Quantification of the apathogenic and ubiquitous torque teno virus (TTV) in the peripheral blood predicts infectious disease in recipients of a kidney allograft. TTV might be a potential tool to tailor immunosuppressive drug therapy to prevent infections after kidney transplantation.
Keywords: torque teno virus, kidney transplantation, infection, biomarker, immunosuppression, cytomegalovirus, polyomavirus nephropathy
Abstract
Background
Drug-induced immunosuppression following kidney transplantation is crucial to prevent allograft rejection, but increases risk for infectious disease. Tailoring of drug dosing to prevent both rejection and infection is greatly desirable. The apathogenic and ubiquitous torque teno virus (TTV) reflects immunocompetence of the host and might be a potential candidate for immunologic monitoring.
Methods
To assess TTV as an infection biomarker, virus load was prospectively quantified in peripheral blood of 169 consecutive renal allograft recipients at the Medical University Vienna.
Results
Patients with infection showed higher TTV levels compared to patients without infection (4.2 × 108 copies/mL [interquartile range, IQR, 2.7 × 107–1.9 × 109] vs 2.9 × 107 [IQR 1.0 × 106–7.2 × 108]; P = .006). Differences in TTV load became evident almost 3 months before infection (median 77 days, IQR 19–98). Each log level of TTV copies/mL increased the odds ratio for infection by 23% (95% confidence interval 1.04–1.45; P = .014). TTV >3.1 × 109 copies/mL corresponded to 90% sensitivity to predict infections. Logistic regression demonstrated independent association between TTV levels and infection.
Conclusions
TTV quantification predicts infection after kidney transplantation and might be a potential tool to tailor immunosuppressive drug therapy.
(See the Editorial Commentary Kotton, on pages 1185–7.)
Organ transplantation is the treatment of choice for patients with end-stage renal disease. In the posttransplant period immunosuppressive drugs are crucial to prevent allograft rejection. Besides this desired effect, reduced immunocompetence of the transplant recipient leads to an increased risk of developing infectious diseases, which are the leading cause of death after kidney transplantation [1]. Currently, monitoring of immunosuppression relies mainly on calcineurin inhibitor drug trough levels in the peripheral blood, which correlate more closely with the risk of drug-related toxicity than the immunosuppressive efficacy [2]. Thus, there is an urgent need for tailored immunosuppressive medication in order to reduce the risk of infection and, at the same time, to prevent allograft rejection.
In this respect, a promising strategy might be the monitoring of peripheral blood levels for the torque teno virus (TTV) [3]. TTV can be detected in up to 90% of healthy individuals [4] and, so far, it has not been linked with any specific human disease [3]. Peripheral blood levels of TTV might mirror the overall strength of innate and specific immunity [5, 6] and thus viral load is closely related to the immunocompetence of the host [7, 8]. The prevalence of TTV in immunocompromised patients after solid-organ transplantation is up to 100% [9] and it is unaffected by conventional antiviral drug therapy used in the posttransplantation setting [10]. TTV levels were shown to be associated with the amount and type of immunosuppressive drugs administered to solid-organ recipients and thus indirectly with allograft rejection and infection [11, 12]. Retrospective cohort studies described associations of low TTV virus levels with graft rejection in lung, liver, kidney, and heart transplantation [10, 13–15] and, conversely, high TTV levels were found to be associated with infection after lung transplantation [11]. Hitherto, the value of TTV for the prediction of infection or organ rejection has not been tested in a prospective study design.
This prospective study was designed to evaluate TTV as a predictor of infectious disease after kidney transplantation. We hypothesize that a high level of TTV reflects an excessive level of immunosuppression and thus precedes infections. To test this hypothesis, TTV was quantified before and longitudinally after transplantation by means of polymerase chain reaction (PCR) in the peripheral blood of consecutive recipients of a kidney allograft in 2016, performed in one academic centre in Vienna, Austria. Assay results were evaluated in the context of a careful monitoring of viral, bacterial, and fungal infections after transplantation.
MATERIAL AND METHODS
Patients
This prospective study includes all 169 consecutive adult (≥18 years of age) recipients of a kidney allograft transplanted at the Medical University of Vienna, Austria, between 1 January and 31 December 2016. Patients were followed at the outpatient clinic of the Medical University of Vienna and follow-up data were included until 1 March 2017. The present study was approved by the institutional review board of the Medical University of Vienna (approval number: 1785/2016) and registered at the German Clinical Trials Registry (register number DRKS00012335).
Initial Immunosuppression and Rejection Therapy
All patients received triple immunosuppression with tacrolimus, mycophenolic acid, and corticosteroids. Calcineurin inhibitor drug trough levels (tacrolimus and cyclosporine) were monitored at every visit to the outpatient clinic. Our transplant protocol allowed for transplantation across immunologic barriers, including both human leukocyte antigen (HLA) and ABO incompatibilities. HLA incompatibility was defined as the presence of donor-specific anti-HLA antibodies detected by single antigen bead assay with a medium fluorescence intensity of 1000 or higher. Recipient of a HLA-incompatible kidney underwent IgG immunoapheresis according to a local protocol [16]. Recipients of an ABO-incompatible kidney underwent ABO blood group antigen specific immunoapheresis (Glycosorb; Glycorex Transplantation AB, Lund, Sweden) and, in cases of high AB antibody titers (>1:256), received additional induction therapy with an anti-CD20 antibody (Rituximab; Hoffmann-La Roche, Basel, Switzerland). Antibody-mediated and T-cellular rejection were scored according to current Banff classification [17]. T-cellular rejection type I and IIA and borderline rejection were treated with dexamethason and rejection type IIB and III were treated with antithymoglobulin. Antibody-mediated rejection was treated with IgG immunoapheresis.
Infection Prophylaxis and Monitoring
All patients received prophylaxis with trimethoprim and sulfamethoxazole for 6 months after transplantation and valganciclovir for 3 months in cytomegalovirus (CMV) IgG-negative recipients of a CMV IgG-positive organ or after treatment with antithymoglobulin and IgG immunoapheresis. Screening for CMV and BK polyomavirus after transplantation was performed by PCR from peripheral blood once per week until discharge from the ward, on the first visit at the outpatient clinic, on month 3 after transplantation, and every 3 months thereafter. The first visit at the outpatient clinic was within 1 week after discharge from the ward. Epstein Barr virus (EBV) PCR from peripheral blood was performed in EBV IgG-negative recipients on the first visit at the outpatient clinic, 1 month after transplantation, monthly until month 6 after transplantation, and every 3 months thereafter. We did not perform specific screening for other viral infections or bacterial and fungal infections.
Primary End Point
The primary end point was any bacterial, fungal, or viral infection requiring antimicrobial or antiviral treatment or reduction of immunosuppressive drugs in case of BK polyomavirus nephropathy (PVN). Asymptomatic bacteriuria was not treated with antibiotic drugs. Severe infections were defined as infections requiring hospitalization or prolongation of a hospital stay or PVN. PVN was defined according to current Banff classification [18].
TTV Quantification
TTV was quantified prospectively in the peripheral blood at the following time points: before transplantation and after transplantation once per week until discharge from the ward, on the first visit at the outpatient clinic, on month 3 after transplantation, and every 3 months thereafter. Attending physicians were unaware of the TTV results. TTV DNA was extracted from 200 μL of plasma using the NucliSENS easyMAG platform (bioMerieux, France), as recommended by the manufacturer, and eluted in 50 μL of elution buffer. TTV DNA was quantitated by TaqMan real time PCR, as described previously [19]. The quantitative PCR reactions were performed in a volume of 25 µL using 2 × TaqMan Universal PCR Master Mix, containing 5 µL of extracted DNA, 400 nM of each primer, and 80 nM of the probe. Thermal cycling was started for 3 minutes at 50°C, followed by 10 minutes at 95°C, and then by 45 cycles at 95°C for 15 seconds, at 55°C for 30 seconds, and at 72°C for 30 seconds, using the 7300 Real Time PCR System (Applied Biosystems, Foster City, CA). Results were recorded in copies/mL. Details on standards, negative and positive controls, and test performance have been described in detail earlier [13].
Statistical Analysis
Summaries for continuous variables are presented as the median and interquartile range (IQR). The Mann-Whitney U test was used for comparing continuous data. Categorical variables are presented as absolute numbers and percentages, and group comparisons were made using the Χ2 test. Logistic regression models were applied to assess whether TTV was independently associated with infection as the outcome. To allow for the correlation of more than 1 event per patient we used a random-effect model. We added the clinically most relevant covariables into the model, including recipient age, donor age, and estimated glomerular filtration rate (eGFR). We also tested if follow-up time influenced the main effect within the model. The likelihood ratio test was used to test for linearity and interaction with diabetes mellitus and recipient sex. Receiver operating curves were applied for calculation of the area under the curve. Generally, a 2-sided P value < .05 was considered statistically significant. Exact tests were used where applicable. We used MS EXCEL 2010 (Redmond, WA), IBM SPSS Statistics 20.0 (SPSS Inc., Chicago, IL) and STATA 14.1 (STAT coop., College Station, TX) for data management and analysis.
RESULTS
Patients and Transplant Outcome
This prospective study includes 169 consecutive adult recipients of a kidney allograft transplanted in 2016 at the Medical University of Vienna. Median follow-up was 218 days (IQR 133–316). Baseline characteristics are listed in Table 1. Median recipient age at transplantation was 55 years (IQR 43–64 years) and 51 (30%) patients were female. Thirty-six (21%) of the patients had a previous kidney transplant and 28 (17%) were recipients of a living donor kidney. Nine patients (5%) were transplanted across ABO and 17 (10%) across HLA antibody barriers. One hundred fifty-three patients (90%) received induction therapy with interleukin-2 receptor antagonist and 16 (10%) with antithymoglobulin.
Table 1.
Baseline Characteristics of the Total Study Cohort and the Event Cohort Stratified According to Patients With and Without Infections
| Characteristics | Total Cohort | Event Cohort | P Value | Infectiona | No Infectiona | P value |
|---|---|---|---|---|---|---|
| (n = 169) | (n = 71) | (n = 22) | (n = 49) | |||
| Recipient characteristics | ||||||
| Recipient age, years (IQR) | 55 (43–64) | 56 (43–64) | .885 | 59 (46–64) | 56 (43–63) | .442 |
| Recipient female, n (%) | 51 (30) | 19 (27) | .761 | 5 (23) | 16 (33) | .421 |
| Recipient BMI, kg/m2 (IQR) | 26 (23–29) | 26 (24–29) | .993 | 25 (23–28) | 26 (24–29) | .558 |
| Renal replacement therapy,b years (IQR) | 2.7 (1.6–4.4) | 2.6 (1.7–4.2) | .977 | 2.4 (1.4–4.5) | 2.7 (2.3–4.3) | .654 |
| Diabetes mellitus, n (%) | 24 (14) | 9 (13) | .839 | 2 (9) | 7 (14) | .711 |
| Major comorbidity,c n (%) | 84 (50) | 33 (47) | .673 | 12 (55) | 21 (43) | .443 |
| CMV IgG positive, n (%) | 120 (71) | 48 (68) | .877 | 15 (68) | 35 (71) | >.99 |
| EBV IgG positive, n (%) | 165 (97) | 68 (96) | >.99 | 20 (91) | 48 (98) | .225 |
| HCV antibody positive, n (%) | 3 (2) | 0 | >.99 | 0 | 0 | NA |
| HBc antibody positive, n (%) | 19 (11) | 8 (11) | >.99 | 3 (14) | 5 (10) | .696 |
| HIV antibody positive, n (%) | 4 (2) | 1 (1) | >.99 | 1 (5) | 0 | .310 |
| Donor characteristics | ||||||
| Living donor, n (%) | 28 (17) | 7 (10) | .329 | 2 (9) | 5 (10) | >.99 |
| Donor after circulatory death, n (%) | 13 (8) | 9 (13) | .327 | 1 (5) | 8 (16) | .257 |
| Donor age, years (IQR) | 55 (43–67) | 56 (45–67) | .542 | 57 (52–68) | 57 (45–68) | .817 |
| Donor female, n (%) | 82 (49) | 33 (46) | >.99 | 9 (41) | 24 (49) | .611 |
| Donor CMV IgG positive, n (%) | 117 (69) | 47 (66) | .648 | 16 (72) | 31 (63) | .589 |
| Transplant characteristics | ||||||
| Retransplantation, n (%) | 36 (21) | 12 (17) | .484 | 4 (18) | 8 (16) | >.99 |
| ABO incompatible transplantation, n (%) | 9 (5) | 2 (3) | .515 | 0 | 2 (4) | >.99 |
| Donor specific antibody, n (%) | 17 (10) | 4 (6) | .325 | 1 (5) | 3 (6) | >.99 |
| HLA A, B, DR mismatch, n (IQR) | 3 (2–4) | 3 (3–4) | .878 | 3 (2–4) | 3 (3–4) | .745 |
| ATG induction, n (%) | 16 (10) | 5 (7) | .625 | 1 (5) | 4 (8) | >.99 |
| CMV prophylaxis, n (%) | 58 (34) | 22 (31) | .882 | 8 (36) | 14 (29) | .583 |
Mann-Whitney U test was used for comparing continuous data and group comparisons were made using the Χ2 test. Exact tests were used where applicable.
Abbreviations: ATG, antithymoglobulin; BMI, body mass index; CMV, cytomegalovirus; EBV, Epstein Barr virus; HBc, hepatitis B-core antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; IgG immunoglobulin G; IQR, inter quartile range; NA, not applicable.
aFor comparison of the clinical baseline data within the event cohort we stratified patients according to their first documented event (infection or no infection).
bTime between the start of renal replacement therapy and transplantation.
cAny history of major cardiovascular, gastrointestinal, pulmonary, or malignant disease.
Patient survival was 94% and death censored graft survival was 92% at the end of the follow-up period (median 7 month, maximum 14 months). The main cause of death was infection (n = 6; 60%; Supplementary Table 1). Acute rejection was detected in 23 (14%) of the allograft recipients. All rejection episodes were documented within 3 months after transplantation (Supplementary Table 2).
Torque Teno Virus Kinetics
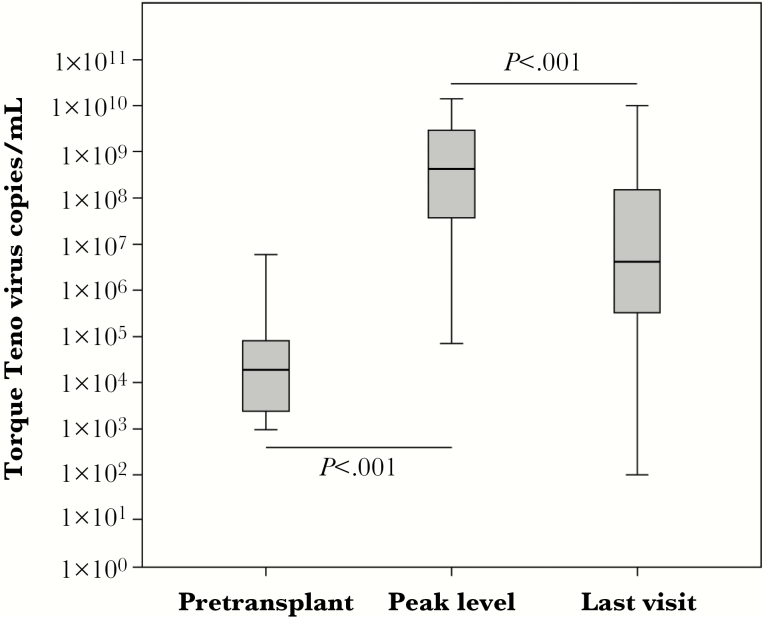
In total, 978 samples for TTV assessment were taken with a mean of 6 measurements per patient (Supplementary Figure 1). Before transplantation, 80% of the patients were TTV positive and all patients except 1 turned TTV positive within month 3 after transplantation. Before transplantation, a median of 1.9 × 104 TTV copies/mL (IQR 2.4 × 103–8.0 × 104) was detected (Figure 1). After transplantation, TTV load increased steeply up to a peak level of 4.3 × 108 TTV copies/mL (IQR 3.6 × 107–2.9 × 109; P < .001 compared to pretransplant levels) at month 3 posttransplantation (median day 92, IQR 87–170). Thereafter, TTV levels decreased modestly to a median of 4.2 × 106 TTV copies/mL (IQR 3.2 × 105–1.7 × 108) at the last available visit (day 269, IQR 183–318; P < .001 compared to TTV at peak level).
Figure 1.
Torque teno virus (TTV) copies/mL in peripheral blood of kidney transplant recipients are displayed in relation to detection time. Individual levels were combined in box plots. TTV load increased steeply after transplantation up to month 3 posttransplantation and showed a modest decrease at the last documented visit. The Mann-Whitney U test was performed for statistical comparison of TTV levels between time points in patients with full dataset.
Infections After Transplantation
To test the value of TTV quantification to predict infectious disease posttransplantation we included all TTV measurements taken after stabilization of TTV viremia (>day 92 posttransplantation) and analyzed the corresponding follow-up periods for the presence of infectious disease events (event cohort). Infectious disease occurred in 31 patients. We allowed for multiple events per patient. In total, 41 TTV measurements followed by infections and 83 TTV measurements with a subsequent follow-up period without infection were registered. Details on the involved pathogens and organ systems of the infections are displayed in Table 2. The majority of the infections were caused by bacteria (n = 26) or viruses (n = 12) and involved the urinary tract (n = 14) or the transplant kidney (n = 11).
Table 2.
Characteristics of Infectious Disease Events
| Type | Localization | Number | Pathogen | Number |
|---|---|---|---|---|
| Bacterial | Urinary tract infection | 14 | Escherichia coli | 7a |
| Enterococcus faecium | 3 | |||
| Pseudomonas aeruginosa | 2 | |||
| Klebsiella pneumoniae | 2b | |||
| Pneumonia | 5 | Streptococcus pneumoniae | 2 | |
| Aspergillus fumigatus | 1 | |||
| Pneumocystis jirovecii | 1 | |||
| Unknown | 1c | |||
| Soft tissue infection | 4 | Staphylococcus aureus | 2 | |
| Group A beta-hemolytic Streptococcus | 1 | |||
| Unknown | 1c | |||
| Colitis | 2 | Campylobacter jejuni | 1 | |
| Clostridium difficile | 1 | |||
| Otitis media | 1 | Streptococcus pneumoniae | 1 | |
| Viral | PVN | 11 | BK virus | 11 |
| Influenza A | 1 | Influenza A virus | 1 | |
| Fungal | Oesophagitis | 1 | Candida albicans | 1 |
| Fever of unknown origin | Unknown | 2 | Unknownd | 2 |
Abbreviation: PVN, polyomavirus nephropathy.
a Two infections were caused by multidrug-resistant bacteria.
b One infection was caused by a multidrug-resistant bacterium.
c Bacterial infection was diagnosed due to clinical and/or radiological findings.
d Clinical course was highly suggestive for bacterial infection.
Baseline characteristics of patients with and without infections at the time of transplantation are displayed in Table 1. No differences concerning risk factors for infections, including recipient age, frequency of diabetes mellitus or other major comorbidities, intensity of induction therapy, or CMV prophylaxis, were found in patients developing infectious diseases compared to patients without infections (Table 1).
Analyzing clinical data after transplantation, at the time of TTV assessment, we found higher C-reactive protein levels and more infections preceding an infection event compared to follow-up periods without infection (Table 3). No differences in allograft rejections or antirejection therapy, CMV viremia, prophylactic virostatic treatment, or type and amount of immunosuppressive drugs and calcineurin inhibitor trough level were noted in patients developing infections compared to patients without infection (Table 3).
Table 3.
Clinical Findings at the Time of Torque Teno Virus Assessment Posttransplantation, Stratified According to the Occurrence of Infection in the Subsequent Follow-up Period
| Characteristics | Infection | No Infection | P Value |
|---|---|---|---|
| (n = 41) | (n = 83) | ||
| Clinical data | |||
| Infection, n (%) | 13 (32) | 4 (5)c | <.001 |
| Allograft rejection,a n (%) | 9 (22) | 16 (19) | .813 |
| Antiviral treatment, n (%) | 1 (2) | 0 (0) | .331 |
| Laboratory data | |||
| CMV load, copies/mL (IQR) | 0 (0–140) | 0 (0–20) | .082 |
| Leukocytes, g/L (IQR) | 6.3 (4.9–8.7) | 6.6 (4.3–7.8) | .423 |
| Neutrophils, g/L (IQR) | 4.6 (3.8–7.5) | 2.9 (4.5–6.0) | .326 |
| Lymphocytes, g/L (IQR) | 1.2 (0.7–1.5) | 1.3 (0.8–1.8) | .229 |
| eGFR, mL/min/m2 (IQR)b | 47 (36–65) | 50 (34–68) | .563 |
| Urinary protein/creatinine ratio, mg/mg (IQR) | 201 (105–395) | 192 (98–350) | .642 |
| CRP, mg/dL (IQR) | 0.66 (0.27–2.81) | 0.27 (0.08–0.70) | .023 |
| Immunosuppression | |||
| Prednisolone, mg (IQR) | 5 (5–10) | 5 (5–11.25) | .174 |
| Cyclosporine, n (%) | 2 (5) | 6 (7) | .719 |
| Tacrolimus, n (%) | 72 (87) | 38 (93) | .384 |
| Tacrolimus once per day, n (%) | 11 (28) | 13 (16) | .149 |
| Tacrolimus trough level, ng/mL (IQR) | 7.3 (5.8–9.7) | 7.3 (5.6–8.8) | .524 |
| Everolimus, n (%) | 1 (2.4) | 2 (2.4) | >.99 |
| Belatacept, n (%) | 1 (2.4) | 4 (4.8) | .665 |
| Mycophenolic acid, n (%) | 40 (98) | 80 (98) | >.99 |
| Mycophenolic acid, g (IQR) | 2 (1–2) | 1 (1–2) | .842 |
Mann-Whitney U test was used for comparing continuous data and group comparisons were made using the Χ2 test. Exact tests were used where applicable.
Abbreviations: CMV, cytomegalovirus; CRP, C reactive protein; eGFR, estimated glomerular filtration rate; IQR, inter quartile range.
aAny episode of biopsy-proven allograft rejection or antirejection therapy preceding torque teno virus assessment.
beGFR was calculated using the Mayo equation [20].
cFour patients had an infection at the time of torque teno virus assessment, but did not develop an infection in the subsequent follow-up period.
Follow-up periods without infection occurred at a later time point posttransplantation compared to infections (264 days posttransplantation [IQR 189–292] vs 192 [IQR 139–267]; P = .003), but there was no difference in the time point of TTV assessment preceding infections and follow-up periods without infections (day 164 posttransplantation [IQR 105–195] vs 174 [IQR 104–189]; P = .292).
Torque Teno Virus Quantification in the Context of Infections After Transplantation
TTV levels detected before the occurrence of an infection were higher compared to levels preceding a follow-up period without infection (4.2 × 108 [IQR 2.7 × 107–1.9 × 109] vs 2.9 × 107 [IQR 1.0 × 106–7.2 × 108]; P = .006). Median time between TTV assessment and the event was 77 days (IQR 19–98). The odds ratio (OR) for an infection increased by 23% per log level increase of TTV copies/mL (95% confidence interval [CI], 1.04–1.45; P = .014) and showed a linear effect. Applying receiver operating statistics an area under the curve of 0.65 (95% CI, 0.55–0.75; P = .006) was calculated for the prediction of an infection by TTV (Supplementary Figure 2). TTV levels above 3.1 × 109 copies/mL corresponded to a sensitivity of 90%, specificity of 20%, negative predictive value of 92%, and positive predictive value of 17% for the prediction of an infection.
Sensitivity analysis in a subgroup of patients with severe infections showed higher TTV levels preceding severe infections compared to follow-up periods without infection (n = 33; 6.4 × 108 [IQR 2.2 × 107–2.0 × 109] vs 2.9 × 107 [IQR 1.0 × 106–7.2 × 108]; P = .012). Such difference was also noted in a second subgroup analysis restricted to all bacterial infections (n = 26; 4.4 × 108 [IQR 5.8 × 107–2.8 × 109] for infections vs 2.9 × 107 [IQR 1.0 × 106–7.2 × 108] for follow-up periods without infection; P = .003).
Patient Characteristic Associated With Torque Teno Virus Level
In order to uncover possible confounder of TTV load we analyzed clinical baseline data at the time of transplantation in the context of TTV quantification (Table 4). Older and male allograft recipients, patients without detectable CMV IgG, recipients of a graft from an older donor, and patients with a previous solid-organ transplant showed higher levels of TTV. TTV levels were not associated with frequency of diabetes mellitus or other major comorbidities, intensity of induction therapy, or CMV prophylaxis (Table 4).
Table 4.
Torque Teno Virus Levels Stratified According to Baseline Characteristics
| Characteristics | TTV Copies/mL (IQR) | P Value | |
|---|---|---|---|
| Variable Positivea | Variable Negativea | ||
| Recipient data | |||
| Recipient age >56 yearsb | 4.2 × 108 (1.8 × 107–2.9 × 109) | 3.2 × 107 (3.6 × 106–4.0 × 108) | .050 |
| Recipient female | 9.3 × 106 (1.8 × 106–3.4 × 108) | 3.4 × 108 (1.8 × 107–2.4 × 109) | .021 |
| Recipient BMI >26 kg/m2 b | 4.2 × 108 (3.4 × 106–1.7 × 109) | 6.8 × 107 (8.3 × 106–4.2 × 108) | .629 |
| Renal replacement therapy >2.6 yearsb, c | 5.2 × 107 (5.3 × 106–8.3 × 108) | 1.8 × 108 (3.3 × 106–2.7 × 109) | .589 |
| Diabetes mellitus | 1.7 × 107 (2.8 × 106–4.3 × 108) | 1.8 × 108 (5.0 × 106–1.5 × 109) | .243 |
| Major comorbidityd | 5.2 × 107 (4.0 × 106–1.5 × 109) | 1.4 × 108 (3.9 × 106–1.1 × 109) | .978 |
| CMV IgG positive | 2.9 × 107 (3.4 × 106–5.3 × 109) | 4.3 × 108 (1.8 × 108–2.9 × 109) | .003 |
| EBV IgG positive | 7.6 × 107 (4.1 × 106–1.4 × 109) | 3.8 × 108 (3.5 × 108–NA) | .415 |
| HBc antibody positive | 7.3 × 106 (3.6 × 106–1.4 × 108) | 2.6 × 108 (8.0 × 106–1.7 × 109) | .074 |
| HIV antibody/antigen positive | NA | NA | .366 |
| Donor data | |||
| Living donor | 9.9 × 107 (1.2 × 107–4.3 × 108) | 1.8 × 108 (4.1 × 106–1.4 × 109) | .884 |
| Donor after circulatory death | 1.7 × 108 (1.6 × 105–7.6 × 108) | 2.4 × 108 (7.3 × 106–1.8 × 109) | .203 |
| Donor age >56 yearsb | 4.2 × 108 (9.3 × 106–2.9 × 109) | 3.0 × 107 (3.8 × 106–4.2 × 108) | .014 |
| Donor female | 3.1 × 107 (3.9 × 106–1.6 × 109) | 2.2 × 108 (7.2 × 106–1.0 × 109) | .699 |
| Donor CMV IgG positive | 3.4 × 107 (3.8 × 106–2.1 × 109) | 4.2 × 108 (1.1 × 107–1.6 × 109) | .164 |
| Transplant data | |||
| Retransplantation | 1.0 × 107 (9.9 × 105–3.3 × 108) | 2.6 × 108 (9.3 × 106–1.7 × 109) | .041 |
| ABO incompatible transplantation | 1.0 × 106 (6.3 × 105–1.8 × 109) | 1.8 × 108 (7.3 × 106–1.3 × 109) | .633 |
| Donor specific antibody | 2.5 × 107 (1.8 × 106–2.1 × 108) | 1.8 × 108 (5.3 × 106–1.4 × 109) | .238 |
| HLA A, B, DR mismatch >3b | 4.7 × 107 (2.8 × 106–1.3 × 109) | 1.8 × 108 (7.3 × 106–1.3 × 109) | .486 |
| ATG induction | 1.9 × 107 (2.3 × 106–1.5 × 108) | 1.8 × 108 (5.2 × 106–1.5 × 109) | .193 |
| CMV prophylaxis | 4.0 × 108 (4.0 × 107–1.8 × 109) | 3.1 × 107 (3.7 × 106–1.2 × 109) | .067 |
Mann-Whitney U test was used for comparing continuous data and group comparisons were made using the Χ2 test. Exact tests were used where applicable.
Abbreviations: ATG, antithymoglobulin; BMI, body mass index; CMV, cytomegalovirus; EBV, Epstein Barr virus; HBc, hepatitis B-core antigen; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; IgG immunoglobulin G; IQR, inter quartile range; NA, not applicable; TTV, torque teno virus.
aTTV levels preceding the first documented event (infection or no infection) per patient were analyzed.
bCutoff defined by median.
cTime between the start of renal replacement therapy and transplantation.
dAny history of major cardiovascular, gastrointestinal, pulmonary, or malignant disease.
Analysis of clinical data posttransplantation, at the time of TTV assessment, revealed an associated of CMV viremia, low lymphocyte counts in the peripheral blood, and the use of high-dose mycophenolic acid (> 1.5 G) as an immunosuppressive drug with higher TTV levels (Table 5). No differences in TTV levels stratified according to allograft rejection and antirejection therapy, virostatic treatment, or type and amount of immunosuppressive drugs other than mycophenolic acid and calcineurin inhibitor trough levels were noted (Table 5). Increased TTV levels before transplantation and timing of TTV assessment or documentation of events (infection or no infection) were not associated with TTV level (Supplementary Table 3).
Table 5.
TTV Levels Stratified According to Clinical Characteristics at the Time of Assessment
| Characteristics | TTV Copies/mL (IQR) | P Value | |
|---|---|---|---|
| Variable Positive | Variable Negative | ||
| Clinical data | |||
| Infection | 1.3 × 108 (1.3 × 107–8.6 × 108) | 9.9 × 107 (2.5 × 106–1.4 × 109) | .953 |
| Allograft rejectiona | 5.2 × 107 (1.3 × 106–5.9 × 108) | 1.7 × 108 (3.4 × 106–1.7 × 109) | .370 |
| Antiviral treatment | NA | 9.9 × 107 (2.5 × 106–1.1 × 109) | >.99 |
| Diabetes mellitus | 4.3 × 107 (2.6 × 106–8.8 × 108) | 1.5 × 108 (2.7 × 106–1.4 × 109) | .806 |
| Laboratory data | |||
| CMV PCR positive | 3.8 × 108 (2.5 × 107–2.8 × 109) | 3.4 × 107 (1.3 × 106–8.5 × 108) | .014 |
| Leukocytes > 6.6 g/Lb | 1.7 × 108 (3.4 × 106–1.1 × 109) | 5.2 × 107 (2.4 × 106–1.2 × 109) | .841 |
| Neutrophiles > 4.5 g/Lb | 3.5 × 107 (1.9 × 106–5.4 × 108) | 2.4 × 107 (4.0 × 105–4.2 × 108) | .896 |
| Lymphocytes > 1.3 g/Lb | 4.4 × 106 (2.4 × 105–1.8 × 108) | 2.6 × 108 (1.2 × 107–1.3 × 109) | <.001 |
| eGFR > 48 mL/min/m2; c | 3.0 × 107 (1.3 × 106–6.4 × 108) | 3.1 × 108 (3.9 × 106–1.8 × 109) | .069 |
| Urinary protein/creatinine ratio >192 mg/mgb | 5.2 × 107 (2.9 × 106–9.6 × 108) | 3.0 × 108 (6.9 × 105–2.5 × 109) | .776 |
| CRP >0.3 mg/dLb | 3.6 × 107 (2.4 × 106–6.4 × 108) | 3.7 × 108 (3.4 × 106–2.5 × 109) | .080 |
| Immunosuppression | |||
| Prednisolone >5 mgb | 1.7 × 108 (1.1 × 107–8.9 × 108) | 9.9 × 107 (1.3 × 106–1.4 × 109) | .584 |
| Cyclosporine | 1.2 × 107 (2.0 × 105–9.2 × 108) | 1.5 × 108 (3.4 × 106–1.2 × 109) | .290 |
| Tacrolimus | 1.1 × 108 (3.4 × 106–1.3 × 109) | 1.0 × 108 (2.3 × 106–8.7 × 108) | .575 |
| Tacrolimus trough level >7.3 ng/mLb | 1.8 × 108 (2.2 × 106–1.9 × 109) | 5.1 × 107 (3.5 × 106–8.8 × 108) | .585 |
| Tacrolimus once per day | 3.8 × 108 (3.8 × 106–3.4 × 109) | 4.9 × 107 (2.0 × 106–8.5 × 108) | .098 |
| Everolimus | 1.8 × 107 (7.6 × 103 – NA) | 1.3 × 108 (2.9 × 106 – 1.2 × 109) | .307 |
| Belatacept | 7.2 × 108 (1.0 × 108 – 1.8 × 109) | 7.5 × 107 (2.3 × 106–1.1 × 109) | .263 |
| Mycophenolic acid | 1.5 × 108 (3.3 × 106–1.3 × 109) | 1.8 × 107 (7.6 × 103 – NA) | .299 |
| Mycophenolic acid > 1.5 gb | 2.6 × 107 (1.8 × 107–9.6 × 108) | 1.8 × 107 (4.8 × 105–1.3 × 109) | .040 |
Mann-Whitney U test was used for comparing continuous data and group comparisons were made using the Χ2 test. Exact tests were used where applicable.
Abbreviations: CMV, cytomegalovirus; CRP, C reactive protein; eGFR, estimated glomerular filtration rate; IQR, interquartile range; NA, not applicable; PCR polymerase chain reaction; TTV, torque teno virus.
aAny episode of biopsy-proven allograft rejection or antirejection therapy preceding torque teno virus assessment.
bCutoff defined by median.
c eGFR was calculated using the Mayo equation [20].
To test whether TTV was independently associated with infection as the outcome we applied logistic regression models (Table 6). After inclusion of the clinically most relevant confounder of TTV load described in the literature or detected in the present study (recipient age, donor age, eGFR at the time of TTV assessment, and follow-up time), the OR for prediction of infection by TTV levels were only marginally changed. In addition, we tested for interaction with the presence of diabetes mellitus or recipient sex and found no significant influence on the association of TTV levels and infections. Taken together logistic regression confirmed a robust and independent association of TTV levels and infection after kidney transplantation.
Table 6.
Logistic Regression Models to Test for an Independent Association of TTV With Infectious Disease
| Method | Variables | Odds Ratio | 95% CI | P Value |
|---|---|---|---|---|
| ln TTV | ||||
| Unadjusted | 1.23 | 1.04–1.45 | .014 | |
| Adjusted | Time to event | 1.23 | 1.04–1.45 | .011 |
| Recipient age, donor age, eGFR | 1.24 | 1.04–1.49 | .017 | |
| Recipient age, donor age, eGFR, DM = 0 | 1.27 | 1.01–1.80 | .043 | |
| Recipient age, donor age, eGFR, DM = 1 | 1.26 | 0.90–1.76 | .175 | |
| Recipient age, donor age, eGFR, recipient female = 0 | 1.22 | 0.99–1.50 | .066 | |
| Recipient age, donor age, eGFR, recipient female = 1 | 1.37 | 0.96–1.95 | .080 | |
Logistic regression models were applied to assess whether TTV was independently associated with infection as the outcome. To allow for the correlation of more than 1 event per patient, a random-effect model was used. The likelihood ratio test was used to test for linearity and interaction.
Abbreviations: CI, confidence interval; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; TTV, torque teno virus.
DISCUSSION
In the present study we were able to demonstrate a linear and independent association of TTV levels in the peripheral blood of kidney transplant recipients and subsequent infectious diseases. Patients with infections showed higher levels of TTV prior to the event compared to patients without infections. This association was still present after adjustment of the most relevant confounder of TTV levels. Moreover, a sensitivity analysis restricted to bacterial infections and severe infections confirmed the association between TTV and infectious disease. In addition, we demonstrated a value of TTV quantification to predict infectious disease. Most interestingly, TTV quantification could detect patients at risk for infections almost 3 months before the infection was clinically apparent. Taken together, our data suggest high levels of TTV reflect a state of intense immunosuppression after kidney transplantation, leading to an increased risk of infectious disease. Thus, TTV quantification might be a promising candidate to tailor immunosuppressive drugs after kidney transplantation and to reduce complications by infections.
Infection is still the main cause of death [1] and organ rejection the main cause of graft dysfunction after kidney transplantation in the contemporary era [21], underlining the importance of improved tailoring of immunosuppressive drugs. Indeed, also in the present cohort, infection was the main cause of death. Currently, surveillances of immunosuppression is guided mainly via measuring of drug trough levels of calcineurin inhibitors, although such measurements might not sufficiently mirror immune function [2]. Accordingly, increased calcineurin inhibitor levels were not associated with infections in our present study. Likewise other known risk factors for infection, including diabetes mellitus, were not useful for selection of patients at risk in our cohort. In this respect, quantification of the ubiquitous and apathogenic TTV might be an interesting strategy, as TTV levels have been associated with immunocompetence of the host [7, 8]. Indeed, in an earlier study we demonstrated an inverse association of TTV levels and allograft rejection at the time of diagnosis in a retrospective analysis of kidney transplant recipients [13]. In addition, Görzer and colleagues described higher TTV levels in the sera of lung transplant recipients developing infections compared to stable patients in a small retrospective study [11]. They calculated a high sensitivity of 90% and a low specificity of 50% for the diagnosis of infections for a cutoff of 2 × 109 TTV copies/mL. In our prospective study a sensitivity of 90% for the diagnosis of infections following kidney transplantation was calculated for a similar cutoff of 3.1 × 109 TTV copies/mL. Both our present study and the report by Görzer and colleagues [11] described a low specificity of TTV to detect infections. Therefore TTV measurement is not sufficient to accurately predict infectious disease after solid-organ transplantation, but rather define patients with a low risk for infection. Interventional studies are needed to test whether tapering of immunosuppressive drugs to reach a TTV level below 3.1 × 109 TTV copies/mL will reduce the occurrence of infectious disease after kidney transplantation.
TTV has a high prevalence of >90% in healthy subjects [4] and up to 100% in the transplant population [9]. Indeed >99% of patients in our cohort were TTV positive after transplantation. Kinetics of TTV levels in our study were similar to those observed after liver [14], heart [10], and lung [9] transplantation, that is a steep increase of TTV load up to month 3 after transplantation, followed by a smooth decline thereafter. Absolute TTV levels were higher in lung and lower in liver transplant recipients compared to patients after kidney transplantation [9, 14]. Such differences might reflect a more intense immunosuppression following lung and a less intense immunosuppression following liver transplantation compared to immunosuppression administered in kidney transplant patients.
TTV levels following kidney transplantation in our study were associated with recipient age and sex, a finding that has been described in previous literature [22]. Interestingly, in our cohort, patients without CMV IgG before transplantation and positive CMV viremia after transplantation had higher levels of TTV, suggesting an association between primary CMV infection and TTV infection. A link between CMV and TTV infection might be supported by earlier findings in healthy individuals, where higher TTV levels were described in CMV IgG-positive compared to IgG-negative individuals [22]. Conflicting data have been presented on the association of CMV and TTV levels in patients early after hematopoietic stem cell transplantation. One study described lower levels [23] and 2 studies higher levels of TTV in patients developing CMV infection [24, 25]. In our cohort TTV was not affected by antiviral treatment with valganciclovir, a finding that has been observed in recipients of a lung and heart allograft also [10]. Recipients of older kidney allografts showed higher TTV levels in our cohort, which might be explained by higher TTV loads within kidneys derived from older donors already prior to transplantation. The higher TTV levels detected in recipients of a retransplant in our cohort might reflect a more intense immunosuppression used in this population to counteract the increased risk for allograft rejection due to HLA presensitization. Higher levels of TTV were also described in our study in a group of patients treated with high doses of mycophenolic acid, which might reflect a more intense immunosuppression due to higher drug dosing. Indeed influence of antimetabolite dosing on TTV following kidney transplantation has been described in an earlier study of our group [13].
The major strength of the present study is the prospective design, the unselected cohort of consecutive kidney transplant recipients, the longitudinal TTV evaluation at predefined time points and the strict definition of the primary end point. Detailed and thorough recording of clinical characteristics allowed for the exclusion of possible confounder. One of the limitations of the study is the short follow-up. TTV levels for prediction of infection were included after TTV viremia stabilization at month 3 posttransplantation and the follow-up was limited to 14 months after transplantation. Secondly, the present data suggest high TTV levels to reflect intense immunosuppression, but did not prove causality.
Taken together our study provides evidence for the value of TTV quantification to predict infectious disease after kidney transplantation. Moreover, we defined a TTV level cutoff for tailoring of immunosuppressive drug dosing. Interventional studies would be required to prove the value of TTV guided immunosuppression compared to calcineurin inhibitor trough level guided immunosuppression to prevent infectious disease.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Authors’ contributions. R. S., M. S., K. D., F. E., Z. K., G. A. G., M. G. V., and S. R. participated in the data collection and writing the manuscript. E. P. and I. G. participated in TTV analysis and writing the manuscript. H. H. and G. A. B. participated in data analysis and writing the manuscript. G. B. participated in the research design, data analysis, and writing the manuscript.
Acknowledgments. The authors wish to thank Frederik Haupenthal and Florentina Dermuth for excellent support in clinical data management.
Financial support. This work was funded by the Medical Scientific Fund of the Major of the City of Vienna (grant number 14058 to G. B. and 17058 to M. S.); and by the Austrian Science Fund (grant number KLI 604-B31 to G. B.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Central European Meeting on Nephrology, Prague, December 2017.
References
- 1. Farrugia D, Cheshire J, Begaj I, Khosla S, Ray D, Sharif A. Death within the first year after kidney transplantation–an observational cohort study. Transpl Int 2014; 27:262–70. [DOI] [PubMed] [Google Scholar]
- 2. Andrews LM, Li Y, De Winter BCM, et al. Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opin Drug Metab Toxicol 2017; 13:1225–36. [DOI] [PubMed] [Google Scholar]
- 3. Focosi D, Antonelli G, Pistello M, Maggi F. Torquetenovirus: the human virome from bench to bedside. Clin Microbiol Infect 2016; 22:589–93. [DOI] [PubMed] [Google Scholar]
- 4. Vasilyev EV, Trofimov DY, Tonevitsky AG, Ilinsky VV, Korostin DO, Rebrikov DV. Torque teno virus (TTV) distribution in healthy Russian population. Virol J 2009; 6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen T, Väisänen E, Mattila PS, Hedman K, Söderlund-Venermo M. Antigenic diversity and seroprevalences of torque teno viruses in children and adults by ORF2-based immunoassays. J Gen Virol 2013; 94:409–17. [DOI] [PubMed] [Google Scholar]
- 6. Rocchi J, Ricci V, Albani M, et al. Torquetenovirus DNA drives proinflammatory cytokines production and secretion by immune cells via toll-like receptor 9. Virology 2009; 394:235–42. [DOI] [PubMed] [Google Scholar]
- 7. Okamoto H. History of discoveries and pathogenicity of TT viruses. Curr Top Microbiol Immunol 2009; 331:1–20. [DOI] [PubMed] [Google Scholar]
- 8. Burra P, Masier A, Boldrin C, et al. Torque teno virus: any pathological role in liver transplanted patients?Transpl Int 2008; 21:972–9. [DOI] [PubMed] [Google Scholar]
- 9. Görzer I, Jaksch P, Kundi M, Seitz T, Klepetko W, Puchhammer-Stöckl E. Pre-transplant plasma torque teno virus load and increase dynamics after lung transplantation. PLoS One 2015; 10:e0122975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Vlaminck I, Khush KK, Strehl C, et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell 2013; 155:1178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Görzer I, Haloschan M, Jaksch P, Klepetko W, Puchhammer-Stöckl E. Plasma DNA levels of torque teno virus and immunosuppression after lung transplantation. J Heart Lung Transplant 2014; 33:320–3. [DOI] [PubMed] [Google Scholar]
- 12. Focosi D, Macera L, Pistello M, Maggi F. Torque teno virus viremia correlates with intensity of maintenance immunosuppression in adult orthotopic liver transplant. J Infect Dis 2014; 210:667–8. [DOI] [PubMed] [Google Scholar]
- 13. Schiemann M, Puchhammer-Stöckl E, Eskandary F, et al. Torque teno virus load-inverse association with antibody-mediated rejection after kidney transplantation. Transplantation 2017; 101:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simonetta F, Pradier A, Masouridi-Levrat S, et al. ; Swiss Transplant Cohort Study (STCS) Torque teno virus load and acute rejection after orthotopic liver transplantation. Transplantation 2017; 101:e219–21. [DOI] [PubMed] [Google Scholar]
- 15. Görzer I, Jaksch P, Strassl R, Klepetko W, Puchhammer-Stöckl E. Association between plasma torque teno virus level and chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant 2017; 36:366–8. [DOI] [PubMed] [Google Scholar]
- 16. Bartel G, Wahrmann M, Regele H, et al. Peritransplant immunoadsorption for positive crossmatch deceased donor kidney transplantation. Am J Transplant 2010; 10:2033–42. [DOI] [PubMed] [Google Scholar]
- 17. Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 2018; 18:293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nickeleit V, Singh HK, Randhawa P, et al. ; Banff Working Group on Polyomavirus Nephropathy The Banff Working Group Classification of definitive polyomavirus nephropathy: morphologic definitions and clinical correlations. J Am Soc Nephrol 2018; 29:680–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maggi F, Pifferi M, Fornai C, et al. TT virus in the nasal secretions of children with acute respiratory diseases: relations to viremia and disease severity. J Virol 2003; 77:2418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004; 141:929–37. [DOI] [PubMed] [Google Scholar]
- 21. Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 2012; 8:348–57. [DOI] [PubMed] [Google Scholar]
- 22. Haloschan M, Bettesch R, Görzer I, Weseslindtner L, Kundi M, Puchhammer-Stöckl E. TTV DNA plasma load and its association with age, gender, and HCMV IgG serostatus in healthy adults. Age (Dordr) 2014; 36:9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Albert E, Solano C, Giménez E, et al. The kinetics of torque teno virus plasma DNA load shortly after engraftment predicts the risk of high-level CMV DNAemia in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2018; 53:180–7. [DOI] [PubMed] [Google Scholar]
- 24. Gilles R, Herling M, Holtick U, et al. Dynamics of torque teno virus viremia could predict risk of complications after allogeneic hematopoietic stem cell transplantation. Med Microbiol Immunol 2017; 206:355–62. [DOI] [PubMed] [Google Scholar]
- 25. Wohlfarth P, Leiner M, Schoergenhofer C, et al. Torquetenovirus dynamics and immune marker properties in patients following allogeneic hematopoietic stem cell transplantation: a prospective longitudinal study. Biol Blood Marrow Transplant 2018; 24:194–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.