Abstract
In normotensive patients with acute symptomatic pulmonary embolism (PE), the effect of undiagnosed obstructive sleep apnea (OSA) on cardiovascular (CV) outcomes lacks clarity. The Prognostic Significance of Obstructive Sleep Apnea in Patients With Acute Symptomatic Pulmonary Embolism (POPE) study is a multicenter, observational study designed to prospectively assess the prognostic significance of concomitant OSA in hemodynamically stable outpatients with acute symptomatic PE. Adult patients with acute stable PE are eligible. Recruited patients undergo an overnight sleep study using a level III portable diagnostic device within 7 days (and preferably within 48 hours) of diagnosis of PE. The sleep tracings are analyzed by a certified sleep technologist and audited by a sleep physician, both of whom are blinded to other study data. The patients are divided into 2 groups based on apnea‐hypopnea index (AHI): OSA (AHI ≥15) and non‐OSA (AHI <15) groups. The study uses a composite of PE‐related death, CV death, clinical deterioration requiring an escalation of treatment, or nonfatal CV events (recurrent venous thromboembolism, acute myocardial infarction, or stroke) within 30 days after the diagnosis of PE as the primary outcome. The projected sample size of 225 patients will provide 80% power to test the hypothesis that OSA will increase the primary outcome from 7% in the non‐OSA group to 20% in the OSA group, with α ≤0.05. The trial results will be important to understand the burden and CV effects of OSA in PE patients.
Keywords: Obstructive Sleep Apnea, Prognosis, Pulmonary Embolism
1. INTRODUCTION
Acute pulmonary embolism (PE), a commonly diagnosed condition, has a morbidity and mortality rate that varies significantly by age, clinical presentation, and the presence of comorbid disease.1, 2, 3, 4, 5, 6 In some cases, patients with PE may safely undergo treatment at home and avoid admission to a hospital.7, 8, 9 However, some patients require immediate admission to an intensive care unit to prevent death.10 The clinical course for a large proportion of patients will fall somewhere between these 2 extremes, making accurate risk stratification a critical component of the initial evaluation.11, 12
Obstructive sleep apnea (OSA) is an emerging cardiovascular (CV) risk factor that is present in about 9% to 15% of middle‐aged adults.13 OSA is characterized by recurrent episodes of complete or partial upper‐airway obstruction due to the collapse of upper‐pharyngeal soft tissue during sleep, resulting in intermittent oxygen deprivation and sleep fragmentation. Sympathetic activation, production of vasoactive substances, and activation of inflammatory and procoagulant pathways contribute to CV pathogenesis,14 including hypertension, coronary artery disease, stroke, and heart failure. A previous study suggested that untreated OSA is independently associated with subsequent cardiac and cerebrovascular events in patients undergoing percutaneous coronary intervention.15 However, no studies have assessed the effect of undiagnosed OSA on CV outcomes in patients with acute symptomatic PE.
Therefore, the multicenter cohort Prognostic Significance of Obstructive Sleep Apnea in Patients With Acute Symptomatic Pulmonary Embolism (POPE) study aims to determine the association between OSA and the incidence of adverse CV outcomes in patients with acute symptomatic PE. We hypothesize that OSA is an independent risk factor for the development of major adverse CV events in PE patients. Results from the POPE study will advance the fundamental understanding of the burden and prognostic implications of OSA in patients with acute PE. In addition, this trial aims to validate the STOP‐Bang score in a Spanish cohort of patients with acute symptomatic PE.
2. METHODS
2.1. Study design
POPE is a prospective, multicenter, observational cohort study designed by the authors and sponsored by Sociedad Española de Neumología y Cirugía Torácica (SEPAR) and Neumomadrid (Figure 1). The main objective of this study is to assess the prognostic significance of untreated OSA in hemodynamically stable outpatients with acute symptomatic PE. Secondary objectives of the study are to assess (1) the prevalence of undiagnosed OSA in normotensive patients with acute PE and (2) the association between OSA and the presence of right ventricular (RV) dysfunction and/or myocardial injury at the time of PE diagnosis. Additionally, we aim to validate the STOP‐Bang questionnaire in this cohort.16 The protocol and consent forms were approved by the institutional review board of each center.
Figure 1.
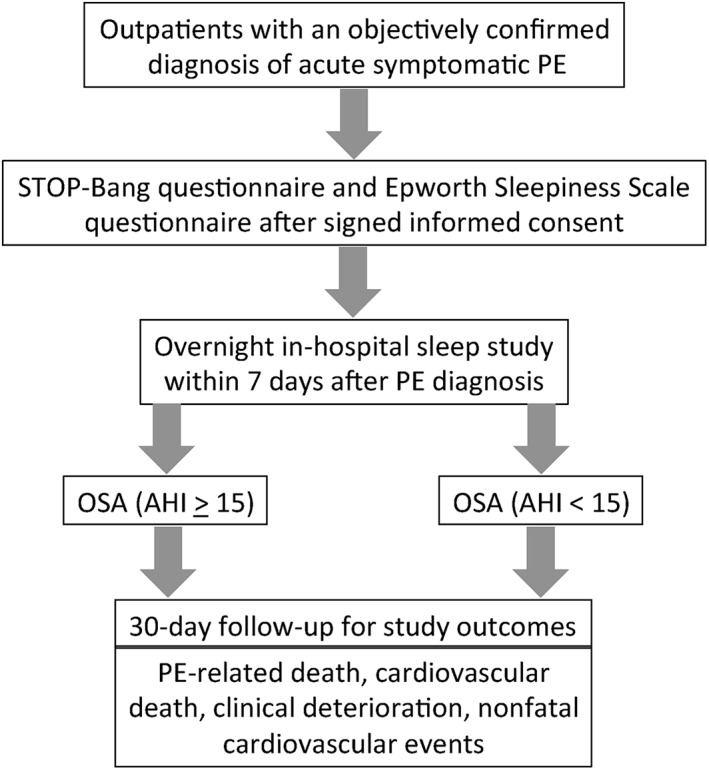
Study flow diagram. Abbreviations: AHI, apnea‐hypopnea index; OSA, obstructive sleep apnea; PE, pulmonary embolism
2.2. Setting
Patients undergo recruitment from the respiratory departments of 3 academic hospitals in Spain.
2.3. Patients
The study aims to enroll consecutive outpatients with an objectively confirmed diagnosis of acute symptomatic PE. The study requires confirmation of the diagnosis of PE with a high‐probability ventilation‐perfusion (V/Q) scintigraphy,17 positive contrast‐enhanced, PE‐protocol, helical chest computerized tomography (single or multidetector) for PE,18 or a nondiagnostic V/Q lung scan and confirmed lower‐limb deep‐vein thrombosis on venous compression ultrasound.19 Table 1 shows study eligibility criteria. The study excludes patients if they have any of the following: known OSA on treatment with thrombolytics at the time of PE diagnosis, life expectancy <3 months, pregnancy, geographic inaccessibility that precludes follow‐up, age < 18 years, or hemodynamic instability at presentation (defined as cardiogenic shock, systolic blood pressure (SBP) <90 mmHg, or use of inotropic support).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| Age ≥ 18 y |
| Acute PE confirmed by objective testing |
| Exclusion criteria |
| Known OSA on treatment |
| Treatment with thrombolytics, embolectomy, or an IVC filter at the time of PE diagnosis |
| Life expectancy <3 mo |
| Pregnancy |
| Geographic inaccessibility that precludes follow‐up |
| Inability to provide informed consent |
| Hemodynamic instability at presentation (defined as cardiogenic shock, SBP <90 mm Hg, or need of CPR, intubation, or inotropic support) |
Abbreviations: CPR, cardiopulmonary resuscitation; IVC, inferior vena cava; OSA, obstructive sleep apnea; PE, pulmonary embolism; SBP, systolic blood pressure.
2.4. OSA screening questionnaire
During the index hospitalization period for acute PE, recruited patients are approached to complete the STOP‐Bang questionnaire and Epworth Sleepiness Scale (ESS) questionnaire (Table 2). The STOP‐Bang questionnaire is a validated screening tool to identify individuals at high risk for OSA.16 It consists of 8 dichotomous (yes/no) items related to the clinical features of sleep apnea. The total score ranges from 0 to 8. Patients can be classified for OSA risk based on their respective scores. A score ≥ 5 determines high risk for OSA. The sensitivity of STOP‐Bang score ≥ 3 to detect moderate to severe OSA (apnea‐hypopnea index [AHI] >15) and severe OSA (AHI >30) is 93% and 100%, respectively. The ESS is a validated questionnaire that identifies the perceived likelihood of falling asleep during 8 everyday situations.20, 21 Patients rate their perceived likelihood of falling asleep during each scenario on a scale of 0 to 3, with a total score between 0 and 24. Excessive daytime sleepiness is defined as an ESS score > 10. Both questionnaires are administered through face‐to‐face interviews by an investigator. However, an inability or failure to complete the questionnaires would not result in exclusion from the POPE study.
Table 2.
STOP‐Bang questionnaire
| STOP | ||
|---|---|---|
| Do you SNORE loudly (louder than talking or loud enough to be heard through closed doors)? | Yes | No |
| Do you often feel TIRED, fatigued, or sleepy during daytime? | Yes | No |
| Has anyone OBSERVED you stop breathing during your sleep? | Yes | No |
| Do you have or are you being treated for high blood PRESSURE? | Yes | No |
| BANG | Yes | No |
| BMI >35 kg/m2? | Yes | No |
| Age > 50 y? | Yes | No |
| Neck circumference > 16 in. (40 cm)? | Yes | No |
| Gender = M? | Yes | No |
Abbreviations: BMI, body mass index; M, male.
2.5. Overnight sleep study
The study schedules recruited patients for an overnight sleep study within 7 days (preferably within the first 48 hours) after PE diagnosis regardless of their results on the 2 screening questionnaires. The sleep studies are conducted with an approved level III portable diagnostic device (NOX T3; Nox Medical Inc., Reykjavík, Iceland) validated for use against in‐laboratory polysomnography. The reported sensitivity and specificity are 100% and 70%, respectively.22 The portable diagnostic device records nasal airflow (nasal pressure transducer), respiratory inductance plethysmography, arterial oxygen saturation (pulse oximetry), snoring episodes (derived from the integrated pressure transducer), and body position. An independent sleep technologist who is blinded to the patients' clinical characteristics manually scores all studies. To ensure the accuracy and consistency of scorings, 50% of all sleep studies will be audited by a blinded investigator with expertise in sleep medicine. To ensure cross‐site consistency in the quality of the overnight sleep studies, they are all graded for quality on the basis of the criteria that were developed after a review of published scoring guidelines.23
The primary measure of the sleep study is the AHI, quantified as the total number of apneas and hypopneas per hour of the total recording time. An apnea is defined as a ≥ 90% decrease in airflow from the baseline value for ≥10 seconds. Apneas are further classified as obstructive or central based on the presence or absence, respectively, of respiratory‐related chest‐wall movement. Hypopnea is defined as a 30% to 90% reduction in airflow from the baseline value lasting ≥10 seconds, in conjunction with ≥3% oxygen desaturation. The patients are divided into 2 groups based on AHI: OSA (AHI ≥15) and non‐OSA (AHI <15) groups. The respiratory event scoring is performed according to the American Academy of Sleep Medicine 2015 guidelines.24 Patients diagnosed with central sleep apnea are excluded from the analyses.
2.6. Transthoracic echocardiography
The study requires that patients undergo transthoracic echocardiography within 24 hours after diagnosis of PE. Patients undergo testing in the left lateral position. Trained and certified local cardiologists, blinded to the patient's clinical data and sleep‐test results, interpret each echocardiogram. The study defines echocardiographic RV dysfunction as the presence of ≥2 among dilatation of the right ventricle (end‐diastolic diameter > 30 mm from the parasternal view or the right ventricle appearing larger than the left ventricle from the subcostal or apical view), hypokinesis of the right ventricle free wall (any view), or systolic pulmonary artery pressure > 30 mm Hg.25
2.7. Cardiac biomarker determinations
Laboratory personnel blinded to the patients' baseline characteristics and clinical outcome measure cardiac troponin I (cTnI) levels quantitatively by using a microparticle enzyme immunoassay (Abbott Laboratories, Abbott Park, IL). The analytical sensitivity for this cTnI is 0.1 ng mL − 1 and represents the lowest measurable concentration of cTnI that can be distinguished from zero. With this assay, we regard cTnI concentrations of >0.1 ng mL − 1 as indicating myocardial injury (cTnI positive). Laboratory personnel measure brain natriuretic peptide (BNP) levels by immunoassay in an AxSYM analyzer (Abbott Laboratories), with detection by microparticle enzyme immunoassay system. With this assay, we regard BNP concentrations of >100 pg mL − 1 as indicating cardiac myocyte stretch (BNP positive).
2.8. Study outcome measures
The study uses a composite of PE‐related death, CV death, clinical deterioration requiring an escalation of treatment, or nonfatal CV events (recurrent venous thromboembolism, acute myocardial infarction, or stroke) within 30 days after the diagnosis of PE as the primary outcome. Investigators assess mortality using patient or proxy interviews and/or hospital chart review. An independent adjudication committee, whose members are blinded to initial prognostic test results, adjudicates all death causes as “fatal PE” or “death from other causes.” The adjudicators classify the cause of death as “fatal PE” if autopsy deems PE as the cause of death, if death follows and is apparently related to a clinically severe PE, or death in a patient who dies suddenly or unexpectedly. The study defines CV death as death due to acute myocardial infarction, sudden cardiac death, heart failure or cardiogenic shock, stroke, or other CV causes, including arrhythmias unrelated to sudden cardiac death, aortic aneurysm rupture, peripheral artery disease, or complications of CV intervention, including cardiac surgery and nonsurgical revascularization.26 The study defines escalation of treatment as need for cardiopulmonary resuscitation, SBP <90 mm Hg for ≥15 minutes, need for catecholamine administration, need for thrombolysis, or need for an insertion of an inferior vena cava filter after initiation of anticoagulation for the index PE event. For the recurrent PE diagnosis, we required the presence of a new perfusion defect involving ≥75% of a lung segment on V/Q scintigraphy or a new intraluminal filling defect or an extension of a previous filling defect on PE‐protocol chest computerized tomography.18 For a new or recurrent deep‐vein thrombosis, we required the appearance of a new noncompressible vein segment or a ≥ 4‐mm increase in the diameter of a thrombus on complete compression ultrasound.27
Secondary outcomes include (1) all‐cause mortality, (2) PE‐related mortality, (3) CV death, (4) escalation of treatment, and (5) nonfatal CV events within the 30 days of follow‐up.
The Data and Safety Monitoring Board, whose members are blinded to initial prognostic test results, adjudicate all serious adverse events. The primary results of the POPE study will be reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement regarding the reporting of results of observational studies in epidemiology.28
2.9. Treatment and follow‐up
The protocol does not mandate a specific approach to treatment. The clinicians typically initiate treatment prior to or within hours of PE diagnosis. Treatment regimens typically consist of therapeutic doses of subcutaneous low‐molecular‐weight heparin, combined with oral vitamin K antagonist therapy; or direct oral anticoagulants. In patients whose clinical status deteriorates after enrollment, the clinicians administer thrombolytic treatment and/or inotropic support as deemed appropriate. Clinicians use an inferior vena cava filter as deemed appropriate (eg, when a contraindication to anticoagulation arises).
2.10. Statistical analyses
We will use χ2 or Fisher exact tests to compare categorical data between groups. We will use the Shapiro–Wilk test to assess continuous data for a normal distribution. We will use 2‐tailed unpaired t tests to compare parametric continuous data between 2 unpaired groups, and we will use the Mann–Whitney U test for nonparametric data comparisons. We will use Cox proportional hazard models to evaluate the association between OSA and the outcome measures. A manual backward stepwise approach will be used to develop the multivariable models. In the full model, covariates determined a priori to be associated with mortality (ie, age, male sex, body mass index, SBP, heart rate, and arterial oxygen saturation) and covariates with imbalance between the groups at baseline will be considered for inclusion. Variables that show evidence of confounding (ie, the coefficient of the variable group changes by >10% when removing that variable from the full model) for the effect of reperfusion on the outcome undergoing analysis will not be removed from the model. Specific candidate variables will be forced, 1 at a time, into the full model to assess their effects.
We will also calculate sensitivity, specificity, positive predictive value, negative predictive value, positive and negative likelihood ratios, and area under the receiver operating characteristic curve of a STOP‐Bang score ≥ 3 for prediction of moderate to severe OSA.
2.10.1. Missing data
Investigators will impute variables' missing data by using the multiple imputations by chained equation method,29 which will result in 10 imputed data sets. We will independently analyze each of the 10 data sets. We will average estimates of the variables to give a single mean estimate and will adjust the SE according to the Rubin rules.30
Statistical significance is defined as a 2‐tailed P value of <0.05 for all analyses. Analyses will be performed using SPSS version 15.0 (SPSS, Inc., Chicago, IL).
2.10.2. Sample‐size calculation
According to a previous study, we assumed a 45% prevalence of OSA, based on an AHI ≥15.15 The expected adverse‐event rate for untreated OSA patients with acute symptomatic PE is 20% and that for non‐OSA patients is 7% within 30 days after the diagnosis of PE. Based on 80% power and a significance level of 5%, the required sample size is 215 (OSA, n = 86; non‐OSA, n = 129). Finally, with a patient loss of 5%,15 the final sample size should be 225 patients.
2.10.3. Interim analysis
According to prespecified criteria, an interim analysis will be performed after enrollment of 50% of the patients to revise sample size.
3. RESULTS
As of July 31, 2017, a total of 62 patients have been recruited (Table 3). Twenty‐six (42%) patients were at high risk for OSA according to the STOP‐Bang questionnaire (ie, a score ≥ 5). Overall, 24 patients (39%; 95% confidence interval: 27%‐52%) had OSA and 38 did not. The mean AHI of the first 62 patients was 18.1. Among these patients, 21 patients (34%) had an AHI <5, 17 patients (27%) had an AHI of 5 to <15, 12 patients (19%) had an AHI of 15 to <30, and 12 patients (19%) had an AHI ≥30.
Table 3.
Baseline characteristics of the first 62 patients
| All Patients, N = 62 | |
|---|---|
| Clinical characteristics | |
| Age, y | 68.3 ± 14.3 |
| Age > 65 y | 39 (63) |
| Male sex | 34 (55) |
| BMI, kg/m2 | 29.4 ± 4.5 |
| Risk factors for VTE | |
| History of VTE | 6 (9.7) |
| Cancera | 10 (16) |
| Recent surgeryb | 8 (13) |
| Immobilizationc | 1 (1.6) |
| Comorbid diseases | |
| Recent major bleedingb | 0 (0) |
| COPD | 7 (11) |
| CHF | 2 (3.2) |
| Previous AMI | 1 (1.6) |
| Previous stroke | 0 (0) |
| Smoking | 31 (50) |
| Hyperlipidemia | 20 (32) |
| HTN | 39 (63) |
| DM | 11 (18) |
| Concomitant DVT | 22 (35) |
| Clinical symptoms and signs at presentation | |
| Syncope | 6 (9.7) |
| Chest pain | 23 (37) |
| Dyspnea | 49 (79) |
| Heart rate ≥ 110 bpm | 22 (35) |
| Arterial SpO2 < 90% | 16 (26) |
| RV hypokinesis | 7 (11) |
| BNP >100 pg/mL | 20 (66) |
| cTnI >0 ng/mL | 19 (32) |
| Laboratory findings | |
| Hb, g/dL | 14.0 ± 1.9 |
| Cr, mg/dL | 1.0 ± 0.5 |
| Sleep‐study characteristics | |
| OSA | 24 (39) |
| AHI | 18.1 ± 21.0 |
| Arterial SpO2 | 90.8 ± 3.1 |
| Total % of time arterial SpO2 < 90% | 24.7 ± 29.0 |
| ESS >10 | 21 (34) |
| High risk based on STOP‐Bang | 26 (42) |
Abbreviations: AHI, apnea‐hypopnea index; AMI, acute myocardial infarction; BMI, body mass index; BNP, brain natriuretic peptide; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; Cr, creatinine; cTnI, cardiac troponin I; DM, diabetes mellitus; DVT, deep‐vein thrombosis; ESS, Epworth Sleepiness Scale; Hb, hemoglobin; HTN, hypertension; OSA, obstructive sleep apnea; RV, right ventricle; SD, standard deviation; SpO2, oxygen saturation; VTE, venous thromboembolism.
Data are presented as n (%) or mean ± SD.
Active or under treatment in the last year.
In the previous month.
Immobilized patients are defined in this analysis as non‐surgical patients who had been immobilized (i.e., total bed rest with bathroom privileges) for ≥4 days in the month prior to PE diagnosis.
4. DISCUSSION
The POPE study investigates the prognostic value of OSA findings in hemodynamically stable PE patients. In addition, this trial assesses the prevalence of undiagnosed OSA in normotensive patients with acute PE and the association between OSA and the presence of RV dysfunction and/or myocardial injury at the time of PE diagnosis. Finally, the study aims to validate the STOP‐Bang questionnaire in a cohort of Spanish patients with proven acute PE.
Different studies have suggested an association between OSA and proven acute venous thromboembolism.31, 32 However, no studies have assessed the prognostic significance of undiagnosed OSA in the acute phase of normotensive PE. Interestingly, one study did not find a relationship between a high‐risk (ie, >6 points) STOP‐Bang questionnaire and 30‐day all‐cause mortality in 137 patients with objectively confirmed PE.33 In this study, the lack of association between OSA and prognosis may have been due to the absence of a confirmatory testing for the diagnosis of sleep apnea, and a lack of statistical power. In the absence of large studies that demonstrate an association between OSA and the short‐term prognosis of patients with acute PE, clinical practice guidelines for the management of acute PE or OSA do not provide any recommendation on this setting.34, 35 The POPE study will provide information on the prognostic significance of OSA diagnosis in this setting.
The findings from this study will have practical implications. A confirmation of the study hypothesis will help to explore the use of continuous positive airway pressure in normotensive patients who have acute PE and concomitant OSA. If, on the other hand, the hypothesis of POPE is rejected, patients with PE will no longer need to be exposed to an overnight sleep study, at least in the acute phase of PE.
4.1. Study limitations
There are some limitations in the POPE trial. Sleep studies in the acute phase of PE might not reflect the real OSA status. However, one study that enrolled 15 OSA patients shortly after acute PE could not demonstrate an impact of PE on the AHI of OSA patients.36 One weakness of the study includes the possibility of using different anticoagulant regimens after diagnosis of PE. It is not apparent whether this will affect the results.
5. CONCLUSION
The POPE study is currently enrolling patients to assess the prognostic significance of untreated OSA in normotensive patients with acute PE. It is anticipated that the findings of this study will enhance our understanding of the prognosis of PE. This rigorously designed study will address the role of OSA in the initial management of patients with PE, potentially leading to better care.
Conflicts of interest
The authors declare no potential conflicts of interest.
1.
Coordinator of the POPE Study: Eva Mañas (Hospital Ramón y Cajal, IRYCIS, Madrid, Spain).
POPE Steering Committee Members: Esther Barbero (Hospital Ramón y Cajal, IRYCIS, Madrid, Spain), David Jiménez (Hospital Ramón y Cajal, IRYCIS, Madrid, Spain), Miguel Ángel Martínez‐García (Hospital La Fe, Valencia, Spain), Miguel Castillo (Hospital Ramón y Cajal, IRYCIS, Madrid, Spain), Roger D. Yusen (Washington University School of Medicine, St. Louis, Missouri).
Data and Safety Monitoring Board: Deisy Barrios (Hospital Ramón y Cajal, IRYCIS, Madrid, Spain), Raquel Morillo (Hospital Ramón y Cajal, IRYCIS, Madrid, Spain).
Statistician: Alfonso Muriel (Hospital Ramón y Cajal, IRYCIS, Madrid, Spain).
Mañas E, Barbero E, Chiluiza D, et al. Impact of obstructive sleep apnea on cardiovascular outcomes in patients with acute symptomatic pulmonary embolism: Rationale and methodology for the POPE study. Clin Cardiol. 2017;40:1182–1188. 10.1002/clc.22834
Funding information This study received funding from Sociedad Española de Neumología y Cirugía Torácica (SEPAR) and Neumomadrid, 2017.
REFERENCES
- 1. Goldhaber SZ. Modern treatment of pulmonary embolism. Eur Respir J Suppl. 2002;35:22s–27s. [DOI] [PubMed] [Google Scholar]
- 2. Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386–1389. [DOI] [PubMed] [Google Scholar]
- 3. Douketis JD, Kearon C, Bates S, et al. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA. 1998;279:458–462. [DOI] [PubMed] [Google Scholar]
- 4. Büller HR, Davidson BL, Decousus H, et al; Matisse Investigators. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism [published correction appears in N Engl J Med. 2004;350:423]. N Engl J Med. 2003;349:1695–1702. [DOI] [PubMed] [Google Scholar]
- 5. Simonneau G, Sors H, Charbonnier B, et al; the THESEE Study Group . A comparison of low‐molecular‐weight heparin with unfractionated heparin for acute pulmonary embolism. N Engl J Med. 1997;337:663–669. [DOI] [PubMed] [Google Scholar]
- 6. Kürkciyan I, Meron G, Sterz F, et al. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med. 2000;160:1529–1535. [DOI] [PubMed] [Google Scholar]
- 7. Moores L, Aujesky D, Jiménez D, et al. Pulmonary Embolism Severity Index and troponin testing for the selection of low‐risk patients with acute symptomatic pulmonary embolism. J Thromb Haemost. 2010;8:517–522. [DOI] [PubMed] [Google Scholar]
- 8. Jiménez D, Yusen RD, Otero R, et al. Prognostic models for selecting patients with acute pulmonary embolism for initial outpatient therapy. Chest. 2007;132:24–30. [DOI] [PubMed] [Google Scholar]
- 9. Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low‐risk patients with pulmonary embolism. J Intern Med. 2007;261:597–604. [DOI] [PubMed] [Google Scholar]
- 10. Jaff MR, MS McMurtry, Archer SL, et al; on behalf of the American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology . Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association [published corrections appear in Circulation. 2012;125:e495 and 2012;126:e104]. Circulation. 2011;123:1788–1830. [DOI] [PubMed] [Google Scholar]
- 11. Goldhaber SZ. Assessing the prognosis of acute pulmonary embolism: tricks of the trade. Chest. 2008;133:334–336. [DOI] [PubMed] [Google Scholar]
- 12. Jiménez D, Aujesky D, Yusen RD. Risk stratification of normotensive patients with acute symptomatic pulmonary embolism. Br J Haematol. 2010;151:415–424. [DOI] [PubMed] [Google Scholar]
- 13. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population: a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaffe LM, Kjekshus J, Gottlieb SS. Importance and management of chronic sleep apnoea in cardiology. Eur Heart J. 2013;34:809–815. [DOI] [PubMed] [Google Scholar]
- 15. Lee CH, Sethi R, Li R, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133:2008–2017. [DOI] [PubMed] [Google Scholar]
- 16. Chung F, Abdullah HR, Liao P. STOP‐Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149:631–638. [DOI] [PubMed] [Google Scholar]
- 17. Investigators PIOPED. Value of ventilation/perfusion scan in acute pulmonary embolism: results of the Prospective Investigation of the Pulmonary Embolism Diagnosis (PIOPED). JAMA. 1990;263:2753–2759. [DOI] [PubMed] [Google Scholar]
- 18. Remy‐Jardin M, Remy J, Wattinne L, et al. Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single‐breath‐hold‐technique—comparison with pulmonary angiography. Radiology. 1992;185:381–387. [DOI] [PubMed] [Google Scholar]
- 19. Kearon C, Ginsberg JS, Hirsh J. The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism. Ann Intern Med. 1998;129:1044–1049. [DOI] [PubMed] [Google Scholar]
- 20. Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest. 1993;103:30–36. [DOI] [PubMed] [Google Scholar]
- 21. Chiner E, Arriero JM, Signes‐Costa J, et al. Validation of the Spanish version of the Epworth Sleepiness Scale in patients with a sleep apnea syndrome [article in Spanish]. Arch Bronconeumol. 1999;35:422–427. [DOI] [PubMed] [Google Scholar]
- 22. Cairns A, Wickwire E, Schaefer E, et al. A pilot validation Study for the NOX T3™ portable monitor for the detection of OSA. Sleep Breath. 2014;18:609–614. [DOI] [PubMed] [Google Scholar]
- 23. Redline S, Sanders MH, Lind BK, et al; Sleep Heart Health Research Group. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 24. Berry RB, Brooks R, Gamaldo CE, et al; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.2. http://aasm.org. Darien, IL: American Academy of Sleep Medicine; 2015. [Google Scholar]
- 25. Grifoni S, Olivotto I, Cecchini P, et al. Short‐term clinical outcome of patients with pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000;101:2817–2822. [DOI] [PubMed] [Google Scholar]
- 26. Hicks KA, Tcheng JE, Bozkurt B, et al; American College of Cardiology; American Heart Association . 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation. 2015;132:302–361. [DOI] [PubMed] [Google Scholar]
- 27. Prandoni P, Cogo A, Bernardi E, et al. A simple ultrasound approach for detection of recurrent proximal‐vein thrombosis. Circulation. 1993;88(4 part 1):1730–1735. [DOI] [PubMed] [Google Scholar]
- 28. von Elm E, Altman DG, Egger M, et al; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies [published correction appears in Ann Intern Med. 2008;148:168]. Ann Intern Med. 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 29. Little RJ, Rubin DB. Statistical Analysis With Missing Data. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 30. Rubin DB, Schenker N. Multiple imputation in health‐care databases: an overview and some applications. Stat Med. 1991;10:585–598. [DOI] [PubMed] [Google Scholar]
- 31. Berghaus TM, Faul C, von Scheidt W, et al. The prevalence of sleep‐disordered breathing among survivors of acute pulmonary embolism. Sleep Breath. 2016;20:213–218. [DOI] [PubMed] [Google Scholar]
- 32. Bosanquet JP, Bade BC, Zia MF, et al. Patients with venous thromboembolism appear to have higher prevalence of obstructive sleep apnea than the general population. Clin Appl Thromb Haemost. 2011;17:E119–E124. [DOI] [PubMed] [Google Scholar]
- 33. Ghiasi F, Ahmadpoor A, Amra B. Relationship between obstructive sleep apnea and 30‐day mortality among patients with pulmonary embolism. J Res Med Sci. 2015;20:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Konstantinides SV, Torbicki A, Agnelli G, et al; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069. [DOI] [PubMed] [Google Scholar]
- 35. Bibbins‐Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force . Screening for obstructive sleep apnea in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:407–414. [DOI] [PubMed] [Google Scholar]
- 36. Berghaus TM, Faul C, Unterer F, et al. Acute pulmonary embolism in patients with obstructive sleep apnoea: does it affect the severity of sleep‐disordered breathing? Sleep Breath. 2012;16:1267–1269. [DOI] [PubMed] [Google Scholar]


