Abstract
Background
Heart rate has been associated with prognosis in patients with heart failure with reduced ejection fraction (HFREF) and sinus rhythm; whether this also holds true in patients with atrial fibrillation (AF) is unknown.
Hypothesis
To evaluate cardiac rhythm and baseline heart rate and the influence of outcome in patients with HFREF enrolled in the Cardiac Insufficiency Bisoprolol Study II.
Methods
In total, 2539 patients were stratified according to their baseline heart rhythm (AF or sinus rhythm) and into quartiles of heart rate (≤70 bpm, 71–78 bpm, 79–90 bpm, and >90 bpm). The primary outcome was all‐cause mortality. Mean follow‐up was 1.3 years.
Results
Mean age was 61 years, mean left ventricular ejection fraction was 28%, and 80% were male. A total of 521 (21%) patients had AF at baseline. The risk associated with all‐cause mortality for each 5 bpm increase in heart rate in patients with sinus rhythm (hazard ratio [HR]: 1.06, 95% confidence interval [CI]: 1.01‐1.11, P = 0.012) was significantly different from those with AF (HR: 1.00, 95% CI: 0.94‐1.07, P = 0.90, P for interaction = 0.041). The risk associated with higher heart rate in sinus rhythm was primarily attributable to excess risk in the highest quartile (HR: 1.64, 95% CI: 1.18–2.30, P = 0.003). Allocation to bisoprolol did not modify the interaction between heart rate, rhythm and outcome.
Conclusions
In HFREF patients with AF, a higher heart rate is not associated with increased event rates in contrast to HFREF patients with sinus rhythm.
Keywords: Atrial Fibrillation, Heart Failure, Heart Rate, Bisoprolol, Cardiovascular Outcome
1. INTRODUCTION
Lower heart rate has been associated with better outcome in general and several cardiovascular diseases.1, 2, 3, 4, 5 Moreover, in patients with heart failure and reduced ejection fraction (HFREF) and sinus rhythm, it has shown that a lower heart rate is associated with a better outcome. However, observations that a lower heart rate for patients with atrial fibrillation (AF) might be more beneficial are indifferent.6, 7, 8, 9 A major concern of a high heart rate in patients with AF has always been the development of heart failure (HF).10, 11, 12 Only 1 trial with predominantly heart failure with a preserved ejection fraction indicated that a low heart rate was not inferior regarding to cardiovascular events.8, 13 Whether this is also applicable to HF patients with reduced ejection fraction is unknown. Two recent meta‐analyses showed that in patients with AF, despite similar heart rate reduction with β‐blocker therapy, β‐blocker therapy did not improve prognosis in contrast to patients with sinus rhythm.14, 15 This suggests that a low heart rate may not be the ultimate therapeutic goal for HFREF patients with AF.14
The relationship between baseline heart rate, and especially the interaction with AF has not been studied in HFREF. Therefore, to further elucidate the association between heart rate, underlying cardiac rhythm (AF vs sinus rhythm) and cardiovascular outcome, we studied the relationship and interaction between baseline heart rate, cardiac rhythm (AF vs sinus rhythm) and cardiovascular morbidity and mortality in the Cardiac Insufficiency Bisoprolol Study (CIBIS) II.
2. METHODS
2.1. Study design
Demographics and the main results of the CIBIS II trial have been published.16 Briefly, CIBIS II was a multicenter, double‐blind, randomized, placebo‐controlled trial in Europe, which enrolled 2647 symptomatic patients in New York Heart Association (NYHA) class III or IV, with left ventricular ejection fraction (LVEF) of <35%, and who were randomly assigned to bisoprolol or placebo.16, 17 In CIBIS II, AF was diagnosed on electrocardiogram (ECG), and heart rate was assessed by pulse rate measurement or on ECG. Heart rate was measured at baseline on ECG recording at rest in the supine position or using pulse‐rate measurements. Each recorded value of heart rate was the mean of 3 measurements at each visit.17 For the present analysis, patients were divided at into 4 groups according to baseline heart rate: ≤70 bpm, 71 to 78 bpm, 79 to 90 bpm, and >90 bpm. Additional stratification was performed according to their underlying cardiac rhythm: AF or sinus rhythm. Patients with a pacemaker or undetermined rhythm were excluded for this analysis. A total of 108 patients had an undefined or pacemaker rhythm and were excluded. Therefore, a total of 2539 patients were available for this analysis. It was previously shown that the mortality risk ratio for bisoprolol vs placebo in sinus rhythm was 0.577 and 1.161 in AF.17
2.2. Outcomes
The primary endpoint was all‐cause mortality. Several secondary endpoints were used similar to the main study. These included a combined endpoint of cardiovascular death and admission to a hospital for cardiovascular events and sudden cardiac death. All endpoints in CIBIS 2 were adjudicated by a critical events committee.16
2.3. Statistical analysis
Baseline descriptive statistics are presented as mean ± standard deviation or median (range) for continuous variables, and counts with percentages for categorical variables. Differences between groups at baseline were evaluated by analysis of variance (ANOVA) or Kruskal‐Wallis tests for continuous variables, depending on normality of the data. A Pearson χ2 test was used for comparison of categorical variables. Cox proportional hazard analysis was used to investigate the prognostic importance of heart rate in the entire study population and after stratification based on heart rhythm (AF and sinus rhythm). To evaluate the interaction between baseline heart rhythm and baseline heart rate, an interaction analysis was carried out using an interaction term of heart rate (continuous) × heart rhythm (AF or sinus rhythm). To evaluate whether allocation to bisoprolol treatment modified the association between heart rate and outcome, we also evaluated the interaction between heart rate and bisoprolol allocation in an interaction analysis (heart rate × bisoprolol allocation) for patients with AF and sinus rhythm separately. Cox proportional hazard assumption was checked by the statistical test of Schoenfeld residuals. Multivariate adjustment was carried out using a panel of covariates as published before,18 and included age, gender, race, LVEF, estimated glomerular filtration rate, systolic and diastolic blood pressure, NYHA class, electrocardiographic abnormalities, history of diabetes, hypertension, cerebrovascular disease, peripheral artery disease or myocardial Infarction, etiology of HF, digoxin treatment, and randomized bisoprolol allocation. Secondary analyses included the prognostic value of quartiles of heart rate in each rhythm group and the interaction with bisoprolol treatment. A 2‐tailed P value <0.05 was considered significant. Statistical analyses were performed using Stata version 12.0 (StataCorp, College Station, TX).
3. RESULTS
3.1. Patients
Patients were divided into 4 groups according to baseline heart rate. Of 2539 patients enrolled, 738 (29.1%) had a heart rate ≤70 bpm, 550 (21.7%) between 71 and 78 bpm, 712 (28.0%) between 79 and 90 bpm, and 539 (21.2%) >90 bpm. Overall, 521 (21%) had AF at baseline. Patients with higher heart rates more often had AF, were younger, had lower LVEF, less often had a history of myocardial infarction, and more frequently used digoxin or amiodarone (Table 1). No differences in the original study arm allocation (bisoprolol vs placebo) was observed among the 4 heart rate categories. The interaction between baseline heart rhythm with diabetes or smoking status was evaluated, and no significant interactions were observed.
Table 1.
Baseline characteristics according to quartiles of baseline heart rate
| Variable | Baseline Heart Rate | P Value | |||
|---|---|---|---|---|---|
| Quartile 1, ≤70 bpm | Quartile 2, 71–78 bpm | Quartile 3, 79–90 bpm | Quartile 4, >90 bpm | ||
| No. | 738 | 550 | 712 | 539 | |
| Atrial fibrillation, % | 14 | 15 | 21 | 35 | <0.001 |
| Heart rate, bpm | 65 (62–68) | 75 (72–76) | 84 (80–88) | 100 (96–109) | <0.001 |
| Age, y | 62 ± 10 | 61 ± 10 | 61 ± 11 | 58 ± 11 | <0.001 |
| Gender, male, % | 84 | 79 | 78 | 81 | 0.011 |
| SBP, mm Hg | 130 ± 19 | 130 ± 18 | 130 ± 19 | 130 ± 21 | 0.88 |
| DBP, mm Hg | 79 ± 11 | 79 ± 11 | 80 ± 11 | 82 ± 12 | <0.001 |
| LVEF, % | 28 ± 6 | 28 ± 6 | 27 ± 6 | 26 ± 6 | <0.001 |
| eGFR, mL/min/1 · 73m2 | 77 ± 28 | 76 ± 31 | 78 ± 33 | 83 ± 33 | <0.001 |
| NYHA class, III/IV | 85/15 | 85/15 | 86/14 | 76/24 | <0.001 |
| Medical history | |||||
| Diabetes | 9% | 11% | 13 | 14 | 0.049 |
| Hypertension | 40% | 47% | 44 | 45 | 0.10 |
| Myocardial infarction | 65% | 61% | 53 | 40 | <0.001 |
| CVA | 8% | 8% | 5 | 9 | 0.036 |
| PAD | 7% | 8% | 7 | 8 | 0.91 |
| Medical treatment | |||||
| Digoxin | 42% | 46% | 56% | 64% | <0.001 |
| Amiodarone | 19% | 15% | 12% | 9% | <0.001 |
| Bisoprolol allocation | 51% | 51% | 49% | 49% | 0.77 |
Abbreviations: CVA, cerebrovascular accident; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PAD, peripheral artery disease; SBP, systolic blood pressure.
3.2. Heart rate and underlying heart rhythm
Table 2 shows the association between heart rate (per 5 beats per minute) and the primary and secondary outcomes adjusted for multiple clinical covariates. Higher heart rate was a prominent predictor of all‐cause mortality in patients with sinus rhythm (hazard ratio [HR] 1.06, 95% confidence interval [CI]: 1.01‐1.11, P = 0.012, per 5 bpm), but was not in patients with AF (HR: 1.00, 95% CI: 0.94‐1.07, P = 0.90), which was significantly different (interaction P value = 0.041). This difference between AF and sinus rhythm was even greater for the secondary (combined) endpoint, where patients in sinus rhythm showed an increased risk (HR: 1.05, P = 0.002), whereas this was absent for patients in AF (HR: 0.97, P = 0.19, interaction P value = 0.001).
Table 2.
Multivariate association between baseline heart rate (per 5 bpm), rhythm, and clinical outcome
| Endpoint | Events, n/N (%) | Atrial Fibrillation | Events, n/N (%) | Sinus Rhythm | P Value Interaction | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) Heart Rate, per 5 bpm | P Value | HR (95% CI) Heart Rate, per 5 bpm | P Value | ||||
| All‐cause mortality | 85/521 (16) | 1.00 (0.94‐1.07) | 0.90 | 275/2018 (14) | 1.06 (1.01‐1.11) | 0.012 | 0.041 |
| Combined endpoint | 177/521 (34) | 0.97 (0.93‐1.02) | 0.19 | 633/2018 (31) | 1.05 (1.02‐1.08) | 0.002 | 0.001 |
| CV death | 58/521 (11) | 1.00 (0.92‐1.08) | 0.92 | 204/2018 (10) | 1.06 (1.00‐1.11) | 0.040 | 0.077 |
| CV hospitalization | 153/521 (29) | 0.96 (0.91‐1.01) | 0.10 | 545/2018 (27) | 1.05 (1.02‐1.08) | 0.003 | <0.001 |
| Sudden death | 29/521 (6) | 0.97 (0.87‐1.08) | 0.57 | 98/2018 (5) | 1.04 (0.97‐1.13) | 0.27 | 0.28 |
Abbreviations: CI, confidence interval; CV, cardiovascular; HR, hazard ratio.
Adjusted for age, gender, race, left ventricular ejection fraction, estimated glomerular filtration rate, systolic and diastolic blood pressure, New York Heart Association class, electrocardiogram abnormalities, history of diabetes, hypertension, cerebrovascular disease, peripheral artery disease or myocardial infarction, etiology of heart failure, digoxin treatment, and randomized bisoprolol allocation.
3.3. Heart rate categories and underlying heart rhythm
Figure 1 shows the univariate HR for patients with AF and sinus rhythm for the combined outcome after stratification into the 4 heart rate categories. The P value for the interaction among bisoprolol allocation, heart rate, and the risk of the combined endpoint was not significantly different (P = 0.73), neither in patients with AF nor sinus rhythm. The interaction P values for all other treatments were also not significantly different, suggesting that the associations observed were independent of randomized bisoprolol allocation.
Figure 1.
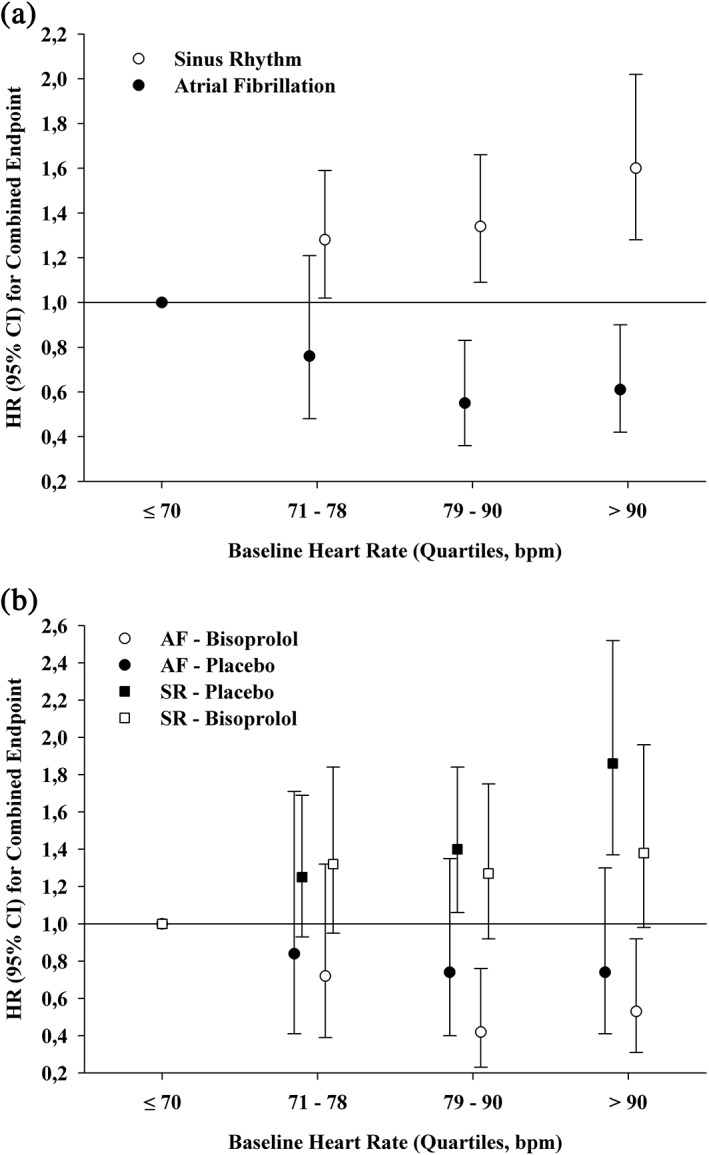
Adjusted hazard ratios for combined endpoint, stratified by rhythm (A) and rhythm and bisoprolol treatment (B). Abbreviations: AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; SR, sinus rhythm.
3.4. Prognosis
Figure 2 shows the HR for all‐cause mortality in patients with AF or sinus rhythm, centered on mean heart rate within each group. Table 3 reveals that in AF patients, in none of the 4 heart rate groups was a significant association with all‐cause mortality observed (see Supporting Figure 1A in the online version of this article). In sinus rhythm patients, the primary outcome was worst in patients with baseline heart rate >90 bpm (HR: 1.40, 95% CI: 0.99‐2.00, P = 0.060 (see Supporting Figure 1B in the online version of this article). Table 3 shows that with multivariable adjustment for the combined endpoint, there was a trend toward a worse outcome in patients with sinus rhythm in the higher heart rate categories, which is also depicted in Supporting Figure 2A,B in the online version of this article.
Figure 2.
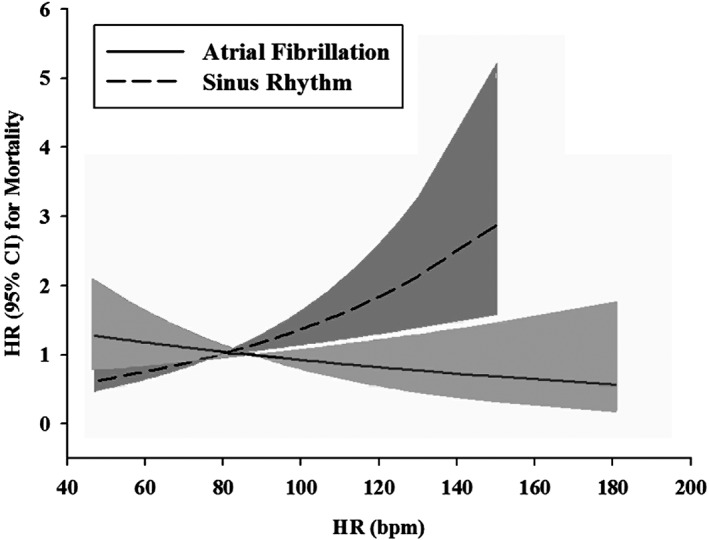
Heart rate, underlying heart rhythm, and mortality. Abbreviations: CI, confidence interval; HR, heart rhythm.
Table 3.
Multivariate association between quartiles of heart rate at baseline, rhythm, and clinical outcome
| Endpoint | Events, n/N | Heart Rate, bpm | Atrial Fibrillation | Sinus Rhythm | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| All‐cause mortality | 93/738 | ≤70 | 1.00 (ref) | — | 1.00 (ref) | — |
| 68/550 | 71–78 | 1.17 (0.59‐2.33) | 0.66 | 0.86 (0.60‐1.24) | 0.42 | |
| 105/712 | 79–90 | 0.93 (0.49‐1.77) | 0.82 | 1.21 (0.88‐1.67) | 0.25 | |
| 94/538 | >90 | 1.12 (0.60‐2.10) | 0.71 | 1.40 (0.99‐2.00) | 0.060 | |
| Combined endpoint | 211/738 | ≤70 | 1.00 (ref) | — | 1.00 (ref) | — |
| 178/550 | 71–78 | 0.75 (0.46‐1.21) | 0.24 | 1.23 (0.98‐1.54) | 0.070 | |
| 229/712 | 79–90 | 0.64 (0.42‐0.98) | 0.042 | 1.30 (1.05‐1.61) | 0.017 | |
| 192/538 | >90 | 0.72 (0.48‐1.08) | 0.12 | 1.48 (1.16‐1.88) | 0.002 | |
| CV death | 71/738 | ≤70 | 1.00 (ref) | — | 1.00 (ref) | — |
| 49/550 | 71–78 | 0.86 (0.38‐1.97) | 0.73 | 0.88 (0.58‐1.33) | 0.54 | |
| 74/712 | 79–90 | 0.62 (0.28‐1.35) | 0.23 | 1.16 (0.80‐1.68) | 0.44 | |
| 68/538 | >90 | 0.88 (0.43‐1.81) | 0.74 | 1.35 (0.90‐2.04) | 0.15 | |
| CV hospitalization | 179/738 | ≤70 | 1.00 (ref) | — | 1.00 (ref) | — |
| 161/550 | 71–78 | 0.79 (0.47‐1.31) | 0.35 | 1.32 (1.04‐1.68) | 0.025 | |
| 194/712 | 79–90 | 0.61 (0.38‐0.97) | 0.035 | 1.32 (1.04‐1.67) | 0.022 | |
| 164/538 | >90 | 0.69 (0.44‐1.08) | 0.11 | 1.52 (1.17‐1.98) | 0.002 | |
| Sudden death | 33/738 | ≤70 | 1.00 (ref) | — | 1.00 (ref) | — |
| 20/550 | 70–78 | 0.95 (0.25‐3.54) | 0.94 | 0.72 (0.38‐1.33) | 0.29 | |
| 41/712 | 79–90 | 1.24 (0.42‐3.66) | 0.70 | 1.15 (0.68‐1.94) | 0.61 | |
| 33/538 | ≥90 | 0.97 (0.33‐2.86) | 0.96 | 1.26 (0.70‐2.27) | 0.44 | |
Abbreviations: CI, confidence interval; CV, cardiovascular; HR, hazard ratio.
Adjusted for age, gender, race, left ventricular ejection fraction, estimated glomerular filtration rate, systolic and diastolic blood pressure, New York Heart Association class, electrocardiogram abnormalities, history of diabetes, hypertension, cerebrovascular disease, peripheral artery disease or myocardial infarction, etiology of heart failure, digoxin treatment, and randomized bisoprolol allocation.
4. DISCUSSION
Lower heart rate is generally associated with better outcome. In patients with HFREF, we found that heart rate and its association with outcome is different in patients with sinus rhythm and AF. In HFREF patients with sinus rhythm, higher heart rate conferred excess risk for most clinical endpoints. However, in contrast, in HFREF patients with AF, heart rate was not associated with increased event rates.
4.1. Heart rate in patients with atrial fibrillation
In the present subanalysis of the CIBIS II trial, we found that in patients with AF, heart rate was not associated with event rates in contrast to patients with sinus rhythm, regardless of bisoprolol allocation. In HFREF patients who also have AF, no prospective data are available on heart rate management. Thus far, only 1 trial addressed optimal heart rate management in patients with AF, with predominantly normal LVEF: the Rate Control Efficacy in Permanent AF: A Comparison between Lenient versus Strict Rate Control II (RACE II) study.13 After a median of 3 years of follow‐up, no differences were observed between lenient (below 110 bpm) and strict (below 80 bpm) rate control in terms of cardiovascular morbidity and mortality.13 Despite the absence of a difference in HF hospitalization in RACE II, one cannot exclude that excessive heart rates (eg, heart rates above 110 bpm) may be harmful, especially in patients with low LVEF, of whom few were included in RACE II.8, 10, 12, 19, 20 Similar results as in our present analysis were found by Castagno et al7 in data coming from CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity). They observed that resting heart rate was not an important predictor of outcome in patients with AF and heart failure, regardless of LVEF.7 Also, in more advanced HF patients, a high heart rate was not associated with an increase in morbidity and mortality.6 Thus, achieving low heart rates may not necessarily be favorable in HFREF patients with AF.
An earlier subanalysis from CIBIS II, which focused on AF, showed that there was no benefit of bisoprolol on prognosis in patients with AF.17 These finding were also observed in MERIT‐HF (Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure) despite the effect on heart rate.21 We found in the present analysis that our findings were independent of the allocation to bisoprolol (ie, there was no significant interaction between bisoprolol, heart rate, and outcome in either patients with AF or sinus rhythm). Also, in 2 recent meta‐analyses it was shown that despite similar heart rate reduction with β‐blocker therapy compared with patients in sinus rhythm, β‐blocker therapy did not improve the prognosis in patients with AF.14, 15 Altogether, it suggests that aggressive rate control with β‐blockers in HFREF AF patients is not warranted. However, this does not imply that β‐blockers have no role in the treatment of HF‐REF and AF, as they may be used for symptom control and reduction. It merely suggest that more research into this subject of rate control is necessary (eg, Rate Control Therapy Evaluation in Permanent Atrial Fibrillation [RATE‐AF]; NCT02391337).
Several underlying mechanisms could explain this interesting finding, including a loss of the atrial kick and the irregularity during AF, implying that patients may need higher heart rates to compensate for similar cardiac output, perhaps even more so during HF.6, 22 As underlying ischemic heart disease is less common in patients with AF, β‐blockers could act differently in these patients compared with patients with nonischemic heart failure. Also, different types of β‐blockers may work by different mechanisms, at different dosages, making a homogenous recommendation difficult.23 Furthermore, AF could lead to more electrical and structural remodeling of the atria that could influence therapy.24 Finally, it could be that the finding of AF in patients with HFREF is far more important than the heart rate of the arrhythmia; in other words, having AF is such an important prognostic indicator, that further stratification based on heart rate is less important.
4.2. Strengths and limitations
Strengths of present analysis include the large population with a large number of patients with AF, which were all confirmed cases by ECG. Several limitations of the present analysis should also be addressed. First, this was a post hoc analysis of the CIBIS II trial, and therefore not specifically designed nor powered to investigate the effect of heart rate in patients with HF and AF in relation to morbidity and mortality. Second, information regarding subclassification of AF (non–self‐terminating, self‐terminating AF) or duration of AF was limited, and outcomes could be different among subcategories. Furthermore, no data on new onset atrial fibrillation were in the original trial. Also, no rate control was applied by study protocol (nor were data available on heart rate during the daytime). Use of amiodarone, anticoagulation, and potentially digoxin could have influenced the outcome for patients with AF. CIBIS II was a trial before cardiac resynchronization therapy was introduced, so the results could potentially be different due to biventricular pacing. Although our models were multivariable adjusted, we cannot exclude any residual confounding. No data on dosages of bisoprolol were available, which could have influenced the results. Due to a relatively short follow‐up, outcome might be different than prolonged follow‐up. Also, no data on quality of life were available. Changes in heart rate over time could not be assessed.25
5. CONCLUSION
In HFREF patients with AF, a higher heart rate is not associated with increased cardiovascular event rates in contrast to HFREF patients with sinus rhythm. Optimal heart rate in HFREF patients may vary according to the underlying cardiac rhythm, and the adage “the lower the better” does not seem to fit HFREF patients with AF. More research is eagerly warranted to study rate control strategies in patients with HFREF.
Conflicts of interest
Dr. Van Veldhuisen has received board membership fees from Amgen, BioControl, Johnson & Johnson, St. Jude, and Vifor. Dr. Van Gelder reports receiving consulting fees from sanofi‐aventis, Boehringer Ingelheim, and Cardiome; grant support from Medtronic, Biotronik, and St. Jude Medical; and lecture fees from sanofi‐aventis, Boehringer Ingelheim, and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
Supporting information
Figure S1. Kaplan Meier of Quartiles of Heart rate and All‐cause mortality in atrial fibrillation (A) and sinus rhythm (B).
Figure S2. Kaplan Meier of Quartiles of Heart rate and Combined Endpoint in atrial fibrillation (A) and sinus rhythm (B).
Mulder BA, Damman K, Van Veldhuisen DJ, Van Gelder IC and Rienstra M. Heart rate and outcome in heart failure with reduced ejection fraction: Differences between atrial fibrillation and sinus rhythm—A CIBIS II analysis. Clin Cardiol. 2017;40:739–744. 10.1002/clc.22725
Funding Information The CIBIS II trial was sponsored by Merck KGaA, Darmstadt, Germany. Funding for the present retrospective analysis was not obtained. Merck KGaA has reviewed this article, but the views and opinions described do not necessarily reflect those of Merck KGaA.
REFERENCES
- 1. Fox K, Borer JS, Camm AJ, et al; Heart Rate Working Group. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. [DOI] [PubMed] [Google Scholar]
- 2. Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of European Society of Cardiology ; Dickstein K, Cohen‐Solal A, Filippatos G, et al; Document Reviewers . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J . 2008;29:2388–2442. [DOI] [PubMed] [Google Scholar]
- 3. Bohm M, Swedberg K, Komajda M, et al; SHIFT Investigators . Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet . 2010;376:886–894. [DOI] [PubMed] [Google Scholar]
- 4. Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long‐term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26:967–974. [DOI] [PubMed] [Google Scholar]
- 5. Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol. 1997;30:1104–1106. [DOI] [PubMed] [Google Scholar]
- 6. Rienstra M, Van Gelder IC, van den Berg MP, Boomsma F, Hillege HL, van Veldhuisen DJ. A comparison of low versus high heart rate in patients with atrial fibrillation and advanced chronic heart failure: effects on clinical profile, neurohormones and survival. Int J Cardiol. 2006;109:95–100. [DOI] [PubMed] [Google Scholar]
- 7. Castagno D, Skali H, Takeuchi M, et al; CHARM Investigators . Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure: results from the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity) program. J Am Coll Cardiol . 2012;59:1785–1795. [DOI] [PubMed] [Google Scholar]
- 8. Mulder BA, Van Veldhuisen DJ, Crijns HJ, et al; for the RACE II investigators . Lenient vs. strict rate control in patients with atrial fibrillation and heart failure: a post‐hoc analysis of the RACE II study. Eur J Heart Fail . 2013;15:1311–1318. [DOI] [PubMed] [Google Scholar]
- 9. Cullington D, Goode KM, Zhang J, Cleland JG, Clark AL. Is heart rate important for patients with heart failure in atrial fibrillation? JACC Heart Fail. 2014;2:213–220. [DOI] [PubMed] [Google Scholar]
- 10. Lazzari JO, Gonzalez J. Reversible high rate atrial fibrillation dilated cardiomyopathy. Heart. 1997;77:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hagens VE, van Veldhuisen DJ, Kamp O, et al. Effect of rate and rhythm control on left ventricular function and cardiac dimensions in patients with persistent atrial fibrillation: results from the RAte Control versus Electrical Cardioversion for Persistent Atrial Fibrillation (RACE) study. Heart Rhythm. 2005;2:19–24. [DOI] [PubMed] [Google Scholar]
- 12. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. [DOI] [PubMed] [Google Scholar]
- 13. Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]
- 14. Rienstra M, Damman K, Mulder BA, Van Gelder IC, McMurray JJV, Van Veldhuisen DJ. Beta‐blockers in patients with heart failure and atrial fibrillation. A meta‐analysis. JACC Heart Fail. 2013;1:21–28. [DOI] [PubMed] [Google Scholar]
- 15. Kotecha D, Holmes J, Krum H, et al; Beta‐Blockers in Heart Failure Collaborative Group. Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual‐patient data meta‐analysis. Lancet . 2014;384:2235–2243. [DOI] [PubMed] [Google Scholar]
- 16. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet . 1999;353:9–13. [PubMed] [Google Scholar]
- 17. Lechat P, Hulot JS, Escolano S, et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation. 2001;103:1428–1433. [DOI] [PubMed] [Google Scholar]
- 18. Damman K, Voors AA, Hillege HL, et al; CIBIS‐2 Investigators and Committees . Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. Eur J Heart Fail. 2010;12:974–982. [DOI] [PubMed] [Google Scholar]
- 19. Hagens VE, Crijns HJ, van Veldhuisen DJ, et al. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J. 2005;149:1106–1111. [DOI] [PubMed] [Google Scholar]
- 20. Van Gelder IC, Wyse DG, Chandler ML, et al; RACE and AFFIRM Investigators . Does intensity of rate‐control influence outcome in atrial fibrillation? An analysis of pooled data from the RACE and AFFIRM studies. Europace . 2006;8:935–942. [DOI] [PubMed] [Google Scholar]
- 21. van Veldhuisen DJ, Aass H, El Allaf D, et al. Presence and development of atrial fibrillation in chronic heart failure. Experiences from the MERIT‐HF Study. Eur J Heart Fail . 2006;8:539–546. [DOI] [PubMed] [Google Scholar]
- 22. Rawles JM. What is meant by a “controlled” ventricular rate in atrial fibrillation? Br Heart J. 1990;63:157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McMurray JJ, van Veldhuisen DJ. Beta blockers, atrial fibrillation, and heart failure. Lancet. 2014;384:2181–2183. [DOI] [PubMed] [Google Scholar]
- 24. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. [DOI] [PubMed] [Google Scholar]
- 25. Vazir A, Claggett B, Jhund P, et al. Prognostic importance of temporal changes in resting heart rate in heart failure patients: an analysis of the CHARM program. Eur Heart J. 2015;36:669–675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan Meier of Quartiles of Heart rate and All‐cause mortality in atrial fibrillation (A) and sinus rhythm (B).
Figure S2. Kaplan Meier of Quartiles of Heart rate and Combined Endpoint in atrial fibrillation (A) and sinus rhythm (B).


