Abstract
Background
Patients with tetralogy of Fallot (TOF) have increased risk of atrial arrhythmias.
Hypothesis
A measure of atrial dispersion, the P‐wave vector magnitude (Pvm), can identify patients at risk for perioperative atrial flutter (AFL) or intra‐atrial re‐entrant tachycardia (IART) in a large TOF cohort.
Methods
We performed a blinded, retrospective analysis of 158 TOF patients undergoing pulmonary valve replacement between 1997 and 2015. History of AFL/IART was documented using electrocardiogram, Holter monitor, exercise stress test, implanted cardiac device, and electrophysiology study. P‐R intervals, Pvm, QRS duration, and QRS vector magnitude were assessed from resting sinus‐rhythm 12‐lead electrocardiograms and identification of those with AFL/IART was determined.
Results
Fourteen patients (8.9%) were found to have AFL/IART. Pvm, QRS duration, and QRS vector magnitude significantly differentiated those with AFL/IART from those without on univariate analysis: 0.09 ± 0.04 vs 0.18 ± 0.07 mV, 161.3 ± 21.9 vs 137.7 ± 31.4 ms, and 1.2 (interquartile range, 1.0–1.2) vs 1.6 mV (1.0–2.3), respectively (P < 0.05 for each). The Pvm had the highest area under the ROC curve (0.88) and was the only significant predictor on multivariate analysis, with odds ratio of 0.02 (95% confidence interval: 0.01‐0.53). P‐R duration, MRI volumes, and right‐heart hemodynamics did not significantly differentiate those with vs those without AFL/IART.
Conclusions
In TOF patients undergoing pulmonary valve replacement, Pvm has significant value in predicting those with perioperative AFL/IART. These clinical features may help further evaluate TOF patients at risk for perioperative atrial arrhythmias. Prospective studies are warranted.
Keywords: Vectorcardiography, Tetralogy of Fallot, Atrial Flutter, Intra‐atrial Re‐entrant Tachycardia
1. INTRODUCTION
Tetralogy of fallot (TOF) patients have significant arrhythmia burden postoperatively, reported to be as high as 43.3% in some series, with association with sudden cardiac death.1 Atrial arrhythmias comprise most of the postoperative arrhythmia burden.1 Invasive risk stratification via programmed ventricular stimulation in TOF patients has been shown to have diagnostic and prognostic value for ventricular arrhythmia prediction; furthermore, some noninvasive measures, such as QRS duration (QRSd) and right ventricular (RV) volumes, have also demonstrated ventricular arrhythmia risk association.1 However, atrial arrhythmia risk association has been limited.2, 3, 4
Age at time of assessment, as well as at secondary repair, have been shown to be independent predictors of atrial arrhythmias, but these have limited predictive value.1, 5 Thus, although the clinical history remains important, it may be insufficient for predicting the risk of atrial arrhythmias. Identification of an independent predictor of atrial arrhythmias from the electrocardiogram (ECG) has yet to be identified in large cohorts of TOF patients in multivariate analyses, although QRSd has been shown to be a weak univariate predictor.1, 5
Vectorcardiographic principles have provided additional diagnostic6, 7 and prognostic8, 9, 10, 11, 12 information, building upon the traditional 12‐lead ECG. The QRS vector magnitude (QRSvm), a measurement of depolarization voltage dispersion, had better predictive value for ventricular arrhythmias compared with the QRSd or spatial QRS‐T angle in a small cohort of adult TOF patients; this association was independent of RV volume (as measured by magnetic resonance imaging [MRI]), late gadolinium enhancement, or hemodynamics.13 However, to date, there are no studies evaluating whether the magnitude of atrial depolarization in 3‐dimensional space, called the P‐wave vector magnitude (Pvm), has perioperative atrial risk stratification. The Pvm is calculated as:
We hypothesize that the Pvm (Figure 1), derived from a sinus‐rhythm ECG prior to pulmonary valve replacement (PVR), will predict an increased risk of atrial flutter (AFL) or intra‐atrial re‐entrant tachycardia (IART) in TOF patients in the perioperative period.
Figure 1.
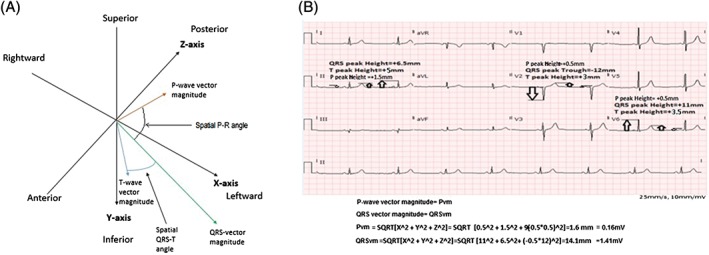
(A) Pvm, QRSvm, and 3‐dimentional angles between them. (B) Example of calculations for QRSvm and Pvm. Abbreviations: Pvm, P‐wave vector magnitude; QRSvm, QRS vector magnitude.
2. METHODS
2.1. Study population
This study was approved by the institutional review boards at the University of Colorado.
A blinded retrospective analysis was performed of 362 TOF patients from 1997 to 2015 at University of Colorado Hospital systems (including the Children's Hospital of Colorado). Of these patients, 158 TOF patients were included who were undergoing surgical PVR. Patients were excluded if they did not have a procedure for PVR or if they did not have an interpretable ECG with adequate baseline measurement within the 6 months prior to their PVR. Patients were also excluded if a diagnosis of TOF or TOF‐ like physiology was not certain or if they had left‐sided obstruction at the time of their PVR.
Patients who had sustained spontaneous perioperative AFL/IART arrhythmia burden (as determined by ECG, Holter monitoring, exercise stress testing, implantable cardioverter‐defibrillator, pacemaker, or telemetry monitoring) were identified. Sustained spontaneous AFL/IART is defined as ≥30 seconds or those that are associated with symptoms. For these patients, the arrhythmia had to have occurred postoperatively or within 6 months of their PVR. The last preoperative sinus‐rhythm ECG was used.
Comparisons were performed between TOF patients with spontaneous sustained AFL/IART vs those without sustained AFL/IART. Given the different mechanisms for atrial fibrillation and AFL/IART, these patient cohorts were separated to not confound the results of the comparisons. Atrioventricular nodal reentry tachycardia (AVNRT) was also separated from the AFL/IART groups, again to eliminate confounding. Power analyses performed demonstrated insufficient power to perform comparisons of the atrial fibrillation or AVNRT groups. Thus, only comparisons were made between those with AFL/IART and those without atrial arrhythmias.
2.2. Electrophysiology studies and ECGs
Sinus‐rhythm ECGs (GE Healthcare, Wauwatosa, WI or Philips, Eindhoven, Netherlands) were performed at a speed of 25 mm/s with 10 mm/mV for limb and precordial leads. The ECGs were analyzed within 6 months of PVR. Measurements of the Pvm (mV), QRSvm (mV), and P‐R and QRS durations (both in ms) were performed. The Pvm was calculated as the square root of the sum of the squared P‐wave magnitudes in leads V5, II, and one‐half of the P‐wave amplitude in V1: . This calculation is based on the P‐wave magnitude as defined by the visually transformed Kors quasi‐orthogonal method.14, 15 The QRSvm was calculated as the square root of the sum of the squared QRS‐wave magnitudes in leads V6, II, and one‐half of the QRS‐wave amplitude in V2 , which is based on the QRS‐wave magnitude as defined by the visually transformed Kors quasi‐orthogonal method.13, 14, 15 Spatial QRST and P‐R angles, as well as principal T‐wave component vector (RMS‐T), are shown in Figure 1A. Calculations of the above parameters are shown in Figure 1B. All measures were assessed based on digital caliper measures. Postoperative assessments were based on ECGs performed within 1 month of PVR.
2.3. MRI, echocardiograms, and cardiac catheterizations
MRI and echocardiographic data within 6 months of a patient's PVR were included in data analysis. MRI measurements of RV volumes were indexed to body surface area. Presence of gadolinium enhancement, pulmonary regurgitant fraction percentage, right and left ventricular end‐diastolic volumes (RVEDV and LVEDV), as well as right and left ventricular ejection fractions (RVEF and LVEF), were also assessed. Echocardiographic measures of LVEF, RA and LA diameters, RV and LV end‐diastolic diameters (RVEDD, LVEDD), physician report of severe pulmonary insufficiency, as well as moderate to severe tricuspid regurgitation, were also assessed. Hemodynamics from cardiac catheterization were included if they were measured within 6 months of the PVR. Cardiac catheterization data included left‐ and right‐sided end‐diastolic pressures and right atrial pressure (RAP).
2.4. Statistical analysis
Data was assessed for normality using Shapiro‐Wilk testing. Non–normally distributed continuous data are presented as median and interquartile range (IQR); normally distributed data are presented as mean ± SD. Student t testing, Mann–Whitney U testing, and contingency table testing were used to identify significant differences between groups. Significance was set at P < 0.05. Odds ratios (OR) were calculated to estimate risk for parameters identified as significantly different by comparative analysis.
Univariate analysis was performed to identify predictors associated with atrial arrhythmias. Multivariate analysis was performed using logistic regression analysis to identify independent risk factors for atrial arrhythmias. Selection of variables for inclusion in the multivariate analysis was made using a stepwise approach (P removal = 0.1).
Receiver operating characteristic (ROC) curves were performed to determine optimal cutoff values for variables, particularly the Pvm, in the prediction of atrial arrhythmias during the follow‐up period. The curve point with the highest sum of sensitivity and specificity was labeled as the optimal cutoff point and was utilized in OR, sensitivity, and specificity analyses.
Pearson and Spearman correlation coefficients were used as appropriate for parametric and nonparametric data. Intraobserver and interobserver variability were estimated by intraclass correlation coefficients based on a 10% sample of the population. Repeatability was performed by DC and NS. Data analysis was performed using SPSS software (IBM, Armonk, NY).
3. RESULTS
3.1. Clinical characteristics
One hundred and fifty‐eight patients with TOF met inclusion criteria; 136 patients with surgical PVR were included. Anteroposterior atriotomy incisions were used in ≥115 patients (72.8%); for the remaining patients, the atriotomy approach was not reported. Fifty‐nine MRIs were performed and 51 cardiac catheterizations were performed prior to PVR. Fourteen patients had AFL/IART (10.3%). Eight patients (4.5%) had atrial fibrillation and 7 patients (4.0%) had AVNRT. Twelve patients (7.1%) had documented sustained ventricular arrhythmias. One hundred and twenty‐two patients (77.2%) did not have any atrial arrhythmia burden.
3.2. Atrial arrhythmia identification
The Pvm significantly differentiated those with sustained AFL/IART (0.09 ± 0.04 mV) vs those without sustained AFL/IART (0.18 ± 0.07 mV; P < 0.001; Table 1 and Figure 2A). The positive and negative predictive values, using a cutoff of 0.12 mV, were 44.8% and 99.1%, respectively, with an OR of 86.1 (95% confidence interval [CI]: 10.5‐703.9). Table 2 provides statistical comparisons between TOF patients with and without perioperative AFL/IART, including area under the ROC curve (Figure 2B).
Table 1.
Clinical characteristics and demographics for patients with perioperative AFL/IART vs those without AFL/IART at time of PVR
| AFL/IART, n = 14 | No AFL/IART, n = 122 | P Value | |
|---|---|---|---|
| Age, y | 33.0 (18.5–40.0) | 15.0 (8.0–26.0) | 0.106 |
| Male sex | 6 (42.8) | 57 (46.7) | 1.000 |
| Syncope1 | 3 (21.4) | 5 (4.1) | 0.024 |
| Repeat PVR | 0 (0.0) | 7 (5.7) | 1.000 |
| Pvm, mV1 | 0.09 ± 0.04 | 0.18 ± 0.07 | <0.001 |
| P‐R, ms | 148.0 (135.0–172.0) | 152.0 (134.0–165.0) | 0.913 |
| QRSvm, mV1 | 1.2 (1.0–2.2) | 1.6 (1.2–2.3) | <0.001 |
| QRSd, ms1 | 161.3 ± 21.9 | 137.7 ± 31.4 | 0.002 |
| Echocardiographic parameters | |||
| Echocardiogram performed | 14 (100) | 134 (96.4) | 0.486 |
| LVEF, %1 | 52.1 ± 13.5 | 54.8 ± 12.8 | 0.578 |
| LVEDD, mm | 47.5 ± 11.7 | 44.8 ± 8.9 | 0.812 |
| RVEDD, mm | 34.9 ± 13.5 | 31.5 ± 10.1 | 0.121 |
| Severe PI | 10 (76.9) | 80 (66.7) | 0.547 |
| Moderate/severe TR | 5 (38.5) | 28 (23.3) | 0.308 |
| LA, mm | 51.2 ± 4.7 | 49.3 ± 5.1 | 0.189 |
| RA, mm | 56.2 ± 6.3 | 49.5 ± 3.9 | 0.071 |
| MRI performed | 6 (42.9) | 53 (43.4) | 1.000 |
| Pulmonary regurgitant fraction % | 41.2 ± 18.9 | 46.4 ± 11.5 | 0.918 |
| RVEDV, mL/m2 | 170.5 (163.3–196.9) | 176.4 (151.0–201.8) | 0.222 |
| LVEDV, mL/m2 | 76.2 ± 22.0 | 80.2 ± 16.7 | 0.556 |
| RVEF, % | 33.0 (25.3–43.0) | 42.5 (37.3–47.0) | 0.782 |
| LVEF, % | 57.0 (53.0–57.0) | 54.5 (48.0–58.3) | 0.089 |
| Gadolinium enhancement | 4/6 (66.7) | 27/53 (50.9) | 0.673 |
| Cardiac catheterization | 10 (71.4) | 41 (33.6) | 0.008 |
| Mean RAP, mm Hg | 11.9 ± 5.4 | 8.0 ± 2.8 | 0.087 |
| RVEDP, mm Hg | 12.0 ± 4.2 | 9.9 ± 3.0 | 0.172 |
| LVEDP, mm Hg | 13.0 (8.0–14.0) | 11.0 (8.0–14.5) | 0.779 |
Abbreviations: AFL, atrial flutter; IART, intra‐atrial re‐entrant tachycardia; IQR, interquartile range; LA, left atrial; LVEDD, left ventricular end‐diastolic diameter; LVEDP, left ventricular end‐diastolic pressure; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; PI, pulmonary insufficiency; P‐R, P‐R interval; Pvm, P‐wave vector magnitude; PVR, pulmonary valve replacement; QRSd, QRS duration; QRSvm, QRS vector magnitude; RA, right atrial; RAP, right atrial pressure; RVEDD, right ventricular end‐diastolic diameter; RVEDP, right ventricular end‐diastolic pressure; RVEDV, right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; SD, standard deviation; TR, tricuspid regurgitation.
Data are presented as n (%), mean ± SD, or median (IQR).
Significant differences.
Figure 2.
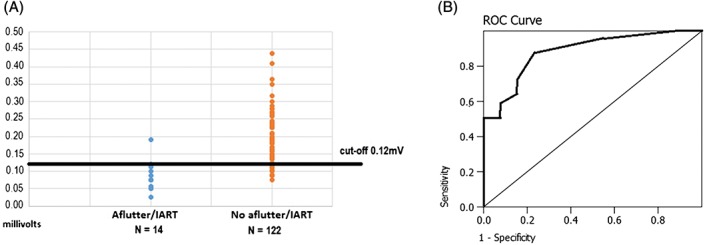
(A) Cutoff values for the Pvm, differentiating TOF patients with AFL/IART vs those patients without AFL/IART. (B) Area under the ROC curve analyses for the Pvm at 0.88 (95% CI: 0.81‐0.94). Abbreviations: AFL, atrial flutter; CI, confidence interval; IART, intra‐atrial re‐entrant tachycardia; Pvm, P‐wave vector magnitude; ROC, receiver operating characteristic; TOF, tetralogy of Fallot.
Table 2.
Statistical comparisons for perioperative AFL/IART vs no arrhythmias
| AFL/IART, n = 14 | No AFL/IART, n = 122 | ||||
|---|---|---|---|---|---|
| Optimum Cutoff Value | Sensitivity, n (%) | Specificity, n (%) | AUROC (95% CI) | P Value | |
| Syncope | Presence of syncope | 3 (21.4) | 117 (95.9) | NA | 0.024 |
| Pvm1 | 0.12 mV | 13 (92.9) | 106 (86.9) | 0.88 (0.81‐0.94) | 0.001 |
| QRSvm1 | 1.30 mV | 13 (92.9) | 87 (71.3) | 0.73 (0.58‐0.83) | 0.002 |
| QRSd1 | 155 ms | 12 (85.7) | 86 (70.5) | 0.65 (0.49‐0.82) | 0.100 |
Abbreviations: AFL, atrial flutter; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; IART, intra‐atrial re‐entrant tachycardia; NA, not applicable; Pvm, P‐wave vector magnitude; QRSd, QRS duration; QRSvm, QRS vector magnitude.
Significant differences.
In addition to the Pvm, the QRSvm also significantly differentiated patients with AFL/IART (1.2 mV; IQR, 1.0 to 1.2 mV) from those without AFL/IART (1.6 mV; IQR, 1.2 to 2.3 mV; P < 0.001). The positive and negative predictive values, using a cutoff of 12.8 mV, were 27.1% and 98.9%, respectively, with an OR of 32.3 (95% CI: 4.1‐256.5; Table 2).
Furthermore, the QRSd was also significantly different between patients with AFL/IART (161.3 ± 21.9 ms) vs those without AFL/IART (137.7 ± 31.4 ms; P = 0.002; Table 1). The positive and negative predictive values, using a cutoff of 155 ms, were 25.0% and 97.7%, respectively, with an OR of 14.3 (95% CI: 3.1‐67.3; Table 2).
Finally, a history of syncope was also statistically different for the 2 groups of patients. Three of 14 patients with a history of AFL/IART also had a history of syncopal events (21.4%), compared with 5 out of 122 (4.1%) patients without AFL/IART (P = 0.024).
P‐R duration, MRI pulmonary regurgitant fraction, MRI measured cardiac volumes, ejection fraction, right/left heart hemodynamics, and echocardiographic parameters were not statistically different for those with and without AFL/IART.
3.3. Univariate and multivariate analyses
Univariate predictors include a history of syncope, Pvm, and QRSvm. On multivariate analysis, only the Pvm remained a significant predictor of AFL/IART (P = 0.038; Table 3). The multivariate OR for the Pvm was 0.02 (95% CI: 0.01‐0.53).
Table 3.
Univariate and multivariate analyses for all parameters evaluated for identification of patients with perioperative AFL/IART vs those without AFL/IART at time of PVR
| OR (95% CI) | P Value | |
|---|---|---|
| Univariate | ||
| Age | 1.03 (0.99‐1.06) | 0.100 |
| Male sex | 1.14 (0.35‐3.73) | 0.828 |
| Syncope1 | 8.50 (2.29‐41.44) | 0.005 |
| Repeat PVR | NA | NA |
| Pvm1 | 0.05 (0.01‐0.63) | <0.001 |
| P‐R, ms | 1.01 (0.99‐1.02) | 0.238 |
| QRSvm1 | 0.87 (0.77‐0.98) | 0.022 |
| QRSd1 | 1.01 (0.99‐1.03) | 0.462 |
| LVEF1 | 0.96 (0.56‐2.19) | 0.819 |
| LVEDD | 1.10 (0.81‐2.56) | 0.520 |
| RVEDD | 1.05 (0.59‐1.35) | 0.121 |
| Severe PI | 1.03 (0.71‐1.89) | 0.739 |
| Moderate/severe TR | 1.04 (0.85‐1.33) | 0.689 |
| LA | 1.02 (0.89‐1.29) | 0.239 |
| RA | 1.33 (0.95‐2.33) | 0.101 |
| Pulmonary regurgitant fraction % | 1.34 (0.85‐2.12) | 0.207 |
| RVEDV | 0.99 (0.96‐1.02) | 0.616 |
| LVEDV | 1.03 (0.95‐1.11) | 0.538 |
| RVEF | 0.92 (0.80‐1.07) | 0.290 |
| LVEF | 0.88 (0.74‐1.06) | 0.174 |
| Gadolinium enhancement | 1.50 (0.14‐15.87) | 0.736 |
| RAP1 | 0.71 (0.30‐1.65) | 0.422 |
| RVEDP | 1.04 (0.77‐1.40) | 0.801 |
| LVEDP | 1.26 (0.99‐1.61) | 0.057 |
| Multivariate | ||
| Age | 1.08 (0.99‐1.18) | 0.069 |
| Pvm1 | 0.02 (0.01‐0.53) | 0.038 |
| QRSvm | 0.74 (0.78‐1.18) | 0.741 |
| LVEDP | 1.25 (0.92‐1.71) | 0.158 |
Abbreviations: AFL, atrial flutter; CI, confidence interval; IART, intra‐atrial re‐entrant tachycardia; LA, left atrium; LVEDD, left ventricular end‐diastolic diameter; LVEDP, left ventricular end‐diastolic pressure; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; NA, not applicable; OR, odds ratio; Pvm, P‐wave vector magnitude; PVR, pulmonary valve replacement; QRSd, QRS duration; QRSvm, QRS vector magnitude; RA, right atrium; RAP, right atrial pressure; RVEDD, right ventricular end‐diastolic diameter; RVEDP, right ventricular end‐diastolic pressure; RVEDV, right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; TR, tricuspid regurgitation.
Significant differences.
3.4. Correlation evaluations
Older age was significantly associated with lower Pvm (r = −0.21), longer P‐R intervals (r = 0.31), lower QRSvm (r = −0.42), and longer QRSd (r = 0.52). Pvm was also significantly inversely associated with longer QRSd (−0.19), higher RAP (−0.41), LVEF (−0.443), and RVEF (−0.33). Those patients with repeat PVRs had significantly longer P‐R interval correlation coefficients (r = −0.50) and lower RVEF correlation coefficients. RVEDV was significantly correlated to RVEF (r = 0.58).
Finally, Pvm significantly correlated with QRSvm (r = 0.27), though it was inversely correlated with QRSd (r = −0.22), LVEDV (r = −0.43), and RAP (r = −0.32).
Intraclass correlation coefficients for the Pvm interobserver and intraobserver variability were 0.91 and 0.94, respectively, based on 10% of the sample. Intraclass correlation coefficients for the QRSvm have previously been described.13 Preoperative and postoperative differences in Pvm were a median value of −0.21 mV (IQR, −0.47 to 0.14) for those without AFL/IART and were a median value of −0.13 mV (IQR, −0.635 to 0.23) for those with AFL/IART, without significant differences noted.
4. DISCUSSION
4.1. Study results
AFL or IART arrhythmias are associated with greater dispersion of atrial depolarization as measured by an electrovectorcardiographic marker, the Pvm. In this study, the Pvm was found to be the only significant predictor on multivariate analysis for perioperative AFL/IART.
Similar to the risk of ventricular arrhythmias, the risk for developing AFL/IART was not associated with end‐diastolic ventricular pressures.13 However, Pvm was significantly associated with RAP. As the magnitude of the P‐wave vector decreases, indicating increased dispersion of the P wave, this correlated to increased RAPs and inversely correlated to LVEF, possibly due to RV‐LV interactions.
4.2. Other electrocardiographic parameters
Ventricular dispersion of depolarization, as measured by the QRSvm, was also a univariate predictor of AF/IART, but with lower ORs. QRSd was not a predictor of atrial arrhythmias on multivariate analysis, as similarly reported.1, 5 Controlling for age, QRSd was not statistically significant; hence, the clinical use of QRSd in predicting AFL/IART may be age‐dependent and may be more useful in adults with TOF undergoing PVR. The P‐R interval was not significantly different in those with AFL/IART, compared with those without AFL/IART, although a trend toward longer P‐R durations was present in those TOF patients with AFL/IART.
4.3. Other studies on perioperative predictors of atrial arrhythmias
Perioperative atrial arrhythmias after coronary artery bypass grafting have been associated with increased age, male sex, low LVEF, RA size, and increased P‐R duration.16 Most of these predictors correlated to the risk of atrial fibrillation rather than AFL/IART, which is more likely to be pertinent in a TOF population. QRSd, as demonstrated in various TOF cohorts, is a univariate predictor of atrial arrhythmias; whereas P‐R prolongation has also been shown to be a univariate predictor of atrial arrhythmias in TOF patients late after repair.1, 5, 17
4.4. Clinical significance
The Pvm has a high negative predictive value and a reasonable positive predictive value for identification of AFL/IART. Thus, if the Pvm is >0.12 mV, the odds of having AFL/IART are low. However, if the Pvm is <0.12 mV, the risk of AFL/IART increases and may inform subsequent management in the perioperative period. Thus, perioperative atrial arrhythmias can be risk‐stratified based on a simple calculation of atrial voltage dispersion (Pvm). Prospective studies are warranted and this association needs to be validated in larger populations. Nevertheless, to our knowledge, this is the first study to demonstrate an association of perioperative atrial arrhythmias to voltage dispersion of the atria in a large cohort of TOF patients undergoing PVR.
4.5. Study limitations
The retrospective nature of this study is a limitation, including limited details regarding surgical approaches given different reporting/documentation by multiple surgeons. Furthermore, not all patients with post‐repair TOF were evaluated; only those undergoing PVR were studied. MRI and catheterization data were not available for all patients. ECG at one time point may not be reflective of a dynamic disease process. Temporal changes in vectorcardiographic parameters may impact the association with atrial arrhythmia risk over longer periods of follow‐up. Other limitations include the fact that first‐time and repeat PVR patients were included.
5. CONCLUSION
The Pvm, a measure of dispersion of atrial depolarization, was a significant independent predictor of perioperative AFL/IART in our cohort of TOF patients undergoing PVR. Prospective studies may help to better define its role in risk stratification for perioperative atrial arrhythmias, both in TOF patients and possibly in other populations.
Conflicts of interest
Drs. Sauer and Nguyen receive significant research grants from Biosense Webster and CardioNXT and educational grants from St. Jude Medical, Boston Scientific, and Medtronic. The authors declare no other potential conflicts of interest.
Cortez D, Barham W, Ruckdeschel E, et al. Noninvasive predictors of perioperative atrial arrhythmias in patients with tetralogy of Fallot undergoing pulmonary valve replacement. Clin Cardiol. 2017;40:591–596. 10.1002/clc.22707
REFERENCES
- 1. Khairy P, Aboulhosn J, Gurvitz M, et al; Alliance for Adult Research in Congenital Cardiology . Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi‐institutional study. Circulation. 2010;122:868–875. [DOI] [PubMed] [Google Scholar]
- 2. Khairy P, Landzberg MJ, Gatzoulis MA, et al. Value of programmed ventricular stimulation after tetralogy of fallot repair: a multicenter study. Circulation. 2004;109:1994–2000. [DOI] [PubMed] [Google Scholar]
- 3. Bassareo PP, Mercuro G. QRS complex enlargement as a predictor of ventricular arrhythmia in tetralogy of Fallot: a comprehensive literature review and historical review. ISRN Cardiol. 2013;782508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Menon SC, Kaza AK, Puchalski MD. Effect of ventricular size and function on exercise performance and the electrocardiogram in repaired tetralogy of Fallot with pure pulmonary regurgitation. Ann Pediatr Cardiol. 2012;5:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gatzoulis MA, Balaji S, Webber S, et al. Risk factors for arrhythmias and sudden cardiac death late after repair of tetralogy of Fallot: a multicenter study. Lancet. 2016;356:975–981. [DOI] [PubMed] [Google Scholar]
- 6. Triola B, Olson MB, Reis SE, et al. Electrocardiographic predictors of cardiovascular outcome in women: the National Heart, Lung, and Blood Institute‐sponsored Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 2005;46:51–56. [DOI] [PubMed] [Google Scholar]
- 7. Voulgari C, Tentolouris N, Moyssakis I, et al. Spatial QRS‐T angle: association with diabetes and left ventricular performance. Eur J Clin Invest. 2006;36:608–613. [DOI] [PubMed] [Google Scholar]
- 8. Borleffs CJ, Scherptong RW, Man SC, et al. Predicting ventricular arrhythmias in patients with ischemic heart disease: clinical application of the ECG‐derived QRS‐T angle. Circ Arrhythm Electrophysiol. 2009;2:548–554. [DOI] [PubMed] [Google Scholar]
- 9. de Torbal A, Kors JA, van Herpen G, et al. The electrical T‐axis and the spatial QRS‐T angle are independent predictors of long‐term mortality in patients admitted with acute ischemic chest pain. Cardiology. 2004;101:199–207. [DOI] [PubMed] [Google Scholar]
- 10. Kardys I, Kors JA, van der Meer IM, et al. Spatial QRS‐T angle predicts cardiac death in a general population. Eur Heart J. 2003;24:1357–1364. [DOI] [PubMed] [Google Scholar]
- 11. Rautaharju PM, Ge S, Nelson JC, et al. Comparison of mortality risk for electrocardiographic abnormalities in men and women with and without coronary heart disease (from the Cardiovascular Health Study). Am J Cardiol. 2006;97:309–315. [DOI] [PubMed] [Google Scholar]
- 12. Yamazaki T, Froelicher VF, Myers J, et al. Spatial QRS‐T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–78. [DOI] [PubMed] [Google Scholar]
- 13. Cortez D, Ruckdeschel E, McCanta AC, et al. Vectorcardiographic predictors of ventricular arrhythmia inducibility in patients with tetralogy of Fallot. J Electrocardiol. 2015;48:141–144. [DOI] [PubMed] [Google Scholar]
- 14. Kors JA, van Herpen G, Sitig AC, et al. Reconstruction of the Frank vectorcardiogram from standard electrocardiographic leads. Eur Heart J. 1990;11:1083–1092. [DOI] [PubMed] [Google Scholar]
- 15. Cortez D, Sharma N, Devers C, et al. Visual transform applications for estimating the spatial QRS‐T angle from the conventional 12‐lead ECG: Kors is still most Frank. J Electrocardiol. 2014;47:12–19. [DOI] [PubMed] [Google Scholar]
- 16. Kolek MJ, Muehlschlegel DJ, Bush WS, et al. A combined genetic and clinical risk prediction model for postoperative atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosianu S, Paprika D, Osztheimer I, et al. Echocardiographic evaluation of patients with undocumented arrhythmias occurring in adults late after repair of tetralogy of Fallot. Eur J Echocardiogr. 2009;10:139–143. [DOI] [PubMed] [Google Scholar]


