Abstract
Background
We evaluated the specific association between a fitness‐fatness index (FFI) and all‐cause mortality among a national sample of US adults with coronary artery disease, congestive heart failure, or myocardial infarction. This FFI has recently emerged in the literature as a novel index of health.
Hypothesis
We hypothesize that FFI will be inversely associated with mortality risk.
Methods
The FFI was calculated as cardiorespiratory fitness divided by waist‐to‐height ratio. Data from the 1999–2006 National Health and Nutrition Examination Survey were used to identify 1206 participants, ages 20 to 85. Person‐months of follow‐up were calculated from the date of interview until date of death or censoring on December 31, 2011, whichever came first.
Results
In a Cox proportional hazards model, for every 1‐FFI‐unit increase, participants had a 6% reduced all‐cause mortality rate (hazard ratio [HR]: 0.94, 95% confidence interval [CI]: 0.91‐0.97, P = 0.001; N = 1206). Results were similar among those diagnosed with coronary artery disease (HR: 0.94, 95% CI: 0.90‐0.98, P = 0.007), congestive heart failure (HR: 0.95, 95% CI: 0.91‐0.99, P = 0.02), or myocardial infarction (HR: 0.96, 95% CI: 0.92‐0.99, P = 0.04). When examined in isolation, only fitness (and not fatness) was linked with survival benefits.
Conclusions
In this national sample, increased FFI was associated with reduced risk of all‐cause mortality; this association was driven by the beneficial effects of fitness. This underscores the importance of tailored cardiac rehabilitation programs designed to promote fitness, in particular, among cardiac populations.
Keywords: Cardiac rehabilitation, chronic disease, Epidemiology, obesity, physical activity
1. INTRODUCTION
Measures of both inadequate cardiorespiratory fitness (CRF) and obesity have been linked to all‐cause mortality.1 Low CRF may be a more influential presage of early death than a host of modifiable risk factors, including smoking, high blood pressure, high cholesterol, and diabetes mellitus (DM).2 The prevalence of obesity has risen to epidemic proportions in the United States, with >35% of citizens currently classified as obese or morbidly obese.3 The deleterious consequences of obesity are multifold, and include heart disease, which is the No. 1 killer in America.4 Body mass index (BMI) measurements are often used to estimate the degree of obesity and are calculated as weight in kilograms divided by height in meters squared.5, 6 Traditionally, BMI measurements have been used to predict cardiovascular disease (CVD) and early mortality;7 however, recent research suggests measurement of a fitness‐fatness index (FFI) may be a more appropriate measure of chronic disease risk.8 The FFI is calculated as CRF divided by waist‐to‐height ratio (WHR).8 Separate measures of fitness and fatness may supply a more robust measure of susceptibility to cardiovascular pathology and isolate specific improvements in metabolic biomarkers. Waist‐to‐height ratio is an anthropometric index known to be universally appropriate across race, age, sex, and genetic predisposition to changes in body weight and fitness level.8 Therefore, it is plausible that WHR is a more comprehensive calculation of fatness than BMI.
Improvements in cardiovascular fitness are also influential in promoting weight management.9 Physical activities expend energy, potentially resulting in weight loss among those who concurrently monitor their dietary intake.10 There is a dearth of existing evidence indicating whether the benefits of physical fitness may counterbalance the negative effects of obesity on various health outcomes.6, 10, 11 Although distinct, an interrelationship between fitness and fatness warrants exploration with respect to incident CVD risk. This expected relationship provides strong rationale for the utility of an FFI in clinical practice. Thus, the aim for this brief report was to assess the clinical value of FFI in predicting mortality risk among adults with CVD. We evaluate this question in a national sample of cardiac patients to maximize generalizability of our findings.
2. METHODS
2.1. Study design and participants
Data from the 1999–2006 National Health and Nutrition Examination Survey (NHANES) were employed. Data from participants in these cycles were linked to death‐certificate data from the National Death Index via a probabilistic algorithm. Person‐months of follow‐up were calculated from the date of the interview until date of death or censoring on December 31, 2011, whichever came first.
After excluding those with missing data for any of the algorithm variables (described below), excluding those who died within the first 24 months of the follow‐up and including those with a physician diagnosis of coronary artery disease (CAD), congestive heart failure (CHF), or myocardial infarction (MI), 1206 participants (age 20–85 years) remained and constituted the analytic sample. In this sample, 676 had a physician diagnosis of CAD (mean, 9.9 years since diagnosis), 687 had an MI (mean, 10.4 years since heart attack), and 452 had a physician diagnosis of CHF (mean, 9.8 years since heart failure). Among the 1206 participants, 425 died over the follow‐up period; the median follow‐up period was 87 months (interquartile range, 65–115 months).
2.2. Fitness‐fatness index
The FFI was calculated as CRF divided by WHR. Fatness was determined by WHR, as measured directly (waist circumference [WC] via measurement tape, just above the ilium) at the NHANES mobile examination center. Fitness was determined via a previously described12 prediction algorithm (including demographic, biological, and behavioral variables) that employs nonexercise testing methods to estimate one's CRF level; these algorithms have been shown to associate with all‐cause and CVD‐specific mortality.12, 13
The specific algorithms used were:
MET represents metabolic equivalent of task, RHR represents resting heart rate (bpm), and WC represents waist circumference (cm). Smoking status was determined based on self‐report; current smoking was coded as 1, and otherwise it was coded as 0. Activity status was determined from self‐report and defined as ≥2000 MVPA MET‐min‐month; MVPA represents moderate‐to‐vigorous physical activity.14 Those above this threshold were coded as 1 and others were coded as 0. The BMI (kg/m2) was determined here from measured height and weight.
2.3. Statistical analysis
A weighted multivariable Cox proportional hazards model was used to evaluate the association between FFI and all‐cause mortality. The proportional hazards assumption was checked and confirmed via Schoenfeld residuals. Given that the CRF algorithm included behavioral and demographic parameters, these parameters were not included as covariates in the model. However, results were stratified by various demographic parameters. Covariates in all models included education status, race‐ethnicity, physician diagnosis of hypertension, and physician diagnosis of DM. Significance was set at P < 0.05.
3. RESULTS
Given that WC was included the CRF algorithm (numerator of FFI) as well as the WHR (denominator of FFI), we evaluated these interrelationships. In the entire sample, the correlation between fitness (CRF) and fatness (WHR) was r = −0.64 (r2 = 0.41) and the correlation between WC and fitness was r = −0.45 (r2 = 0.20).
3.1. Survival curve
The Figure 1 displays the Kaplan‐Meier survival curve evaluating the probability of survival among those below (<13 METs) and above (≥13 METs) the median FFI for the entire sample.
Figure 1.
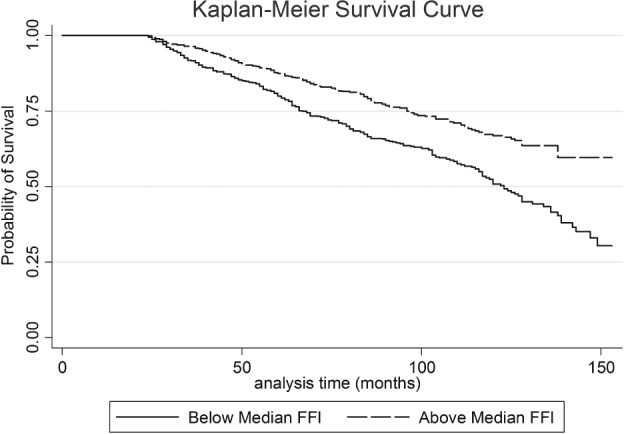
Kaplan‐Meier survival curve evaluating the probability of survival among those below (<13 METs) and above (≥13 METs) the median FFI for the entire sample. Abbreviations: FFI, fitness‐fatness index; MET, metabolic equivalent.
3.2. Sample characteristics
With regard to the entire sample, the mean (SE) age of the sample was 64.3 (0.47) years; 60.1% were male and 80.9% were non‐Hispanic white; 22.7% had DM and 63.9% had hypertension. The mean FFI was 14.1 (0.2). In the sample, 425 deaths occurred across the median follow‐up of 87 months (interquartile range, 65–115 months).
Sample characteristics stratified by CVD status are shown in Table 1. When comparing CAD, CHF, and MI patients, CAD patients were the oldest, had the highest FFI and CRF, were more likely to be male and white, had the most education, and had the lowest mortality rate.
Table 1.
Weighted characteristics of the study variables
| CAD | CHF | MI | |
|---|---|---|---|
| N | 676 | 452 | 687 |
| Mean age, y | 65.1 (0.5) | 64.2 (0.8) | 63.8 (0.5) |
| Mean FFI | 14.4 (0.2) | 13.2 (0.4) | 14.3 |
| CRF, mean METs | 8.5 (0.1) | 7.9 (0.1) | 8.4 (0.1) |
| WHR, mean | 0.61 (0.01) | 0.63 (0.01) | 0.61 (0.003) |
| Male sex, % | 65.2 | 58.0 | 64.3 |
| White race, % | 84.1 | 75.7 | 82.4 |
| DM, % | 23.6 | 27.7 | 22.6 |
| HTN, % | 66.6 | 68.3 | 62.4 |
| Some college or more, % | 42.9 | 35.4 | 38.6 |
| MI, % | 51.1 | 47.5 | 100 |
| Died, % | 26.7 | 37.8 | 27.3 |
| Mean duration of disease, y | 9.4 (0.5) | 9.0 (0.6) | 9.5 (0.5) |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; CRF, cardiorespiratory fitness; DM, diabetes mellitus; FFI, fitness‐fatness index; HTN, hypertension; MET, metabolic equivalent of task; MI, myocardial infarction; SE, standard error; WHR, waist‐to‐height ratio.
Values in parentheses represent SE.
3.3. FFI on mortality risk
Weighted Cox proportional hazards analyses examining the association between FFI and all‐cause mortality across CVD status are shown in Table 2. With regard to CAD patients, and after adjustment, higher FFI was associated with a reduced all‐cause mortality risk (hazard ratio [HR]: 0.94, 95% confidence interval [CI]: 0.90‐0.98, P = 0.004). This association remained significant for men, those age ≥50 years, and those age ≥65 years, but not for women.
Table 2.
Weighted Cox proportional hazard analyses examining the association between FFI and all‐cause mortality across CVD status
| Model | 1‐Unit Increase in FFI | P Value | |
|---|---|---|---|
| HR | 95% CI | ||
| CAD patients | 0.94 | 0.90‐0.98 | 0.004 |
| Men | 0.92 | 0.87‐0.97 | 0.005 |
| Women | 0.94 | 0.87‐1.02 | 0.15 |
| 50+ years | 0.93 | 0.89‐0.97 | 0.001 |
| 65+ years | 0.95 | 0.90‐0.99 | 0.04 |
| CHF patients | 0.95 | 0.91‐0.99 | 0.02 |
| Men | 0.94 | 0.89‐0.98 | 0.01 |
| Women | 0.94 | 0.86‐1.03 | 0.23 |
| 50+ years | 0.96 | 0.91‐1.01 | 0.11 |
| 65+ years | 0.99 | 0.94‐1.04 | 0.80 |
| MI patients | 0.95 | 0.92‐0.99 | 0.03 |
| Men | 0.94 | 0.89‐1.00 | 0.05 |
| Women | 0.93 | 0.85‐1.02 | 0.17 |
| 50+ years | 0.95 | 0.92‐0.99 | 0.02 |
| 65+ years | 0.97 | 0.93‐1.01 | 0.16 |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; CVD, cardiovascular disease; DM, diabetes mellitus; FFI, fitness‐fatness index; HR, hazard ratio; HTN, hypertension; MI, myocardial infarction.
All models are adjusted for education status, race–ethnicity, physician diagnosis of HTN, and physician diagnosis of DM.
With regard to CHF patients, and after adjustment, higher FFI was associated with reduced all‐cause mortality risk (HR: 0.95, 95% CI: 0.91‐0.99, P = 0.002). This association remained significant for men, but not for women or those age ≥50 years or age ≥65 years.
With regard to MI patients, and after adjustment, higher FFI was associated with reduced all‐cause mortality risk (HR: 0.95, 95% CI: 0.92‐0.99, P = 0.03). This association remained significant for men and those age ≥50 years, but not for women or those specifically age ≥65 years.
3.4. CRF/WHR on mortality risk
Weighted Cox proportional hazard analyses examining the association between FFI/CRF/WHR and all‐cause mortality across CVD status are shown in Table 3. Identical to the results shown in Table 2, FFI was independently associated with reduced all‐cause mortality risk for CAD patients, CHF patients, and MI patients. Similarly, across these 3 groups, CRF was inversely associated with mortality risk. However, for all 3 groups, WHR was not associated with reduced mortality risk.
Table 3.
Weighted Cox proportional hazards analyses examining the association between FFI/CRF/WHR and all‐cause mortality across CVD status
| Model | HR | 95% CI | P Value |
|---|---|---|---|
| CAD patients | |||
| FFI, 1‐unit increase | 0.94 | 0.90‐0.98 | 0.004 |
| CRF, 1‐unit increase | 0.84 | 0.77‐0.93 | 0.001 |
| WHR, 1‐unit increase | 0.98 | 0.10‐9.2 | 0.99 |
| CHF patients | |||
| FFI, 1‐unit increase | 0.95 | 0.91‐0.99 | 0.02 |
| CRF, 1‐unit increase | 0.86 | 0.78‐0.94 | 0.003 |
| WHR, 1‐unit increase | 0.60 | 0.06‐5.6 | 0.65 |
| MI patients | |||
| FFI, 1‐unit increase | 0.95 | 0.92‐0.99 | 0.03 |
| CRF, 1‐unit increase | 0.86 | 0.79‐0.94 | 0.001 |
| WHR, 1‐unit increase | 0.25 | 0.03‐2.0 | 0.19 |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; CRF, cardiorespiratory fitness; CVD, cardiovascular disease; DM, diabetes mellitus; FFI, fitness‐fatness index; HR, hazard ratio; HTN, hypertension; MI, myocardial infarction; WHR, waist‐to‐height ratio.
All models adjusted for education status, race‐ethnicity, physician diagnosis of HTN, and physician diagnosis of DM.
4. DISCUSSION
Measurement of an FFI has been shown to correlate with incident DM8; however, to our knowledge, research has yet to examine FFI as a predictive and preventive tool for predicting mortality among individuals with prior diagnosis of heart disease, which was the purpose of this brief report. The main finding of this study was that cardiac patients with a higher FFI had a decreased rate of all‐cause mortality. FFI increases were similarly associated with reduced mortality among those with a diagnosis of CAD, CHF, or MI. This finding may guide tailored health‐promotion efforts to improve physical fitness and reduce the astronomical burden of obesity within populations requiring inpatient or outpatient cardiac rehabilitation.
4.1. Study limitations
Choice of a nonobjective measure of CRF is a potential limitation of this study; however, the algorithm we used is a valid indirect estimate of CRF and supplies adequate evidence of all‐cause and CVD‐specific mortality.12, 13 Other limitations include an inability to confirm heart disease with an objective measure, although self‐reported diagnosis of ischemic heart disease is suitably proximate to validated hospital records.15 We were additionally unable to determine if cardiac “patients” were actively enrolled in cardiac rehabilitation programs or to evaluate CVD‐specific mortality due to cell‐size issues. Future experimental research, as well as clinical practice in cardiac‐rehabilitation settings, should consider the 6‐Minute Walk Test as an appropriate measure of CRF, as this test is often used as an indication of heart function in obese individuals.16, 17 A strong correlation exists between anthropometric status and 6‐Minute Walk Test performance.17 Other brief functional‐related clinical‐based tests to consider within this cardiac population include the 20‐meter walking test.18 Further, WC, in particular, was included in the calculation of both fitness (numerator of FFI) and fatness (denominator of FFI). Notably, however, the association between fitness and fatness was modest at best (see first paragraph of Results section), suggesting that collinearity was not a concern. To overcome this limitation, future research on this topic should employ an objective measure of fitness, in particular. Strengths of this study include the evaluation of fitness and fatness in synergy, a prospective study design, and identification of a national sample of at‐risk individuals.
We calculated WHR from an objective measurement of WC for all participants. Waist circumference is easily calculable with a tape measure, and thus it can be readily replicated for use in cardiac rehabilitation. The reliability of the National Death Index has also been previously cited,19, 20 which allows us to express confidence in the legitimacy of our outcome variable. Results were similar across varying subpopulations, reinforcing our hypothesis that FFI has strong clinical and experimental potential as an integratory quantification of CRF and adiposity. Subsequent research should further examine the interplay of fitness and fatness in isolation and when assimilated. Our findings, in particular, demonstrated that fitness, as opposed to fatness, was a more consistent predictor of mortality risk. Consistent with our current findings that enhanced fitness is linked with reduced mortality risk, past work has shown that CRF has the potential to overcome severe health risks associated with obesity.21 Namely, for obese individuals diagnosed with CVD, or even heart failure, sustaining a higher degree of CRF may markedly improve health status when compared with their inactive counterparts.22, 23 This underscores the importance of fitness promotion, particularly among individuals susceptible to the negative impacts of chronic disease. Describing the importance of these factors within the lens of the American obesity epidemic will communicate critical evidence capable of better informing cardiac rehabilitation pedagogy and exercise prescription.
5. CONCLUSION
Our findings demonstrated that a higher FFI (favorable fitness‐to‐fatness ratio) was associated with reduced risk of all cause‐mortality among individuals with a diagnosis of CVD. A fitness‐to‐fatness ratio is a vital anthropometric measurement offering powerful implications for not only cardiac rehabilitation, but also mainstream public health. Future research on this topic should explore the link between fitness and fatness in cardiac rehabilitation programs. These programs should linearly track changes in each measure to inform subsequent research, prevent widespread morbidity and mortality, and promote cardiovascular health to the general population.
Conflicts of interest
The authors declare no potential conflicts of interest.
Frith E and Loprinzi PD. The protective effects of a novel fitness‐fatness index on all‐cause mortality among adults with cardiovascular disease. Clin Cardiol. 2017;40:469–473. 10.1002/clc.22679
REFERENCES
- 1. Yerrakalva D, Mullis R, Mant J. The associations of “fatness,” “fitness,” and physical activity with all‐cause mortality in older adults: a systematic review. Obesity (Silver Spring) . 2015;23:1944–1956. [DOI] [PubMed] [Google Scholar]
- 2. Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. [DOI] [PubMed] [Google Scholar]
- 3. Flegal KM, Kruszon‐Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauer UE, Briss PA, Goodman RA, et al. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384:45–52. [DOI] [PubMed] [Google Scholar]
- 5. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol . 2014;63(25 part B):2985–3023. [DOI] [PubMed] [Google Scholar]
- 6. Lee DC, Sui X, Artero EG, et al. Long‐term effects of changes in cardiorespiratory fitness and body mass index on all‐cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation. 2011;124:2483–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atique SM, Shadbolt B, Marley P, et al. Association between body mass index and age of presentation with symptomatic coronary artery disease. Clin Cardiol. 2016;39:653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sloan RA, Haaland BA, Sawada SS, et al. A fit‐fat index for predicting incident diabetes in apparently healthy men: a prospective cohort study. PLoS One. 2016;11:e0157703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee DC, Artero EG, Sui X, et al. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol . 2010;24(4 suppl):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fogelholm M. Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors. A systematic review. Obes Rev . 2010;11:202–221. [DOI] [PubMed] [Google Scholar]
- 11. Dankel SJ, Loenneke JP, Loprinzi PD. Health outcomes in relation to physical activity status, overweight/obesity, and history of overweight/obesity: a review of the WATCH paradigm. Sports Med . 2016. doi: 10.1007/s40279-016-0641-7. [DOI] [PubMed] [Google Scholar]
- 12. Addoh O, Edwards MK, Loprinzi PD. Predictive validity of a medical‐related cardiorespiratory fitness algorithm in predicting cardiovascular disease‐ and all‐cause mortality: implications for integration into clinical practice. Mayo Clin Proc. 2016;91:1320–1321. [DOI] [PubMed] [Google Scholar]
- 13. Artero EG, Jackson AS, Sui X, et al. Longitudinal algorithms to estimate cardiorespiratory fitness: associations with nonfatal cardiovascular disease and disease‐specific mortality. J Am Coll Cardiol. 2014;63:2289–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loprinzi PD. Dose–response association of moderate‐to‐vigorous physical activity with cardiovascular biomarkers and all‐cause mortality: considerations by individual sports, exercise and recreational physical activities. Prev Med. 2015;81:73–77. [DOI] [PubMed] [Google Scholar]
- 15. Bergmann MM, Byers T, Freedman DS, et al. Validity of self‐reported diagnoses leading to hospitalization: a comparison of self‐reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147:969–977. [DOI] [PubMed] [Google Scholar]
- 16. Hamilton DM, Haennel RG. Validity and reliability of the 6‐minute walk test in a cardiac rehabilitation population. J Cardiopulm Rehabil. 2000;20:156–164. [DOI] [PubMed] [Google Scholar]
- 17. Baillot A, Baillargeon JP, Brown C, et al. The 6‐min walk test reflects functional capacity in primary care and obese patients. Int J Sports Med. 2015;36:503–509. [DOI] [PubMed] [Google Scholar]
- 18. Loprinzi PD. Brief walking test and cognitive function among congestive heart failure patients: effect modification by duration of congestive heart failure. Int Cardiovasc Forum J. 2015;6:76–78. [Google Scholar]
- 19. Rich‐Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019. [DOI] [PubMed] [Google Scholar]
- 20. Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119:837–839. [DOI] [PubMed] [Google Scholar]
- 21. Lavie CJ, De Schutter A, Parto P, et al. Obesity and prevalence of cardiovascular diseases and prognosis—the obesity paradox updated. Prog Cardiovasc Dis. 2016;58:537–547. [DOI] [PubMed] [Google Scholar]
- 22. Myers J, McAuley P, Lavie CJ, et al. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis. 2015;57:306–314. [DOI] [PubMed] [Google Scholar]
- 23. Lavie CJ. Analyzing 2015 impact factors—Special Editor's Commentary. Prog Cardiovasc Dis. 2016;59:323–324. [DOI] [PubMed] [Google Scholar]


