine, and severity of illness at ICU admission." Page S12 |
||
Nakadai 2013.
| Study characteristics | |||
| Patient sampling | Not stated | ||
| Patient characteristics and setting | 207 patients with SIRS | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis; blood cultures | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "to evaluate the clinical application of measuring procalcitonin (PCT) level for diagnosis of bacterial sepsis in patients with and without systemic inflammatory response syndrome (SIRS), we studied the relationship between blood culture (BC) and serum PCT level in clinical 207 cases. In addition, we evaluated the time courses of PCT and other inflammatory markers: tumour necrosis factor‐alpha (TNF‐alpha), interleukin 6 (IL‐6), E‐selectin, white blood cell count and C‐reactive protein (CRP) in 5 bacterial septic patients with SIRS" Page 781 |
||
Nishida 2012.
| Study characteristics | |||
| Patient sampling | Single‐centre, prospective, observational study. Patients who had 1 or more SIRS criteria were included. 84 patients were enrolled from June 2010 to June 2011. | ||
| Patient characteristics and setting | Not stated | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | The blood samples for measuring the markers were collected, and the severity of sepsis was evaluated at the time of admission and every other day for a week. | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "the aims of this study were to investigate the most useful biomarkers which are serum levels of soluble CD14 subtype (sCD14‐ST) named presepsin, procalcitonin (PCT), IL‐6, and C‐reactive protein (CRP) as markers for early diagnosis of sepsis" Page S11 |
||
Oberhoffer 2000.
| Study characteristics | |||
| Patient sampling | Prospective consecutive case series | ||
| Patient characteristics and setting | Setting: surgical ICU of a university hospital. 243 patients experiencing ICU stays of longer than 48 hours categorized for sepsis were included. | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis; ACCP/SCCM Consensus Conference criteria | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "to determine the correlations and predictive strength of surrogate markers (body temperature, leukocyte count, C‐reactive protein (CRP) and procalcitonin (PCT)) with elevated levels of tumour necrosis factor‐alpha (TNF‐alpha) and interleukin‐6 (IL‐6) in septic patients on randomly chosen days" Page S11 |
||
Pyle 2011.
| Study characteristics | |||
| Patient sampling | Not stated | ||
| Patient characteristics and setting | 63 leftover plasma samples collected from ICU patients on the first day they had SIRS (identified though an automated electronic medical record scan) were included. Of these, 26 had culture‐confirmed sepsis and 37 had no bacterial infection within ±3 days of specimen collection. | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "our objective was to identify a panel of biomarkers that detects sepsis in ICU patients with SIRS" Page 468 |
||
Rotar 2017.
| Study characteristics | |||
| Patient sampling | Not stated | ||
| Patient characteristics and setting | 70 patients with severe acute necrotizing pancreatitis in a single intensive care department of regional hospital were assessed. | ||
| Index tests | Levels of presepsin as well as procalcitonin, IL‐6, and C‐reactive protein at admission | ||
| Target condition and reference standard(s) | Infection | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "to establish utility of presepsin (PSP) for diagnosis of local and systemic infection during ANP" Page S57 |
||
Tong 2014.
| Study characteristics | |||
| Patient sampling | Retrospective clinical study | ||
| Patient characteristics and setting | 131 patients in the surgical ICU who had SIRS but not sepsis from March 2012 to March 2013 | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "this study was designed to analyse and compare the ability of 8 biomarkers with their accuracy in an early prediction of sepsis" Page S153 |
||
Uchimido 2017.
| Study characteristics | |||
| Patient sampling | Prospective, observational study of a convenience sample of adult ED patients with suspected infection and non‐infected ED controls, conducted from September 2009 to April 2014 | ||
| Patient characteristics and setting | Not stated | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "to define the association of circulating endocan levels with inflammation, endothelial cell signalling, sepsis severity, and organ dysfunction (SOFA score) in Emergency Department (ED) patients with sepsis" Page S28 |
||
Woo 2015.
| Study characteristics | |||
| Patient sampling | Retrospective cohort study conducted from January 2013 to December 2013 | ||
| Patient characteristics and setting | 122 patients with SIRS, 55 of which were classified as the older age group (> 65 years). 33 (60%) patients in the older group and 40 (59.7%) patients in the other group had sepsis. | ||
| Index tests | IL‐6 | ||
| Target condition and reference standard(s) | Sepsis | ||
| Flow and timing | Not stated | ||
| Comparative | Not stated | ||
| Notes | Conference proceeding Objective (quote): "we assessed the diagnostic value of PCT and IL‐6 in older patients and other patients with SIRS and sepsis in the ED" Page S21 |
||
ACCP: American College of Chest Physicians; ED: emergency department; ICU: intensive care unit; IL‐1b: interleukin‐1b; IL‐6: interleukin‐6; IL‐10: interleukin‐10; PCT: procalcitonin; SBP: systolic blood pressure; SCCM: Society of Critical Care Medicine; SIRS: systemic inflammatory response syndrome; SOFA: Sequential Organ Failure Assessment; TNF‐alpha: tumour necrosis factor alpha
Differences between protocol and review
We made the following changes to the information published in the protocol of this review (Molano Franco 2015).
Title and objectives: following feedback received from the peer reviewers, we modified the title and objectives to include the source of measurement of interleukin‐6.
General: several paragraphs related to the Background and Reference standards sections were updated, taking into account updated information about definition and clinical criteria for sepsis diagnosis, as well as management of sepsis. We included several appendices to reflect clinical information relevant to this review.
Methods/included studies: case‐control studies were excluded, taking into account the considerable risk of bias involved in their development.
-
Statistical analysis: several methods planned in the protocol were not performed due to the high heterogeneity of the collected data.
We planned to obtain summary sensitivity and summary specificity estimates using the bivariate model (Reitsma 2005), analysing information of most common thresholds when data with more than one positive threshold were reported within the same study (Molano Franco 2015). However, we were unable to perform this analysis because studies largely varied in the thresholds they used to define a positive test result, and we observed high heterogeneity in data that prevented the estimation of summary accuracy estimates. Instead, we estimated a summary receiver operating characteristic (SROC) curve by fitting a hierarchical summary ROC (HSROC) non‐linear mixed model (Rutter 2001).
In addition, using the information from the HSROC model, we derived sensitivity at the median value of specificity along with corresponding 95% confidence intervals calculated using the delta method as implemented in R package. We performed this analysis to provide additional information about the possible consequences of the use of the test. However, we recognize that this analysis has several limitations, and advise caution in its interpretation.
We planned to investigate potential sources of heterogeneity using the bivariate random‐effects model; however, for the reasons mentioned above, we used an HSROC model for analysis of these sources of heterogeneity. We were unable to analyse several covariates due to the heterogeneity of the information collected.
Following the advice of a peer reviewer, we analysed the effect of year of publication ≥ 2011 in order to assess if the most recent evidence about the performance of interleukin‐6 has an impact in the HSROC parameters.
Sensitivity analysis: we planned to report the results of the sensitivity analysis for each risk of bias domain using a summary table. However, as all studies were judged as at high risk of bias for index test issues, we were unable to perform this analysis.
Assessment of reporting bias. We planned to explore publication bias by regressing log (diagnostic odds ratios (DORs)) on inverse root squared of effective sample size (Deeks 2005). However, we reconsidered performing this analysis given the absence of consensus about adequate methods to detect reporting bias in diagnostic test accuracy reviews at present (Deeks 2013).
Search strategies: we worked with the Cochrane Emergency and Critical Care Information Specialist in the modification of the search strategies in order to fulfil all the recommendations provided by the peer reviewers. As a result, the final searches differ from those published in the protocol of this review.
Contributions of authors
Daniel Molano Franco (DM), Ingrid Arevalo‐Rodriguez (IAR), Marta Roqué i Figuls (MR), Nadia Montero‐Oleas (NMO), Xavier Nuvials (XN), Javier Zamora (JZ)
Draft the protocol: IAR, DM, JZ, MR
Develop and run the search strategy: IAR, DM
Obtain copies of studies: DM, IAR, NMO
Select which studies to include (two review authors): IAR, DM, MR, NMO, XN, JZ
Extract data from studies (two review authors): IAR, MR, NMO
Enter data into Review Manager 5: DM, IAR, NMO
Carry out the analysis: IAR, JZ
Interpret the analysis: IAR, DM, JZ, MR, NMO, XN
Draft the final review: IAR, DM, JZ, MR, NMO, XN
Update the review: IAR, DM, JZ, MR, NMO, XN
Sources of support
Internal sources
Fundación Universitaria de Ciencias de la Salud, Hospital San José/Hospital Infantil de San José, Bogotá D.C., Colombia.
Cochrane Ecuador. Centro de Investigacion en Salud Publica y Epidemiologia Clinica (CISPEC). Universidad Tecnologica Equinoccial, Ecuador.
Cochrane Collaborative Unit, Hospital Ramon y Cajal, Spain.
External sources
No sources of support supplied
Declarations of interest
Daniel Molano Franco has no conflicts of interest to declare.
Ingrid Arevalo‐Rodriguez is funded by a Sara Borrell contract from the Instituto de Salud Carlos III (CD17/00219; Acción Estrategica en Salud 2013‐2016, co‐funded by European Social Fund 2014‐2020, "Investing in your future").
Marta Roqué i Figuls has no conflicts of interest to declare.
Nadia Montero‐Oleas has no conflicts of interest to declare.
Xavier Nuvials has no conflicts of interest to declare.
Javier Zamora has no conflicts of interest to declare.
New
References
References to studies included in this review
Aalto 2004 {published data only}
- Aalto H, Takala A, Kautiainen H, Repo H. Laboratory markers of systemic inflammation as predictors of bloodstream infection in acutely ill patients admitted to hospital in medical emergency. European Journal of Clinical Microbiology and Infectious Diseases 2004;23(9):699‐704. [PUBMED: 15309668] [DOI] [PubMed] [Google Scholar]
Anand 2015 {published data only}
- Anand D, Das S, Bhargava S, Srivastava LM, Garg A, Tyagi N, et al. Procalcitonin as a rapid diagnostic biomarker to differentiate between culture‐negative bacterial sepsis and systemic inflammatory response syndrome: a prospective, observational, cohort study. Journal of Critical Care 2015;30(1):218.e7‐12. [PUBMED: 25263339] [DOI] [PubMed] [Google Scholar]
Du 2003 {published data only}
- Du B, Li Y, Chen D, Pan J. Serum procalcitonin and interleukin‐6 help differentiate between severe sepsis and systemic inflammatory response syndrome of non‐infectious origin. Zhonghua Yi Xue Za Zhi 2002;82(16):1111‐4. [PUBMED: 12425821] [PubMed] [Google Scholar]
- Du B, Pan J, Chen D, Li Y. Serum procalcitonin and interleukin‐6 levels may help to differentiate systemic inflammatory response of infectious and non‐infectious origin. Chinese Medical Journal 2003;116(4):538‐42. [PUBMED: 12875718] [PubMed] [Google Scholar]
Endo 2012 {published data only}
- Endo S, Suzuki Y, Takahashi G, Shozushima T, Ishikura H, Murai A, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. Journal of Infection and Chemotherapy 2012;18(6):891‐7. [PUBMED: 22692596] [DOI] [PubMed] [Google Scholar]
Fu 2013 {published data only}
- Fu Y, Chen J, Cai B, Zhang J, Li L, Wang L. Serum procalcitonin, interleukin‐6, C‐reactive protein and serum amyloid a measurement in ICU department for diagnostic evaluation and risk‐analysis of prolonged ICU stay in patients with sepsis. Clinical Chemistry 2013;59(10 Suppl 1):A199. [Embase: 72249200] [Google Scholar]
- Fu Y, Chen J, Cai B, Zhang JL, Li LX, Wang LL. Diagnostic values of procalcitonin, interleukin‐6, C reactive protein and serum amyloid A in sepsis. Sichuan da Xue Xue Bao Yi Xue Ban 2012;43(5):702‐5. [PUBMED: 23230743] [PubMed] [Google Scholar]
- Fu Y, Chen J, Li L, Wang L. The usefulness of procalcitonin (PCT), interleukin‐6 (IL‐6), C reactive protein (CRP) and serum amyloid protein a (SAA) in diagnosis of nonviral infection of liver/kidney transplanted patients. Transplant International 2013;26 Suppl 2:165. [Google Scholar]
- Fu Y, Jiang H, Li L, Chen J, Zhang JL, Wang LL. Application value of procalcitonin and immune inflammatory factors for prediction of bacteraemia in patients with hematologic malignancy combined with febrile neutropenia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2013;21(5):1296‐300. [PUBMED: 24156453] [DOI] [PubMed] [Google Scholar]
Gao 2018 {published data only}
- Gao L, Yang B, Zhang H, Ou Q, Lin Y, Zhang M, et al. DcR3, a new biomarker for sepsis, correlates with infection severity and procalcitonin. Oncotarget 2018;9(13):10934‐44. [PUBMED: 29541387] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomez 2010 {published data only}
- Gomez JA, Ortiz‐Espejo M, Torrealba‐Rodríguez MI, Gordillo‐Álvarez J, Castellanos‐Ortega A, Suberviola B, et al. Evaluation of the diagnostic and prognostic capacity of procalcitonin, C reactive protein, interleukin‐6 and lipopolysaccharide binding protein in patients suspected of having sepsis [Evaluación de la capacidad diagnóstica y pronóstica de procalcitonina, proteína C reactiva, interleucina‐6 y proteína ligadora del lipopolisacárido en pacientes con sospecha de sepsis]. Revista del Laboratorio Clinico 2010;3(1):12‐9. [ibc‐85192] [Google Scholar]
Harbarth 2001 {published data only}
- Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, et al. Diagnostic value of procalcitonin, interleukin‐6, and interleukin‐8 in critically ill patients admitted with suspected sepsis. American Journal of Respiratory and Critical Care Medicine 2001;164(3):396‐402. [PUBMED: 11500339] [DOI] [PubMed] [Google Scholar]
Hou 2016 {published data only}
- Hou YQ, Liang DY, Lou XL, Zhang M, Zhang ZH, Zhang LR. Branched DNA‐based Alu quantitative assay for cell‐free plasma DNA levels in patients with sepsis or systemic inflammatory response syndrome. Journal of Critical Care 2016;31(1):90‐5. [PUBMED: 26589770] [DOI] [PubMed] [Google Scholar]
Jekarl 2013 {published data only}
- Jekarl DW, Kim JY, Lee S, Kim M, Kim Y, Han K, et al. Diagnosis and evaluation of severity of sepsis via the use of biomarkers and profiles of 13 cytokines: a multiplex analysis. Clinical Chemistry and Laboratory Medicine 2015;53(4):575‐81. [PUBMED: 25274957] [DOI] [PubMed] [Google Scholar]
- Jekarl DW, Lee SY, Lee J, Park YJ, Kim Y, Park JH, et al. Procalcitonin as a diagnostic marker and IL‐6 as a prognostic marker for sepsis. Diagnostic Microbiology and Infectious Disease 2013;75(4):342‐7. [PUBMED: 23391607] [DOI] [PubMed] [Google Scholar]
Jiang 2015 {published data only}
- Jiang YN, Cai X, Zhou HM, Jin WD, Zhang M, Zhang Y, et al. Diagnostic and prognostic roles of soluble CD22 in patients with Gram‐negative bacterial sepsis. Hepatobiliary and Pancreatic Diseases International 2015;14(5):523‐9. [PUBMED: 26459729] [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li L, Zhu Z, Chen J, Ouyang B, Chen M, Guan X. Diagnostic value of soluble triggering receptor expressed on myeloid cells‐1 in critically‐ill, postoperative patients with suspected sepsis. American Journal of the Medical Sciences 2013;345(3):178‐84. [PUBMED: 22739556] [DOI] [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu XL, Du B, Pan JQ, Xu Y, Hua BL. Role of procalcitonin in the differentiation and surveillance of systemic inflammatory response syndrome. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2005;27(1):48‐52. [PUBMED: 15782493] [PubMed] [Google Scholar]
Llewelyn 2013 {published data only}
- Llewelyn MJ, Berger M, Gregory M, Ramaiah R, Taylor AL, Curdt I, et al. Sepsis biomarkers in unselected patients on admission to intensive or high‐dependency care. Critical Care 2013;17(2):R60. [PUBMED: 23531337] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mat‐Nor 2016 {published data only}
- Mat‐Nor MB, Ralib AMD, Abdulah NZ, Pickering JW. The diagnostic ability of procalcitonin and interleukin‐6 to differentiate infectious from noninfectious systemic inflammatory response syndrome and to predict mortality. Journal of Critical Care 2016;33:245‐51. [PUBMED: 26851139] [DOI] [PubMed] [Google Scholar]
Meynaar 2011 {published data only}
- Meynaar IA, Droog W, Batstra M, Vreede R, Herbrink P. In critically ill patients, serum procalcitonin is more useful in differentiating between sepsis and SIRS than CRP, Il‐6, or LBP. Critical Care Research and Practice 2011;2011:594645. [PUBMED: 21687569] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynaar IA, Droog W, Batstra M, Vreede R, Herbrink P. Procalcitonin is more useful in diagnosing sepsis than CRP, IL‐6 and LBP. Intensive Care Medicine 2010;36 Suppl 2:S134. [Google Scholar]
Moscovitz 1994 {published data only}
- Moscovitz H, Shofer F, Mignott H, Behrman A, Kilpatrick L. Plasma cytokine determinations in emergency department patients as a predictor of bacteremia and infectious disease severity. Critical Care Medicine 1994;22(7):1102‐7. [PUBMED: 8026198] [DOI] [PubMed] [Google Scholar]
Ramirez 2009 {published data only}
- Ramirez P, Ferrer M, Gimeno R, Tormo S, Valencia M, Piner R, et al. Systemic inflammatory response and increased risk for ventilator‐associated pneumonia: a preliminary study. Critical Care Medicine 2009;37(5):1691‐5. [PUBMED: 19325465] [DOI] [PubMed] [Google Scholar]
Sakr 2008 {published data only}
- Sakr Y, Burgett U, Nacul FE, Reinhart K, Brunkhorst F. Lipopolysaccharide binding protein in a surgical intensive care unit: a marker of sepsis?. Critical Care Medicine 2008;36(7):2014‐22. [PUBMED: 18552695 ] [DOI] [PubMed] [Google Scholar]
Tromp 2012 {published data only}
- Tromp M, Lansdorp B, Bleeker‐Rovers CP, Gunnewiek JM, Kullberg BJ, Pickkers P. Serial and panel analyses of biomarkers do not improve the prediction of bacteremia compared to one procalcitonin measurement. Journal of Infection 2012;65(4):292‐301. [PUBMED: 22710263] [DOI] [PubMed] [Google Scholar]
Tsalik 2012 {published data only}
- Tsalik EL, Jaggers LB, Glickman SW, Langley RJ, Velkinburgh JC, Park LP, et al. Discriminative value of inflammatory biomarkers for suspected sepsis. Journal of Emergency Medicine 2012;43(1):97‐106. [PUBMED: 22056545] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsantes 2013 {published data only}
- Tsantes A, Tsangaris I, Kopterides P, Kapsimali V, Antonakos G, Zerva A, et al. The role of procalcitonin and IL‐6 in discriminating between septic and non‐septic causes of ALI/ARDS: a prospective observational study. Clinical Chemistry and Laboratory Medicine 2013;51(7):1535‐42. [PUBMED: 23314554] [DOI] [PubMed] [Google Scholar]
Zhao 2014 {published data only}
- Zhao Y, Li C. Diagnostic value of a combination of biomarkers in patients with sepsis and severe sepsis in emergency department. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014;26(3):153‐8. [PUBMED: 24598287] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aikawa 2005 {published data only}
- Aikawa N, Fujishima S, Endo S, Sekine I, Kogawa K, Yamamoto Y, et al. Multicenter prospective study of procalcitonin as an indicator of sepsis. Journal of Infection and Chemotherapy 2005;11(3):152‐9. [PUBMED: 15990980] [DOI] [PubMed] [Google Scholar]
Angeletti 2015 {published data only}
- Angeletti S, Dicuonzo G, Fioravanti M, Cesaris M, Fogolari M, Lo Presti A, et al. Procalcitonin, MR‐proadrenomedullin, and cytokines measurement in sepsis diagnosis: advantages from test combination. Disease Markers 2015;2015:Epub 2015 Nov 9. [PUBMED: 26635427] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aznar‐Oroval 2010 {published data only}
- Aznar‐Oroval E, Sánchez‐Yepes M, Lorente‐Alegre P, San Juan‐Gadea MC, Ortiz‐Muñoz B, Pérez‐Ballestero P, et al. Diagnostic value of procalcitonin, interleukin 8, interleukin 6, and C‐reactive protein for detecting bacteremia and fungemia in cancer patients. Enfermedades Infecciosas y Microbiologia Clinica 2010;28(5):273‐7. [PUBMED: 20097454] [DOI] [PubMed] [Google Scholar]
BalcI 2003 {published data only}
- BalcI C, Sungurtekin H, Gürses E, Sungurtekin U, Kaptanoglu B. Usefulness of procalcitonin for diagnosis of sepsis in the intensive care unit. Critical Care 2003;7(1):85‐90. [PUBMED: 12617745] [DOI] [PMC free article] [PubMed] [Google Scholar]
Behnes 2014 {published data only}
- Behnes M, Bertsch T, Lepiorz D, Lang S, Trinkmann F, Brueckmann M, et al. Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Critical Care 2014;18(5):507. [PUBMED: 25190134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Byl 1997 {published data only}
- Byl B, Devière J, Saint‐Hubert F, Zech F, Gulbis B, Thys JP, et al. Evaluation of tumor necrosis factor‐α, interleukin‐6 and C‐reactive protein plasma levels as predictors of bacteremia in patients presenting signs of sepsis without shock. Clinical Microbiology and Infection 1997;3(3):306‐13. [PUBMED: 11864125] [DOI] [PubMed] [Google Scholar]
Carlyn 2015 {published data only}
- Carlyn CJ, Andersen NJ, Baltch AL, Smith R, Reilly AA, Lawrence DA. Analysis of septic biomarker patterns: prognostic value in predicting septic state. Diagnostic Microbiology and Infectious Disease 2015;83(3):312‐8. [PUBMED: 26272282] [DOI] [PubMed] [Google Scholar]
Chalupa 2011 {published data only}
- Chalupa P, Beran O, Herwald H, Kasprikova N, Holub M. Evaluation of potential biomarkers for the discrimination of bacterial and viral infections. Infection 2011;39(5):411‐7. [PUBMED: 21720792] [DOI] [PubMed] [Google Scholar]
Chen 2018 {published data only}
- Chen X, Yong CY. Significance of changes of serum IL‐6, IL‐8, and PCT levels in patients with infection secondary to severe acute pancreatitis. World Chinese Journal of Digestology 2018;26(10):623‐7. [DOI: 10.11569/wcjd.v26.i10.623] [DOI] [Google Scholar]
de Bont 1999 {published data only}
- Bont ES, Vellenga E, Swaanenburg JC, Fidler V, Visser‐van Brummen PJ, Kamps WA. Plasma IL‐8 and IL‐6 levels can be used to define a group with low risk of septicaemia among cancer patients with fever and neutropenia. British Journal of Haematology 1999;107(2):375‐80. [PUBMED: 10583227] [DOI] [PubMed] [Google Scholar]
de Mendonca‐Filho 2005 {published data only}
- Mendonça‐Filho HT, Gomes GS, Nogueira PM, Fernandes MA, Tura BR, Santos M, et al. Macrophage migration inhibitory factor is associated with positive cultures in patients with sepsis after cardiac surgery. Shock 2005;24(4):313‐7. [PUBMED: 16205314] [DOI] [PubMed] [Google Scholar]
Durila 2012 {published data only}
- Durila M, Bronský J, Haruštiak T, Pazdro A, Pechová M, Cvachovec K. Early diagnostic markers of sepsis after oesophagectomy (including thromboelastography). BMC Anesthesiology 2012;12:12. [PUBMED: 22742451] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durila M, Bronský J, Haruštiak T, Pazdro A, Pechová M, Cvachovec K, et al. Biochemical and hematological parameters (including thromboelastography) differ in patients with sepsis and SIRS after esophagectomy. Critical Care 2011;15 Suppl 1:S156. [Embase: 70587680] [Google Scholar]
Endo 2008 {published data only}
- Endo S, Aikawa N, Fujishima S, Sekine I, Kogawa K, Yamamoto Y, et al. Usefulness of procalcitonin serum level for the discrimination of severe sepsis from sepsis: a multicenter prospective study. Journal of Infection and Chemotherapy 2008;14(3):244‐9. [PUBMED: 18574663] [DOI] [PubMed] [Google Scholar]
Feng 2016 {published data only}
- Feng M, Sun T, Zhao Y, Zhang H. Detection of serum interleukin‐6/10/18 levels in sepsis and its clinical significance. Journal of Clinical Laboratory Analysis 2016;30(6):1037‐43. [PUBMED: 27184083] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fink‐Neuboeck 2016 {published data only}
- Fink‐Neuboeck N, Lindenmann J, Bajric S, Maier A, Riedl R, Weinberg AM, et al. Clinical impact of interleukin 6 as a predictive biomarker in the early diagnosis of postoperative systemic inflammatory response syndrome after major thoracic surgery: a prospective clinical trial. Surgery 2016;160(2):443‐53. [PUBMED: 27206334] [DOI] [PubMed] [Google Scholar]
Gaini 2006 {published data only}
- Gaïni S, Koldkjaer OG, Pedersen C, Pedersen SS. Procalcitonin, lipopolysaccharide‐binding protein, interleukin‐6 and C‐reactive protein in community‐acquired infections and sepsis: a prospective study. Critical Care 2006;10(2):R53. [PUBMED: 16569262] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaini 2007 {published data only}
- Gaïni S, Koldkjaer OG, Møller HJ, Pedersen C, Pedersen SS. A comparison of high‐mobility group‐box 1 protein, lipopolysaccharide‐binding protein and procalcitonin in severe community‐acquired infections and bacteraemia: a prospective study. Critical Care 2007;11(4):R76. [PUBMED: 17625012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaïni S, Pedersen SS, Koldkjaer OG, Pedersen C, Møller HJ. High mobility group box‐1 protein in patients with suspected community‐acquired infections and sepsis: a prospective study. Critical Care 2007;11(2):R32. [PUBMED: 17346334] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giamarellos 2008 {published data only}
- Giamarellos‐Bourboulis EJ, Mouktaroudi M, Tsaganos T, Koutoukas P, Spyridaki E, Pelekanou A, et al. Evidence for the participation of soluble triggering receptor expressed on myeloid cells‐1 in the systemic inflammatory response syndrome after multiple trauma. Journal of Trauma, Injury, Infection and Critical Care 2008;65(6):1385‐90. [PUBMED: 19077631] [DOI] [PubMed] [Google Scholar]
Gille‐Johnson 2012 {published data only}
- Gille‐Johnson P, Hansson KE, Gardlund B. Clinical and laboratory variables identifying bacterial infection and bacteraemia in the emergency department. Scandinavian Journal of Infectious Diseases 2012;44(10):745‐52. [PUBMED: 22803656 ] [DOI] [PubMed] [Google Scholar]
Groeneveld 2001 {published data only}
- Groeneveld AB, Bossink AW, Mierlo GJ, Hack CE. Circulating inflammatory mediators in patients with fever: predicting bloodstream infection. Clinical and Diagnostic Laboratory Immunology 2001;8(6):1189‐95. [PUBMED: 11687462] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grover 2014 {published data only}
- Grover V, Kelleher P, Soni N, Singh S. Use of soluble and surface triggering receptor expressed on myeloid cells‐1 as markers of ventilator‐associated pneumonia in intensive care. British Journal of Anaesthesia 2010;104(4):526P‐7P. [Google Scholar]
- Grover V, Pantelidis P, Soni N, Takata M, Shah PL, Wells AU, et al. A biomarker panel (Bioscore) incorporating monocytic surface and soluble TREM‐1 has high discriminative value for ventilator‐associated pneumonia: a prospective observational study. PLOS ONE 2014;9(10):e109686. [PUBMED: 25289689] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurlich 1998 {published data only}
- Gurlich R, Maruna P, Cermak J. The significance of cytokines in the early diagnosis of postoperative intraabdominal sepsis. Rozhledy V Chirurgii 1998;77(4):146‐9. [PUBMED: 9658957] [PubMed] [Google Scholar]
Hamed 2017 {published data only}
- Hamed S, Behnes M, Pauly D, Lepiorz D, Barre M, Becher T, et al. Diagnostic value of Pentraxin‐3 in patients with sepsis and septic shock in accordance with latest sepsis‐3 definitions. BMC Infectious Diseases 2017;17(1):554. [PUBMED: 28793880] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoenigl 2014 {published data only}
- Hoenigl M, Raggam RB, Wagner J, Prueller F, Grisold AJ, Leitner E, et al. Procalcitonin fails to predict bacteremia in SIRS patients: a cohort study. International Journal of Clinical Practice 2014;68(10):1278‐81. [PUBMED: 24898888] [DOI] [PubMed] [Google Scholar]
- Hoenigl M, Raggam RB, Wagner J, Valentin T, Leitner E, Seeber K, et al. Diagnostic accuracy of soluble urokinase plasminogen activator receptor (suPAR) for prediction of bacteremia in patients with systemic inflammatory response syndrome. Clinical Biochemistry 2013;46(3):225‐9. [PUBMED: 23159293] [DOI] [PubMed] [Google Scholar]
Huang 2016 {published data only}
- Huang L, Li J, Han Y, Zhao S, Zheng Y, Sui F, et al. Serum calprotectin expression as a diagnostic marker for sepsis in postoperative intensive care unit patients. Journal of Interferon and Cytokine Research 2016;36(10):607‐16. [PUBMED: 27610929] [DOI] [PubMed] [Google Scholar]
Iapichino 2010 {published data only}
- Iapichino G, Marzorati S, Umbrello M, Baccalini R, Barassi A, Cainarca M, et al. Daily monitoring of biomarkers of sepsis in complicated long‐term ICU‐patients: can it support treatment decisions?. Minerva Anestesiologica 2010;76(10):814‐23. [PUBMED: 20935617] [PubMed] [Google Scholar]
Kweon 2014 {published data only}
- Kweon OJ, Choi JH, Park SK, Park AJ. Usefulness of presepsin (sCD14 subtype) measurements as a new marker for the diagnosis and prediction of disease severity of sepsis in the Korean population. Journal of Critical Care 2014;29(6):965‐70. [PUBMED: 25042676] [DOI] [PubMed] [Google Scholar]
Lin 2015 {published data only}
- Lin S, Huang Z, Wang M, Weng Z, Zeng D, Zhang Y, et al. Interleukin‐6 as an early diagnostic marker for bacterial sepsis in patients with liver cirrhosis. Journal of Critical Care 2015;30(4):732‐8. [PUBMED: 25891645] [DOI] [PubMed] [Google Scholar]
Linder 2009 {published data only}
- Linder A, Christensson B, Herwald H, Björck L, Akesson P. Heparin‐binding protein: an early marker of circulatory failure in sepsis. Clinical Infectious Diseases 2009;49(7):1044‐50. [PUBMED: 19725785] [DOI] [PubMed] [Google Scholar]
Lukaszewski 2008 {published data only}
- Lukaszewski RA, Yates AM, Jackson MC, Swingler K, Scherer JM, Simpson AJ, et al. Presymptomatic prediction of sepsis in intensive care unit patients. Clinical and Vaccine Immunology: CVI 2008;15(7):1089‐94. [PUBMED: 18480235]18480235 [Google Scholar]
Mandi 2000 {published data only}
- Mandi Y, Farkas G, Takacs T, Boda K, Lonovics J. Diagnostic relevance of procalcitonin, IL‐6, and sICAM‐1 in the prediction of infected necrosis in acute pancreatitis. International Journal of Pancreatology 2000;28(1):41‐9. [PUBMED: 11185709] [DOI] [PubMed] [Google Scholar]
Mardi 2010 {published data only}
- Mardi D, Fwity B, Lobmann R, Ambrosch A. Mean cell volume of neutrophils and monocytes compared with C‐reactive protein, interleukin‐6 and white blood cell count for prediction of sepsis and nonsystemic bacterial infections. International Journal of Laboratory Hematology 2010;32(4):410‐8. [PUBMED: 19919621] [DOI] [PubMed] [Google Scholar]
Marie 2018 {published data only}
- Marie Relster M, Gaini S, Møller HJ, Johansen IS, Pedersen C. The macrophage activation marker sMR as a diagnostic and prognostic marker in patients with acute infectious disease with or without sepsis. Scandinavian Journal of Clinical and Laboratory Investigation 2018;78(3):180‐6. [PUBMED: 29383956] [DOI] [PubMed] [Google Scholar]
Maruna 2002 {published data only}
- Maruna P, Gurlich R, Frasko R, Chachkhiani I, Marunova M, Owen K, et al. Procalcitonin in the diagnosis of postoperative complications. Sbornik Lekarsky 2002;103(2):283‐95. [PUBMED: 12688153] [PubMed] [Google Scholar]
Maruna 2011 {published data only}
- Maruna P, Kunstyr J, Plocova KM, Mlejnsky F, Hubacek J, Klein AA, et al. Predictors of infection after pulmonary endarterectomy for chronic thrombo‐embolic pulmonary hypertension. European Journal of Cardiothoracic Surgery 2011;39(2):195‐200. [PUBMED: 20615721] [DOI] [PubMed] [Google Scholar]
Mickiewicz 2015 {published data only}
- Mickiewicz B, Tam P, Jenne CN, Leger C, Wong J, Winston BW, et al. Alberta Sepsis Network. Integration of metabolic and inflammatory mediator profiles as a potential prognostic approach for septic shock in the intensive care unit. Critical Care 2015;19:11. [PUBMED: 25928796] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mokart 2005 {published data only}
- Mokart D, Merlin M, Sannini A, Brun JP, Delpero JR, Houvenaeghel G, et al. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. British Journal of Anaesthesia 2005;94(6):767‐73. [PUBMED: 15849208] [DOI] [PubMed] [Google Scholar]
Muller 2000 {published data only}
- Muller B, Becker KL, Schachinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Critical Care Medicine 2000;28(4):977‐83. [PUBMED: 10809269] [DOI] [PubMed] [Google Scholar]
Nierhaus 2012 {published data only}
- Nierhaus A, Klatte S, Linssen J, Eismann NM, Wichmann D, Hedke J, et al. Revisiting the white blood cell count: immature granulocytes count as a diagnostic marker to discriminate between SIRS and sepsis ‐ a prospective, observational study. BMC Immunology 2013;14:8. [PUBMED: 23398965] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus A, Linssen J, Wichmann D, Braune S, Kluge S. Use of a weighted, automated analysis of the differential blood count to differentiate sepsis from non‐infectious systemic inflammation: the intensive care infection score (ICIS). Inflammation and Allergy Drug Targets 2012;11(2):109‐15. [PUBMED: 22280231] [DOI] [PubMed] [Google Scholar]
- Nierhaus A, Thiele S, Linssen J, Eismann N, Wichmann D, Hedke J, et al. Revisiting the white blood cell count: immature granulocytes as a diagnostic marker in adult sepsis. Infection 2011;39 Suppl 2:S114. [Embase: 70660880] [Google Scholar]
- Nierhaus A, Thiele S, Linssen J, Eismann N, Wichmann D, Hedke J, et al. Revisiting the white blood cell count: immature granulocytes as a diagnostic marker in adult sepsis ‐ an observational study. International Journal of Laboratory Hematology 2012;34 Suppl 1:125‐6. [Embase: 70868445 ] [Google Scholar]
- Nierhaus A, Thiele S, Linssen J, Wichmann D, Braune S, Kluge S, et al. The intensive care infection score (ICIS): a new approach for discrimination between sepsis and non‐infectious systemic inflammation. Infection 2011;39 Suppl 2:S113‐4. [Embase: 70660879] [Google Scholar]
Parenica 2014 {published data only}
- Pařenica J, Maláska J, Jarkovský J, Helánová K, Jabandžiev P, Michálek J, et al. Dynamics of interleukin 6 levels in the patients with cardiogenic and septic shock and in a control group of patients with uncomplicated AMI. Vnitrni Lekarstvi 2014;60(2):114‐22. [PUBMED: 24754415] [PubMed] [Google Scholar]
Pauly 2016 {published data only}
- Pauly D, Hamed S, Behnes M, Lepiorz D, Lang S, Akin I, et al. Endothelial cell‐specific molecule‐1/endocan: diagnostic and prognostic value in patients suffering from severe sepsis and septic shock. Journal of Critical Care 2016;31(1):68‐75. [PUBMED: 26489483] [DOI] [PubMed] [Google Scholar]
Prat 2008 {published data only}
- Prat C, Sancho JM, Dominguez J, Xicoy B, Gimenez M, Ferra C, et al. Evaluation of procalcitonin, neopterin, C‐reactive protein, IL‐6 and IL‐8 as a diagnostic marker of infection in patients with febrile neutropenia. Leukemia and Lymphoma 2008;49(9):1752‐61. [PUBMED: 18661397] [DOI] [PubMed] [Google Scholar]
Qu 2015 {published data only}
- Qu J, Lü X, Liu Y, Wang X. Evaluation of procalcitonin, C‐reactive protein, interleukin‐6 & serum amyloid a as diagnostic biomarkers of bacterial infection in febrile patients. Indian Journal of Medical Research 2015;141(3):315‐21. [PUBMED: 25963492] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rau 2010 {published data only}
- Rau M, Schiller M, Krienke S, Heyder P, Lorenz H, Blank N. Clinical manifestations but not cytokine profiles differentiate adult‐onset Still's disease and sepsis. Journal of Rheumatology 2010;37(11):2369‐76. [PUBMED: 20810496] [DOI] [PubMed] [Google Scholar]
Ravishankaran 2011 {published data only}
- Ravishankaran P, Shah AM, Bhat R. Correlation of interleukin‐6, serum lactate, and C‐reactive protein to inflammation, complication, and outcome during the surgical course of patients with acute abdomen. Journal of Interferon and Cytokine Research 2011;31(9):685‐90. [PUBMED: 21923250] [DOI] [PubMed] [Google Scholar]
Reichsoellner 2014 {published data only}
- Reichsoellner M, Raggam RB, Wagner J, Krause R, Hoenigl M. Clinical evaluation of multiple inflammation biomarkers for diagnosis and prognosis for patients with systemic inflammatory response syndrome. Journal of Clinical Microbiology 2014;52(11):4063‐6. [PUBMED: 25187630] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rettig 2016 {published data only}
- Rettig TC, Verwijmeren L, Dijkstra IM, Boerma D, Garde EM, Noordzij PG. Postoperative interleukin‐6 level and early detection of complications after elective major abdominal surgery. Annals of Surgery 2016;263(6):1207‐12. [PUBMED: 26135695] [DOI] [PubMed] [Google Scholar]
Sander 2006 {published data only}
- Sander M, Heymann C, Dossow V, Spaethe C, Konertz WF, Jain U, et al. Increased interleukin‐6 after cardiac surgery predicts infection. Anesthesia and Analgesia 2006;102(6):1623‐9. [PUBMED: 16717298] [DOI] [PubMed] [Google Scholar]
Schefold 2008 {published data only}
- Schefold JC, Hasper D, Haehling S, Meisel C, Reinke P, Schlosser H‐G. Interleukin‐6 serum level assessment using a new qualitative point‐of‐care test in sepsis: a comparison with ELISA measurements. Clinical Biochemistry 2008;41(10‐1):893‐8. [PUBMED: 18395522] [DOI] [PubMed] [Google Scholar]
Shozushima 2011 {published data only}
- Shozushima T. Presepsin (sCD14‐ST) as a new diagnostic biomarker of sepsis: development of diagnostic tools using the whole blood. Critical Care 2011;15 Suppl 3:S2. [Embase: 70601572] [Google Scholar]
- Shozushima T, Masuda T, Suzuki Y, Takahashi G, Endo S. Presepsin (SCD14‐ST): a new marker for diagnosis of bacterial infection. Intensive Care Medicine 2011;37 Suppl 1:S142. [Embase: 70639354] [Google Scholar]
- Shozushima T, Suzuki Y, Masuda T, Takahashi G, Endo S. Usefulness of presepsin (sCD14‐ST) measurements as a marker for the diagnosis and severity of sepsis in systemic inflammatory response syndrome. Critical Care 2011;15 Suppl 1:S147. [Embase: 70587651] [DOI] [PubMed] [Google Scholar]
- Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14‐ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. Journal of Infection and Chemotherapy 2011;17(6):764‐9. [PUBMED: 21560033] [DOI] [PubMed] [Google Scholar]
Takahashi 2014 {published data only}
- Takahashi W, Nakada T‐a, Abe R, Tanaka K, Matsumura Y, Oda S. Usefulness of interleukin 6 levels in the cerebrospinal fluid for the diagnosis of bacterial meningitis. Journal of Critical Care 2014;29(4):693.e1‐6. [PUBMED: 24636923] [DOI] [PubMed] [Google Scholar]
Takahashi 2016 {published data only}
- Takahashi W, Nakada TA, Yazaki M, Oda S. Interleukin‐6 levels act as a diagnostic marker for infection and a prognostic marker in patients with organ dysfunction in intensive care units. Shock 2016;46(3):254‐60. [PUBMED: 27172160] [DOI] [PubMed] [Google Scholar]
Talebi‐Taher 2014 {published data only}
- Talebi‐Taher M, Babazadeh S, Barati M, Latifnia M. Serum inflammatory markers in the elderly: are they useful in differentiating sepsis from SIRS?. Acta Medica Iranica 2014;52(6):438‐42. [PUBMED: 25130150] [PubMed] [Google Scholar]
Tasabehji 2008 {published data only}
- Tasabehji WR, Al‐Quobaili FA, Al‐Daher NA. Usefulness of procalcitonin and some inflammatory parameters in septic patients. Saudi Medical Journal 2008;29(4):520‐5. [PUBMED: 18382791] [PubMed] [Google Scholar]
Uusitalo‐Seppala 2011 {published data only}
- Uusitalo‐Seppala R, Koskinen P, Leino A, Peuravuori H, Vahlberg T, Rintala EM. Early detection of severe sepsis in the emergency room: diagnostic value of plasma C‐reactive protein, procalcitonin, and interleukin‐6. Scandinavian Journal of Infectious Diseases 2011;43(11‐2):883‐90. [PUBMED: 21892899] [DOI] [PubMed] [Google Scholar]
Uusitalo‐Seppälä 2012 {published data only}
- Uusitalo‐Seppälä R, Huttunen R, Tarkka M, Aittoniemi J, Koskinen P, Leino A, et al. Soluble urokinase‐type plasminogen activator receptor in patients with suspected infection in the emergency room: a prospective cohort study. Journal of Internal Medicine 2012;272(3):247‐56. [PUBMED: 22755554] [DOI] [PubMed] [Google Scholar]
Vanska 2012 {published data only}
- Vanska M, Koivula I, Jantunen E, Hamalainen S, Purhonen A‐K, Pulkki K, et al. IL‐10 combined with procalcitonin improves early prediction of complications of febrile neutropenia in hematological patients. Cytokine 2012;60(3):787‐92. [PUBMED: 22902948] [DOI] [PubMed] [Google Scholar]
von Dossow 2005 {published data only}
- Dossow V, Rotard K, Redlich U, Hein OV, Spies CD. Circulating immune parameters predicting the progression from hospital‐acquired pneumonia to septic shock in surgical patients. Critical Care 2005;9(6):R662‐9. [PUBMED: 16280065] [DOI] [PMC free article] [PubMed] [Google Scholar]
von Lilienfeld‐Toal 2004 {published data only}
- Lilienfeld‐Toal M, Dietrich MP, Glasmacher A, Lehmann L, Breig P, Hahn C, et al. Markers of bacteremia in febrile neutropenic patients with hematological malignancies: procalcitonin and IL‐6 are more reliable than C‐reactive protein. European Journal of Clinical Microbiology and Infectious Disease 2004;23(7):539‐44. [PUBMED: 15221617] [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang JF, Yu ML, Yu G, Bian JJ, Deng XM, Wan XJ, et al. Serum miR‐146a and miR‐223 as potential new biomarkers for sepsis. Biochemical and Biophysical Research Communications 2010;394(1):184‐8. [PUBMED: 20188071] [DOI] [PubMed] [Google Scholar]
Weiss 2006 {published data only}
- Weiss G, Benedix F, Lippert H. Diagnostic problems of nosocomial infections in patients with severe sepsis and ongoing antimicrobial treatment ‐ efficiency and value of serum inflammatory markers and routine microbiological monitoring. Clinical Intensive Care 2006;17(3‐4):113‐23. [Embase: 352248643] [Google Scholar]
Wutzler 2009 {published data only}
- Wutzler S, Maier M, Lehnert M, Henrich D, Walcher F, Maegele M, et al. Suppression and recovery of LPS‐stimulated monocyte activity after trauma is correlated with increasing injury severity: a prospective clinical study. Journal of Trauma: Injury, Infection and Critical Care 2009;66(5):1273‐80. [PUBMED: 19430226] [DOI] [PubMed] [Google Scholar]
Yang 2016 {published data only}
- Yang Y, Xie J, Guo F, Longhini F, Gao Z, Huang Y, et al. Combination of C‐reactive protein, procalcitonin and sepsis‐related organ failure score for the diagnosis of sepsis in critical patients. Annals of Intensive Care 2016;6(1):51. [PUBMED: 27287669] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yousef 2010 {published data only}
- Yousef AA, Abdulmomen G. The assessment of selective biomarkers improves the diagnosis of critically ill patients. Anesthesia and Analgesia 2010;110(3 Suppl 1):S106. [Embase: 71788330] [Google Scholar]
- Yousef AA, Amr YM, Suliman GA. The diagnostic value of serum leptin monitoring and its correlation with tumor necrosis factor‐α in critically ill patients: a prospective observational study. Critical Care 2010;14(2):R33. [PUBMED: 20230641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2018 {published data only}
- Zhao JJ, Lou XL, Chen HW, Zhu FT, Hou YQ. Diagnostic value of decoy receptor 3 combined with procalcitonin and soluble urokinase‐type plasminogen activator receptor for sepsis. Cellular and Molecular Biology Letters 2018;23(1):22. [PUBMED: 29760745] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Balci 2017 {published data only}
- Balci C. The research on effects of heparin‐binding protein levels on early diagnosis in sepsis, severe sepsis and septic shock. Critical Care 2017;21(Suppl 1):1. [Embase: 619526589] [Google Scholar]
Blouin 2018 {published data only}
- Blouin A, Pastores S, Halpern N, Fleisher M, Ramanathan L. Procalcitonin and cytokine levels in cancer patients with neutropenia and bloodstream infections. Critical Care Medicine 2018;46(Suppl 1):698. [Embase: 620080794] [Google Scholar]
Cate 2013 {published data only}
- Cate F, Colón‐Franco J, Litt S, Rice T, Wheeler A, Plummer D, et al. Identification of models to predict sepsis in emergency department patients. Clinical Chemistry 2013;59(10 Suppl 1):A197. [Embase: 72249192] [Google Scholar]
Cebreiros‐Lopez 2017 {published data only}
- Cebreiros‐Lopez I, Noguera‐Velasco J, Martinez‐Lopez De Castro A, Castillo‐Guardiola V. Contribution of interleukin‐6 (IL6) in the diagnosis of sepsis in the emergency department. Clinical Chemistry and Laboratory Medicine 2017;55 Suppl 1:S147. [Embase: 616773040] [Google Scholar]
Das 2012 {published data only}
- Das S, Anand D, Ray S, Bhargava S, Manocha A, Kankra M, et al. Diagnostic accuracy of procalcitonin in proven and clinically suspected systemic infection. Critical Care 2012;16(Suppl 3):1. [Embase: 70925883] [Google Scholar]
Fukui 2012 {published data only}
- Fukui Y. Evaluation of a soluble CD14 subtype in patients with surgical sepsis. Critical Care 2012;16(Suppl 3):2. [Embase: 70925884] [Google Scholar]
Hakobyan 2012 {published data only}
- Hakobyan G, Yeghiazaryan M. Informativeness of current markers of sepsis. British Journal of Anaesthesia 2012;108 Suppl 2:ii85‐6. [Embase: 70719093] [Google Scholar]
Kawano 2016 {published data only}
- Kawano Y, Hoshino K, Irie Y, Izutani Y, Muranishi K, Tamura T, et al. Usefulness of sepsis biomarkers according to the new definitions of sepsis. Critical Care Medicine 2016;44(12 Suppl 1):412. [Embase: 613523828] [Google Scholar]
Kriplani 2017 {published data only}
- Kriplani D, Udwadia F, Antia VP, Laurenco E. Evaluation of the significance of IL‐6 and procalcitonin as a biomarker for infection and correlation with haematological parameters for early detection of sepsis. Indian Journal of Hematology and Blood Transfusion 2017;33(1 Suppl 1):S67. [Embase: 619504756] [Google Scholar]
Mendu 2015 {published data only}
- Mendu DR, Sherard L, Kostelecky N, Sun C, Pastores S, Halpern NA, et al. Plasma cytokines augment procalcitonin levels in septic patients admitted to oncological intensive care unit. Clinical Chemistry 2015;61(10 Suppl 1):S11‐2. [Embase: 72264945] [Google Scholar]
Mikaszewska‐Sokolewicz 2010 {published data only}
- Mikaszewska‐Sokolewicz MA, Tomasiuk R, Lazowski T. Bed‐site biomarkers: IL‐6, IL‐8 and s100b in diagnostic and treatment decision making process of sepsis. Intensive Care Medicine 2010;36(Suppl 2):S314. [Google Scholar]
Murai 2012 {published data only}
- Murai A, Ishikura H, Nishida T, Irie Y, Kamitani T, Yuge R, et al. Clinical usefulness of measuring endotoxin activity on ICU admission. Critical Care 2012;16 Suppl 1:S12. [Embase: 70734974] [Google Scholar]
Nakadai 2013 {published data only}
- Nakadai H, Yoshizawa S, Miyamoto H, Ikeda Y, Akizuki S, Ohnishi A. Clinical verification of serum procalcitonin measurement in sepsis. Rinsho Byori 2013;61(9):781‐6. [PUBMED: 24369589] [PubMed] [Google Scholar]
Nishida 2012 {published data only}
- Nishida T, Ishikura H, Murai A, Irie Y, Yuge R, Kamitani T, et al. Assessment of the usefulness of presepsin (soluble CD14 subtype) in septic patients. Critical Care 2012;16 Suppl 1:S11‐2. [Embase: 70734972] [Google Scholar]
Oberhoffer 2000 {published data only}
- Oberhoffer M, Russwurm S, Bredle D, Chatzinicolaou K, Reinhart K. Discriminative power of inflammatory markers for prediction of tumor necrosis factor‐alpha and interleukin‐6 in ICU patients with systemic inflammatory response syndrome (SIRS) or sepsis at arbitrary time points. Intensive Care Medicine 2000;26 Suppl 2:S170‐4. [Embase: 30186577] [DOI] [PubMed] [Google Scholar]
Pyle 2011 {published data only}
- Pyle AL, Anderson DA, Hooper M, Rice T, Wheeler A, Moffett C, et al. A multi‐marker approach to differentiate sepsis from SIRS. American Journal of Clinical Pathology 2011;136(3):468‐9. [Embase: 71304607] [Google Scholar]
- Pyle AL, Anderson DA, Hooper M, Rice T, Wheeler A, Woodworth A, et al. Multiplex cytokine analysis for the differentiation of SIRS and sepsis. Clinical Chemistry 2011;57(10 Suppl 1):A164‐5. [Embase: 71304607] [Google Scholar]
- Pyle AL, Hooper M, Rice T, Wheeler A, Woodworth A. Multiplex cytokine analysis for the differentiation of SIRS and sepsis. American Journal of Clinical Pathology 2010;134(3):509. [Embase: 71304768] [Google Scholar]
Rotar 2017 {published data only}
- Rotar O, Khomiak I, Petrovsky G, Nazarchuk M, Rotar V. Presepsin for early infection diagnosis during acute necrotizing pancreatitis. Pancreatology 2017;17(3 Suppl 1):S57. [DOI: 10.1016/j.pan.2017.05.182] [DOI] [Google Scholar]
Tong 2014 {published data only}
- Tong L, Liu HE, Tang XC. Construction and analysis of early prediction model of sepsis: a retrospective clinical study. Intensive Care Medicine 2014;40(1 Suppl 1):S153. [Embase: 71630367] [Google Scholar]
Uchimido 2017 {published data only}
- Uchimido R, Lopez GJ, Lu S, Ghiran I, Aird W, Shapiro I, et al. The association of endocan with inflammation, endothelial cell signalling, illness severity, and organ dysfunction in sepsis. Academic Emergency Medicine 2017;24 Suppl 1:S28‐9. [Google Scholar]
Woo 2015 {published data only}
- Woo SH. Diagnostic value of procalcitonin and IL‐6 for sepsis of older patients and other patients in the emergency department. Critical Care 2015;19 Suppl 1:S21‐2. [Google Scholar]
Additional references
Abraham 2000
- Abraham E, Matthay MA, Dinarello CA, Vincent JL, Cohen J, Opal SM, et al. Consensus conference definitions for sepsis, septic shock, acute lung injury, and acute respiratory distress syndrome: time for a reevaluation. Critical Care Medicine 2000;28(1):232‐5. [PUBMED: 10667529] [DOI] [PubMed] [Google Scholar]
Andriolo 2017
- Andriolo BNG, Andriolo RB, Salomão R, Atallah ÁN. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD010959.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aneja 2011
- Aneja R, Carcillo J. Differences between adult and pediatric septic shock. Minerva Anestesiologica 2011;77(10):986‐92. [PUBMED: 21952599] [PubMed] [Google Scholar]
Angus 2001
- Angus DC, Linde‐Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine 2001;29(7):1303‐10. [PUBMED: 11445675] [DOI] [PubMed] [Google Scholar]
Bloos 2014
- Bloos F, Reinhart K. Rapid diagnosis of sepsis. Virulence 2014;5(1):154‐60. [PUBMED: 24335467] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bone 1992
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101(6):1644‐55. [PUBMED: 1303622] [DOI] [PubMed] [Google Scholar]
Boucher 1999
- Boucher BA, Hanes SD. Searching for simple outcome markers in sepsis: an effort in futility?. Critical Care Medicine 1999;27(7):1390‐1. [PUBMED: 10446842] [DOI] [PubMed] [Google Scholar]
Carson 1999
- Carson WE, Yu H, Dierksheide J, Pfeffer K, Bouchard P, Clark R, et al. A fatal cytokine‐induced systemic inflammatory response reveals a critical role for NK cells. Journal of Immunology 1999;162(8):4943‐51. [PUBMED: 10202041] [PubMed] [Google Scholar]
Castellanos‐Ortega 2010
- Castellanos‐Ortega A, Suberviola B, García‐Astudillo LA, Holanda MS, Ortiz F, Llorca J, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Critical Care Medicine 2010;38(4):1036‐43. [PUBMED: 20154597] [DOI] [PubMed] [Google Scholar]
Cheong 2002
- Cheong YC, Shelton JB, Laird SM, Richmond M, Kudesia G, Li TC, et al. IL‐1, IL‐6 and TNF‐alpha concentrations in the peritoneal fluid of women with pelvic adhesions. Human Reproduction (Oxford, England) 2002;17(1):69‐75. [PUBMED: 11756364] [DOI] [PubMed] [Google Scholar]
Christaki 2014
- Christaki E, Giamarellos‐Bourboulis EJ. The complex pathogenesis of bacteremia: from antimicrobial clearance mechanisms to the genetic background of the host. Virulence 2014;5(1):57‐65. [PUBMED: 24067507] [DOI] [PMC free article] [PubMed] [Google Scholar]
Danai 2006
- Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest 2006;129(6):1432‐40. [PUBMED: 16778259] [DOI] [PubMed] [Google Scholar]
de Montmollin 2014
- Montmollin E, Annane D. Year in review 2013: critical care ‐ sepsis. Critical Care 2014;18(5):578. [PUBMED: 25673430] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882‐93. [PUBMED: 16085191] [DOI] [PubMed] [Google Scholar]
Deeks 2013
- Deeks JJ, Bossuyt PM, Gatsonis C (editors). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
Dellinger 2013
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical Care Medicine 2013;41(2):580‐637. [PUBMED: 23353941] [DOI] [PubMed] [Google Scholar]
Dinarello 1997
- Dinarello CA. Proinflammatory and anti‐inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 1997;112(6 Suppl):321S‐9S. [PUBMED: 9400897] [DOI] [PubMed] [Google Scholar]
Dowdy 2005
- Dowdy DW, Eid MP, Sedrakyan A, Mendez‐Tellez PA, Pronovost PJ, Herridge MS, et al. Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Medicine 2005;31(5):611‐20. [DOI] [PubMed] [Google Scholar]
Ferrer 2009
- Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, et al. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. American Journal of Respiratory and Critical Care Medicine 2009;180(9):861‐6. [PUBMED: 19696442] [DOI] [PubMed] [Google Scholar]
Finfer 2004
- Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult‐population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Medicine 2004;30(4):589‐96. [PUBMED: 14963646] [DOI] [PubMed] [Google Scholar]
Finnerty 2007
- Finnerty CC, Herndon DN, Jeschke MG. Inhalation injury in severely burned children does not augment the systemic inflammatory response. Critical Care 2007;11(1):R22. [PUBMED: 17306027] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleischmann 2016
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital‐treated sepsis. Current estimates and limitations. American Journal of Respiratory and Critical Care Medicine 2016;193(3):259‐72. [PUBMED: 26414292] [DOI] [PubMed] [Google Scholar]
Gamez‐Diaz 2011
- Gamez‐Diaz LY, Enriquez LE, Matute JD, Velasquez S, Gomez ID, Toro F, et al. Diagnostic accuracy of HMGB‐1, sTREM‐1, and CD64 as markers of sepsis in patients recently admitted to the emergency department. Academic Emergency Medicine 2011;18(8):807‐15. [PUBMED: 21762470] [DOI] [PubMed] [Google Scholar]
Gentile 2013
- Gentile LF, Cuenca AG, Vanzant EL, Efron PA, McKinley B, Moore F, et al. Is there value in plasma cytokine measurements in patients with severe trauma and sepsis?. Methods 2013;61(1):3‐9. [PUBMED: 23669589] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2012
- Glanville J, Cikalo M, Crawford F, Dozier M, McIntosh H. Handsearching did not yield additional unique FDG‐PET diagnostic test accuracy studies compared with electronic searches: a preliminary investigation. Research Synthesis Methods 2012;3(3):202‐13. [DOI] [PubMed] [Google Scholar]
Green 2008
- Green RS, Djogovic D, Gray S, Howes D, Brindley PG, Stenstrom R, et al. Canadian Association of Emergency Physicians Sepsis Guidelines: the optimal management of severe sepsis in Canadian emergency departments. Canadian Journal of Emergency Medicine 2008;10(5):443‐59. [PUBMED: 18826733] [DOI] [PubMed] [Google Scholar]
Hauptmann 1991
- Hauptmann B, Damme J, Dayer JM. Modulation of IL‐1 inflammatory and immunomodulatory properties by IL‐6. European Cytokine Network 1991;2(1):39‐46. [PUBMED: 1651782] [PubMed] [Google Scholar]
Heney 1995
- Heney D, Whicher JT. Factors affecting the measurement of cytokines in biological fluids: implications for their clinical measurement. Annals of Clinical Biochemistry 1995;32 (Pt 4):358‐68. [PUBMED: 7486794] [DOI] [PubMed] [Google Scholar]
Hou 2015
- Hou T, Huang D, Zeng R, Ye Z, Zhang Y. Accuracy of serum interleukin (IL)‐6 in sepsis diagnosis: a systematic review and meta‐analysis. International Journal of Clinical and Experimental Medicine 2015;8(9):15238‐45. [PUBMED: 26629009] [PMC free article] [PubMed] [Google Scholar]
Jekarl 2015
- Jekarl DW, Kim JY, Lee S, Kim M, Kim Y, Han K, et al. Diagnosis and evaluation of severity of sepsis via the use of biomarkers and profiles of 13 cytokines: a multiplex analysis. Clinical Chemistry and Laboratory Medicine 2015;53(4):575‐81. [PUBMED: 25274957] [DOI] [PubMed] [Google Scholar]
Kishimoto 1995
- Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin‐6 family of cytokines and gp130. Blood 1995;86(4):1243‐54. [PUBMED: 7632928] [PubMed] [Google Scholar]
Kumar 2011
- Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, et al. Nationwide trends of severe sepsis in the 21st century (2000‐2007). Chest 2011;140(5):1223‐31. [PUBMED: 21852297] [DOI] [PubMed] [Google Scholar]
Latronico 2011
- Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurology 2011;10(10):931‐41. [PUBMED: 21939902] [DOI] [PubMed] [Google Scholar]
Levy 2003
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Critical Care Medicine 2003;31(4):1250‐6. [PUBMED: 12682500] [DOI] [PubMed] [Google Scholar]
Levy 2018
Littenberg 1993
- Littenberg B, Moses LE. Estimating diagnostic accuracy from multiple conflicting reports: a new meta‐analytic method. Medical Decision Making: an International Journal of the Society for Medical Decision Making 1993;13(4):313‐21. [PUBMED: 8246704] [DOI] [PubMed] [Google Scholar]
Liu 2016
- Liu Y, Hou JH, Li Q, Chen KJ, Wang SN, Wang JM. Biomarkers for diagnosis of sepsis in patients with systemic inflammatory response syndrome: a systematic review and meta‐analysis. SpringerPlus 2016;5(1):2091. [PUBMED: 28028489] [DOI] [PMC free article] [PubMed] [Google Scholar]
Livaditi 2006
- Livaditi O, Kotanidou A, Psarra A, Dimopoulou I, Sotiropoulou C, Augustatou K, et al. Neutrophil CD64 expression and serum IL‐8: sensitive early markers of severity and outcome in sepsis. Cytokine 2006;36(5‐6):283‐90. [PUBMED: 17368039] [DOI] [PubMed] [Google Scholar]
Ma 2016
- Ma L, Zhang H, Yin YL, Guo WZ, Ma YQ, Wang YB, et al. Role of interleukin‐6 to differentiate sepsis from non‐infectious systemic inflammatory response syndrome. Cytokine 2016;88:126‐35. [PUBMED: 27599258] [DOI] [PubMed] [Google Scholar]
McGuire 2014
- McGuire TR, Reardon NT, Bogard K, Plumb TJ, Bultsma CJ, Nissen SW, et al. IL6 plasma concentrations in patients with sepsis receiving SLED and antibiotics: a predictor for survival. In Vivo 2014;28(6):1131‐4. [PUBMED: 25398811] [PubMed] [Google Scholar]
Moses 1993
- Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data‐analytic approaches and some additional considerations. Statistics in Medicine 1993;12(14):1293‐316. [PUBMED: 8210827] [DOI] [PubMed] [Google Scholar]
Onyenekwu 2017
- Onyenekwu CP, Okwundu CI, Ochodo EA. Procalcitonin, C‐reactive protein, and presepsin for the diagnosis of sepsis in adults and children. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD012627] [DOI] [Google Scholar]
Pallas 2016
- Pallas Beneyto LA, Rodriguez Luis O, Saiz Sanchez C, Cotell Simon O, Bautista Rentero D, Miguel Bayarri V. Prognostic value of interleukin 6 for death of patients with sepsis [Valor pronostico de la interleucina 6 en la mortalidad de pacientes con sepsis]. Medicina Clinica 2016;147(7):281‐6. [PUBMED: 27422737] [DOI] [PubMed] [Google Scholar]
Patel 1994
- Patel RT, Deen KI, Youngs D, Warwick J, Keighley MR. Interleukin 6 is a prognostic indicator of outcome in severe intra‐abdominal sepsis. British Journal of Surgery 1994;81(9):1306‐8. [PUBMED: 7953393] [DOI] [PubMed] [Google Scholar]
Pinsky 1993
- Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple‐system organ failure and mortality. Chest 1993;103(2):565‐75. [PUBMED: 8432155] [DOI] [PubMed] [Google Scholar]
Puthucheary 2013
- Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310(15):1591‐600. [PUBMED: 24108501] [DOI] [PubMed] [Google Scholar]
R Development Core Team 2008 [Computer program]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2008.
Rangel‐Frausto 1995
- Rangel‐Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 1995;273(2):117‐23. [PUBMED: 7799491] [PubMed] [Google Scholar]
Reinhart 2010
- Reinhart K, Brunkhorst FM, Bone HG, Bardutzky J, Dempfle CE, Forst H, et al. Prevention, diagnosis, therapy and follow‐up care of sepsis: 1st revision of S‐2k guidelines of the German Sepsis Society (Deutsche Sepsis‐Gesellschaft e.V. (DSG)) and the German Interdisciplinary Association of Intensive Care and Emergency Medicine (Deutsche Interdisziplinare Vereinigung fur Intensiv‐ und Notfallmedizin (DIVI)). German Medical Science 2010;8:14. [PUBMED: 20628653] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [PUBMED: 16168343] [DOI] [PubMed] [Google Scholar]
Rello 2017
- Rello J, Valenzuela‐Sanchez F, Ruiz‐Rodriguez M, Moyano S. Sepsis: a review of advances in management. Advances in Therapy 2017;34(11):2393‐411. [PUBMED: 29022217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhodes 2017
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Medicine 2017;43(3):304‐77. [DOI] [PubMed] [Google Scholar]
Rios‐Toro 2017
- Rios‐Toro JJ, Marquez‐Coello M, Garcia‐Alvarez JM, Martin‐Aspas A, Rivera‐Fernandez R, Saez de Benito A, et al. Soluble membrane receptors, interleukin 6, procalcitonin and C reactive protein as prognostic markers in patients with severe sepsis and septic shock. PLOS ONE 2017;12(4):e0175254. [PUBMED: 28380034] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rizoli 2002
- Rizoli SB, Marshall JC. Saturday night fever: finding and controlling the source of sepsis in critical illness. Lancet Infectious Diseases 2002;2(3):137‐44. [PUBMED: 11944183] [DOI] [PubMed] [Google Scholar]
Romero‐Bermejo 2011
- Romero‐Bermejo FJ, Ruiz‐Bailen M, Gil‐Cebrian J, Huertos‐Ranchal MJ. Sepsis‐induced cardiomyopathy. Current Cardiology Reviews 2011;7(3):163‐83. [PUBMED: 22758615] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutter 2001
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20(19):2865‐84. [PUBMED: 11568945] [DOI] [PubMed] [Google Scholar]
SAS 2014 [Computer program]
- SAS Institute. SAS software. Version 9.4. Cary (NC): SAS Institute, 2014.
Shankar‐Hari 2015
- Shankar‐Hari M, Deutschman CS, Singer M. Do we need a new definition of sepsis?. Intensive Care Medicine 2015;41(5):909‐11. [PUBMED: 25643905] [DOI] [PubMed] [Google Scholar]
Simon 2004
- Simon L, Gauvin F, Amre DK, Saint‐Louis P, Lacroix J. Serum procalcitonin and C‐reactive protein levels as markers of bacterial infection: a systematic review and meta‐analysis. Clinical Infectious Diseases 2004;39(2):206‐17. [PUBMED: 15307030] [DOI] [PubMed] [Google Scholar]
Singer 2016
- Singer M, Deutschman CS, Seymour CW, Shankar‐Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA 2016;315(8):801‐10. [PUBMED: 26903338] [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2005
- Song M, Kellum J. Interleukin‐6. Critical Care Medicine 2005;33(12):S463‐5. [PUBMED: 16340422] [DOI] [PubMed] [Google Scholar]
Stoppelkamp 2015
- Stoppelkamp S, Veseli K, Stang K, Schlensak C, Wendel HP, Walker T. Identification of predictive early biomarkers for sterile‐SIRS after cardiovascular surgery. PLOS ONE 2015;10(8):e0135527. [PUBMED: 26263001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanaka 2014
- Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harbor perspectives in biology 2014;6(10):a016295. [PUBMED: 25190079] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2007
- Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta‐analysis. Lancet Infectious Diseases 2007;7(3):210‐7. [PUBMED: 17317602] [DOI] [PubMed] [Google Scholar]
Thomas 2016
- Thomas R, Cheah HM, Creaney J, Turlach BA, Lee YC. Longitudinal measurement of pleural fluid biochemistry and cytokines in malignant pleural effusions. Chest 2016;149(6):1494‐500. [PUBMED: 26836920] [DOI] [PubMed] [Google Scholar]
Thompson 2012
- Thompson DK, Huffman KM, Kraus WE, Kraus VB. Critical appraisal of four IL‐6 immunoassays. PLOS ONE 2012;7(2):e30659. [PUBMED: 22347395] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Poll 1990
- Poll T, Buller HR, Cate H, Wortel CH, Bauer KA, Deventer SJ, et al. Activation of coagulation after administration of tumor necrosis factor to normal subjects. New England Journal of Medicine 1990;322(23):1622‐7. [PUBMED: 2188129] [DOI] [PubMed] [Google Scholar]
van Engelen 2018
- Engelen TSR, Wiersinga WJ, Scicluna BP, Poll T. Biomarkers in sepsis. Critical Care Clinics 2018;34(1):139‐52. [PUBMED: 29149935] [DOI] [PubMed] [Google Scholar]
Waage 1989
- Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. Journal of Experimental Medicine 1989;169(1):333‐8. [PUBMED: 2783334] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wacker 2013
- Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta‐analysis. Lancet Infectious Diseases 2013;13(5):426‐35. [PUBMED: 23375419] [DOI] [PubMed] [Google Scholar]
Whiting 2011a
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. Journal of Clinical Epidemiology 2011;64(6):602‐7. [PUBMED: 21075596] [DOI] [PubMed] [Google Scholar]
Whiting 2011b
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [PUBMED: 22007046] [DOI] [PubMed] [Google Scholar]
WHO 2017
- World Health Organization. Resolution WHA70.7: Improving the prevention, diagnosis and clinical management of sepsis. apps.who.int/gb/ebwha/pdf_files/WHA70/A70_R7‐en.pdf?ua=1&ua=1 (accessed 10 October 2018).
Wong 2015
- Wong HR, Walley KR, Pettila V, Meyer NJ, Russell JA, Karlsson S, et al. Comparing the prognostic performance of ASSIST to interleukin‐6 and procalcitonin in patients with severe sepsis or septic shock. Biomarkers: Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals 2015;20(2):132‐5. [PUBMED: 25578228] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zamora 2006
- Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta‐DiSc: a software for meta‐analysis of test accuracy data. BMC Medical Research Methodology 2006;6:31. [PUBMED: 16836745] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Molano Franco 2015
- Molano Franco D, Arevalo‐Rodriguez I, Roqué i Figuls M, Zamora J. Interleukin‐6 for diagnosis of sepsis in critically ill adult patients. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD011811] [DOI] [PMC free article] [PubMed] [Google Scholar]


