Abstract
Background
Data on temporal trends of heart failure (HF) in acute coronary syndrome (ACS) are scarce.
Hypothesis
Improved treatment options may have led to lower case‐fatality rates (CFRs) during the last years in ACS complicated by HF.
Methods
Patients of the nationwide Acute Myocardial Infarction in Switzerland (AMIS)–Plus ACS registry were analyzed from 2000 to 2014.
Results
Of 36 366 ACS patients, 3376 (9.3%) had acute or chronic HF, 2111 (5.8%) de novo acute HF (AHF), 964 (2.7%) chronic HF (CHF), and 301 (0.8%) acute decompensated CHF (ADCHF). In‐hospital CFRs were highest in patients with ADCHF (32.6%) and de novo AHF (29.7%), followed by patients with CHF (12.9%) and without HF (3.2%, P < 0.001). Although in‐hospital CFRs gradually decreased in CHF patients (14.3% to 4.5%, P = 0.003) and patients without HF (3.5% to 2.2%, P < 0.001), they remained high in patients with ADCHF (36.4% to 40.0%, P = 0.45) and de novo AHF (50.0% to 29.4%, P = 0.37). Although there was an increase in specific ACS therapies in the cohort over time, ACS patients with HF received significantly less pharmacological and interventional ACS therapies than patients without HF. There was no significant change in HF medication rates except less frequent use of β‐blockers and diuretics in de novo AHF patients in recent years.
Conclusions
HF is present in 1 out of 10 patients presenting with ACS and is associated with high in‐hospital CFRs, particularly in acute HF. Although advances in ACS therapy improved in‐hospital CFRs in patients with no HF or CHF, CFRs remained unchanged and high in patients with acute HF and ACS over the last decade.
Keywords: Acute Coronary Syndrome, Heart Failure
1. INTRODUCTION
There is a close relationship between coronary artery disease (CAD) and heart failure (HF). Although CAD is the single most important cause of HF,1, 2, 3 HF in the setting of an acute coronary syndrome (ACS) is strongly associated with reduced in‐hospital and long‐term survival.4
According to its temporal presentation, HF can be classified as acute de novo HF (de novo AHF), acutely decompensated chronic HF (ADCHF), or chronic HF (CHF). In patients with stable CHF, pharmacological and device therapy has improved survival during the last decades,1, 5 whereas in AHF, this was not the case. However, in patients with CAD, coronary revascularization may be an additional and established strategy to improve survival in certain clinical situations with both acute6 and chronic HF.7, 8 Proposed mechanisms of recovery of left ventricular ejection fraction (LVEF) after revascularization include the recruitment of viable but stunned or hibernating myocardium and the prevention of progressive ventricular dysfunction and remodeling.7 However, previous studies not only excluded patients with a history of HF but were mostly older and therefore not reflecting contemporary management.
Despite advances in treatment, acute and chronic HF carry an increased risk when they coincide with ACS. Previous data from various large population‐based studies and randomized controlled trials show both an underuse of adequate therapy and increased mortality rate in patients with ACS and de novo HF, irrespective of specific electrocardiogram (ECG) changes such as ST elevation.4, 9, 10, 11, 12, 13, 14 However, previous studies excluded patients with a history of HF and, thus, only reflect the impact of de novo AHF on the prognosis of ACS.
Therefore, the aim of this study was to compare HF subpopulations with patients with no HF over time, that is, to study incidence, therapeutic management, outcome, and prognostic determinants of patients with ACS complicated by de novo HF, ADCHF, or CHF over the years 2000 to 2014 using the nationwide Acute Myocardial Infarction in Switzerland (AMIS)‐Plus Registry.
2. METHODS
The AMIS‐Plus Registry is a Swiss nationwide registry of patients with ACS endorsed by the Swiss Societies of Internal Medicine, Cardiology, and Intensive Care.15 Currently, more than 50 000 patients have been enrolled in 83 of the 106 (78%) acute cardiac care hospitals in Switzerland since the beginning of data collection in 1997.16 Participating hospitals report data in an anonymized fashion to a central database where they are checked for plausibility and consistency. In addition, monitoring visits are performed on a regular basis. Currently, 230 variables are obtained in the case report form that include medical history, clinical presentation, hospital course, immediate drug treatment, and discharge medication.15 The registry complies with the Declaration of Helsinki and has been approved by the Swiss Over‐Regional Ethics Committee for Clinical Studies, the Swiss Board for Data Security, and all cantonal ethic commissions.15 Informed written consent has been obtained from all subjects.
Patients were enrolled into the registry based on their final ACS diagnosis. Based on available data, patients between 2000 and 2014 were used for this analysis. For the registry, diagnosis of ACS is made using clinical symptoms, ECG alterations, and characteristic changes of cardiac enzymes such as troponin and creatine kinase. Myocardial infarction is defined as typical clinical symptoms and a significant raise and decrease of biomarkers with a further subdivision based on ECG characteristics into non–ST‐elevation or ST‐elevation myocardial infarction.17 Unstable angina pectoris was defined as typical symptoms of ischemia without elevation of biomarkers. For this analysis, CHF is defined according to the Charlson Comorbidity Index (ie, as exertional or paroxysmal nocturnal dyspnea responding symptomatically to digitalis, diuretics, or afterload‐reducing drugs), and dyspnea NYHA III and IV.18 AHF is defined as Killip class III or IV at admission (ie, as patients with overt pulmonary edema or cardiogenic shock, hypotension [systolic blood pressure <90 mm Hg], and evidence of peripheral vasoconstriction [oliguria, cyanosis, sweating]).19 Patients are divided into 4 groups: (1) patients without HF (ie, with neither CHF nor AHF), (2) patients with CHF, (3) patients with de novo AHF, and (4) patients with ADCHF (ie, with both CHF and AHF).
In‐hospital outcome was assessed directly in all patients. For this analysis, the primary endpoint was defined as in‐hospital all‐cause mortality (ie, case fatality rates [CFRs]), whereas the secondary endpoints were (1) in‐hospital major adverse cardiac and cerebrovascular events (MACCE) (ie, myocardial infarction, cerebrovascular events, and/or all‐cause mortality), (2) recurrent in‐hospital myocardial infarction as defined previously, and (3) in‐hospital cerebrovascular events.15
Categorical variables are presented as percentages and analyzed with the nonparametric Pearson χ2 test or Fisher exact test, as appropriate. Normally distributed continuous variables are presented as mean ± standard deviation and analyzed using the analysis of variance test with the Bonferroni correction for the mean differences between the groups, whereas non‐normally distributed continuous variables are presented as median with interquartile ranges and analyzed with the Mann–Whitney U test. All statistical tests are 2‐tailed. A P value of <0.05 is considered statistically significant. To analyze if varying types of HF were independent predictors of in‐hospital mortality, multivariate logistic regression analysis was done using no HF as the reference and adjusting for the following baseline variables: age, sex, ST‐elevation myocardial infarction, and comorbidities according to Charlson Comorbidity Index >1. SPSS version 19 (IBM, Armonk, NY) was used for all statistical analyses.
3. RESULTS
3.1. Patient population
The total population consisted of 41 801 ACS patients, of whom 36 366 (87%) had data on HF available (Figure 1). These patients form the basis of the present analysis. Of these patients, 3376 (9.3%) had HF, of whom 964 (2.7%) had CHF, 2111 (5.8%) had de novo AHF, and 301 (0.8%) had ADCHF. The percentages of the different groups remained stable over the years (P = 0.36).
Figure 1.
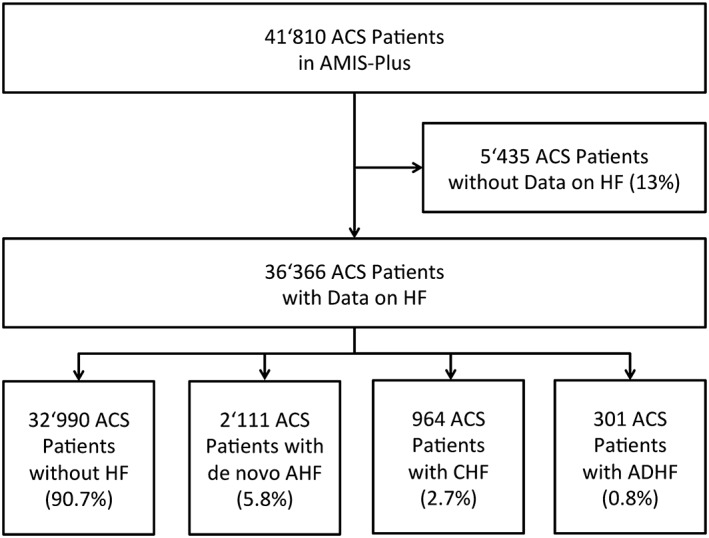
Flowchart of the patient population. Abbreviations: ACS, acute coronary syndrome; ADHF, acutely decompensated heart failure; AHF, acute heart failure; AMIS, acute myocardial infarction in Switzerland; CHF, chronic heart failure; HF, heart failure.
3.2. Baseline characteristics
Patients with CHF and ADCHF were older with more cardiovascular risk factors (hypertension, diabetes, dyslipidemia) and a higher burden of cardiovascular comorbidities (CAD, cerebrovascular disease, chronic kidney disease) (Table 1). LVEF was significantly decreased in patients with HF with the severest reduction in the group with ADCHF. Regarding ACS treatment, patients with a history of HF (CHF and ADCHF) had lower treatment rates of aspirin, P2Y12 inhibitors, glycoprotein IIb/IIIa antagonists, and percutaneous coronary intervention. This underuse of ACS treatment was particularly evident in ADCHF patients. In contrast, patients with de novo AHF had the highest rates of ST‐elevation myocardial infarction and resuscitation prior to admission, and were treated with the highest rates of vasopressors of all patient groups.
Table 1.
Baseline characteristics of ACS patients according to heart failure groups
| No HF | CHF | De novo AHF | ADCHF | P | |
|---|---|---|---|---|---|
| No. of patients | 32 990 | 964 | 2111 | 301 | |
| Demographic data | |||||
| Sex, female, no. (%) | 8704 (26.4) | 324 (33.6) | 690 (32.7) | 119 (39.5) | <0.001 |
| Age, y, mean (SD) | 65.5 (13.1) | 76.9 (10.7) | 70.9 (12.9) | 77.8 (9.7) | <0.001 |
| Clinical data | |||||
| Delay, median (IQR), min | 225 (110–629) | 240 (125–655) | 170 (80–453) | 228 (86–780) | <0.001 |
| Resuscitation prior admission, no. (%) | 935/32 784 (2.9) | 20/954 (2.1) | 581/2099 (27.7) | 35/297 (11.8) | <0.001 |
| STEMI, no. (%) | 18 076 (54.8) | 428 (44.4) | 1321 (62.6) | 154 (51.2) | <0.001 |
| Risk factors, no. (%) | |||||
| Hypertension | 19 064/31 511 (60.4) | 778/931 (83.6) | 1303/1898 (68.7) | 248/284 (87.3) | <0.001 |
| Diabetes | 6099/31 893 (19.1) | 349/942 (37.0) | 645/1954 (33.0) | 140/288 (48.6) | <0.001 |
| Dyslipidemia | 17 222/29 479 (58.4) | 543/825 (65.8) | 884/1696 (52.1) | 166/250 (66.4) | 0.059 |
| Obesity, BMI >30 | 5935/28 530 (20.8) | 192/809 (23.7) | 314/1557 (20.2) | 66/240 (27.5) | 0.204 |
| Smoking | 11 744/30 276 (38.8) | 176/855 (20.6) | 625/1676 (37.3) | 51/253 (20.2) | <0.001 |
| Known CAD | 11 202/32 638 (34.3) | 689/950 (72.5) | 742/2020 (36.7) | 211/295 (71.5) | <0.001 |
| Echo LVEF <30%a | 857/13 648 (6.3) | 66/228 (28.9) | 222/666 (33.3) | 37/59 (62.7) | <0.001 |
| Angio LVEF <35%a | 148/3123 (4.7) | 12/48 (25.0) | 84/277 (30.3) | 7/20 (35.0) | <0.001 |
| Cerebrovascular disease | 1747 (5.3) | 139 (14.4) | 214 (10.1) | 65 (21.6) | <0.001 |
| Renal disease | 1957 (5.9) | 233 (23.1) | 291 (13.8) | 81 (26.9) | <0.001 |
| Cancer | 1765 (5.4) | 92 (9.5) | 164 (7.8) | 39 (13.0) | <0.001 |
| Medicaments, no. (%) | |||||
| Aspirin | 31 501/32 890 (95.8) | 828/961 (86.2) | 1881/2101 (89.5) | 235/299 (84.6) | <0.001 |
| P2Y12 inhibitors | 26 169/32 814 (79.7) | 526/956 (55.0) | 1322/2091 (63.2) | 132/298 (44.3) | <0.001 |
| GP IIb/IIIa antagonist | 8739/32 394 (27.0) | 114/953 (12.0) | 432/2077 (20.8) | 33/297 (11.1) | <0.001 |
| Heparin | 28 496/32 763 (87.0) | 746/960 (77.7) | 1752/2099 (83.5) | 247/300 (82.3) | <0.001 |
| β‐Blocker | 21 633/32 673 (66.2) | 594/959 (61.9) | 760/2074 (36.6) | 127/297 (42.8) | <0.001 |
| ACEI/ARB antagonist | 17 476/32 677 (53.5) | 565/959 (58.9) | 777/2080(37.4) | 148/299 (49.5) | <0.001 |
| Nitrate | 17 454/32 456 (53.8) | 550/958 (57.4) | 924/2072 (44.6) | 178/298 (59.7) | <0.001 |
| Diuretic | 4837/25 545 (18.9) | 408/669 (61.0) | 882/1671 (52.8) | 161/207 (77.8) | <0.001 |
| Statin | 25 127/32 697 (76.8) | 597/955 (62.5) | 1132/2083 (54.3) | 158/298 (54.3) | <0.001 |
| Vasopressors | 1269/25 427 (5.0) | 39/664 (5.9) | 679/1674 (40.6) | 59/202 (29.2) | <0.001 |
| Intervention, no. (%) | |||||
| Any PCI | 25 966/32 036 (81.1) | 458/935 (49.0) | 1352/2050 (66.0) | 107/295 (36.3) | <0.001 |
| Primary PCI in STEMI | 15 423/17 786 (86.7) | 215/417 (51.6) | 962/1298 (74.1) | 63/152 (41.4) | <0.001 |
| CABG | 864/32 248 (2.7) | 19/945 (2.0) | 73/2061 (3.5) | 6/298 (2.0) | 0.228 |
| Mechanical support device | 871/32 302 (2.7) | 33/941 (3.5) | 461/2071 (22.3) | 63/152 (9.7) | <0.001 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitors; ACS, acute coronary syndrome; ADCHF, acute decompensated heart failure; AHF, acute heart failure; ARB, angiotensin receptor blockers; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CHF, chronic heart failure; GP, glycoprotein; HF, heart failure; IQR, interquartile range; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention, SD, standard deviation; STEMI, ST‐elevation myocardial infarction.
Values for LVEF were measured in different patients (no double counting).
3.3. In‐hospital outcome
In‐hospital CFRs were highest in patients with ADCHF (32.6%), followed by patients with de novo AHF (29.7%) and patients with CHF (12.9%), whereas patients without HF had the lowest in‐hospital CFRs of 3.2% (P < 0.001) (Table 2). Similarly, as seen for in‐hospital CFRs, MACCE were highest in ADCHF, followed by de novo AHF and CHF (P < 0.001). Patients with HF, notwithstanding whether they were acutely decompensated, de novo, or chronic, developed significantly more recurrent myocardial infarctions during hospitalization (P < 0.001), whereas cerebrovascular events were mainly affecting patients with AHF (P < 0.001).
Table 2.
Outcome of ACS patients according to heart failure groups
| No HF | CHF | De novo AHF | ADCHF | P | |
|---|---|---|---|---|---|
| In hospital, no. of patients | 32 990 | 964 | 2111 | 301 | |
| All‐cause mortality (%) | 1060 (3.2) | 124 (12.9) | 627 (29.7) | 98 (32.6) | <0.001 |
| Myocardial infarction (%) | 311/32 743 (0.9) | 29/956 (3.0) | 44/2092 (2.1) | 7/297 (2.4) | <0.001 |
| Cerebrovascular event (%) | 185/32 546 (0.6) | 6/949 (0.6) | 52/2090 (2.5) | 10/297 (3.4) | <0.001 |
| MACCE (%) | 1412/32 559 (4.3) | 141/950 (14.8) | 671/2097 (32.0) | 106/297 (35.7) | <0.001 |
Abbreviations: ACS, acute coronary syndrome; ADCHF, acute decompensated heart failure; AHF, acute heart failure; CHF, chronic heart failure; HF, heart failure; MACCE, major adverse cardiac and cerebrovascular events.
3.4. Multivariate analysis
The presence of AHF on admission was an independent predictor of mortality for patients with CHF (odds ratio 3.08, 95% confidence interval 2.24‐4.22) and also for patients without preexisting heart failure (odds ratio 9.9, 95% confidence interval 8.85‐11.2).
3.5. Therapeutic management and mortality 2000–2014
Between 2000 and 2014, the use of a specific ACS therapy increased from below 40% in 2000 to over 70% in 2014 for P2Y12 inhibitors, and from below 50% in 2000 to 65% in 2014 for percutaneous coronary intervention in all HF patients (both P < 0.001) (Figure 2A). There was little change in HF medication rates except for β‐blockers and diuretics, which were less frequently used in patients without HF and patients with de novo AHF in recent years (both P < 0.001). In addition, angiotensin‐converting enzyme (ACE) inhibitors or angiotensin‐receptor blockers were used in approximately 50% of patients, with an increase in patients with no HF (P < 0.001) or CHF (P = 0.005), but no significant change over time in patients with de novo AHF (P = 0.091) or ADCHF (P = 0.94) (Figure 2B). The odds ratio with 95% confidence intervals for an additional admission year for in‐hospital mortality adjusted for age, sex, ACS type, and comorbidities for patients with no HF was 0.95 (0.93‐0.96; P < 0.001), for patients with CHF 0.92 (0.87‐0.98; P = 0.004), for patients with de novo AHF 1.01 (0.98‐1.04; P = 0.45), and for patients with ADCHF 1.01 (0.94‐1.08; P = 0.70). Therefore, the decrease in adjusted CFRs in patients with no HF was 5% and in patients with CHF 8% per year, without decrease in de novo AHF and ADCHF patients.
Figure 2.
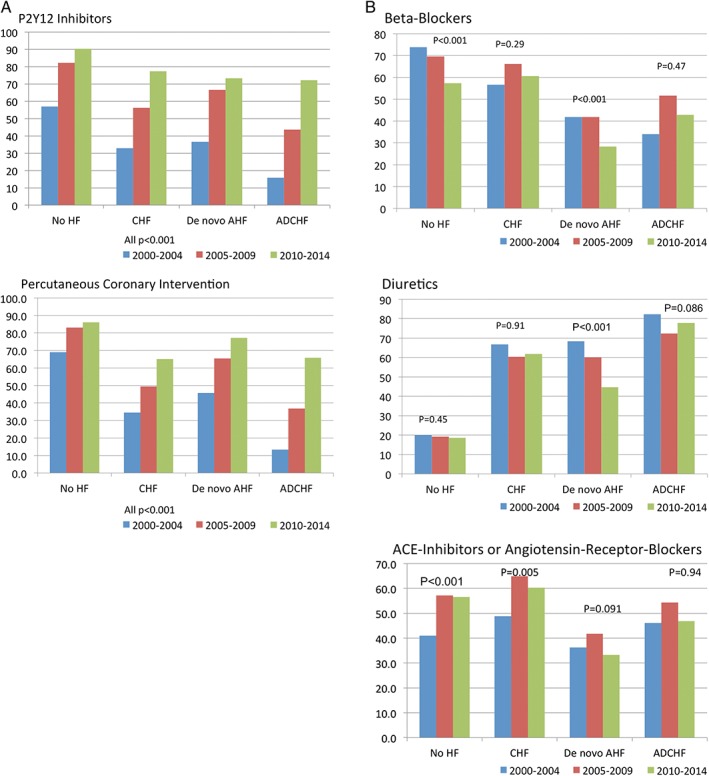
Rates of specific treatment over time for the 4 groups (no HF, CHF, de novo AHF, and ADCHF): (A) ACS therapy. (B) HF therapy. Abbreviations: ACE, angiotensin‐converting enzyme; ACS, acute coronary syndrome; ADHF, acutely decompensated heart failure; AHF, acute heart failure; CHF, chronic heart failure; HF, heart failure.
Between 2000 and 2014, in‐hospital mortality gradually decreased in ACS patients without HF (3.5% to 2.2%, P < 0.001) and in those with CHF (14.3% to 4.5%, P = 0.03). In contrast, patients with ACS and concomitant AHF, be it de novo AHF or ADCHF, exhibited an unchanged high in‐hospital mortality over time (36.4% in 2000 and 40.0% in 2014, P = 0.45 for ADCHF; and 50.0% in 2000 and 29.4% in 2014, P = 0.37 for de novo AHF) (Figure 3).
Figure 3.
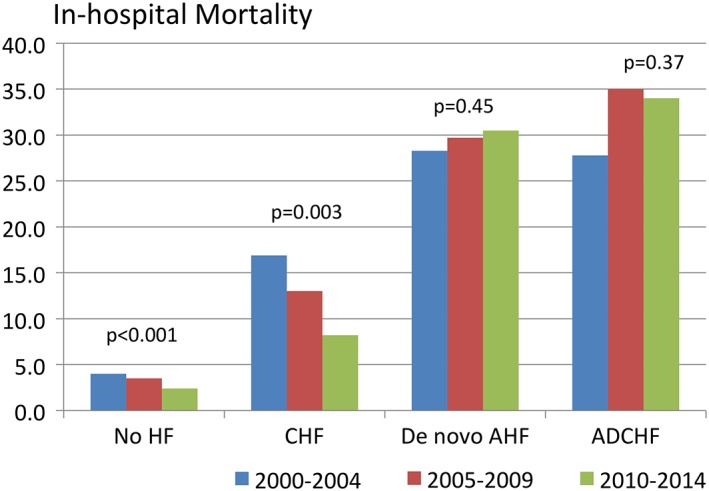
In‐hospital all‐cause mortality rates over time for the 4 groups (no HF, CHF, de novo AHF, and ADCHF). Abbreviations: ADHF, acutely decompensated heart failure; AHF, acute heart failure; CHF, chronic heart failure; HF, heart failure.
4. DISCUSSION
In ACS, HF has an important impact on short‐ and long‐term outcomes. The present data from a large nationwide longitudinal observational study demonstrate unchanged, high in‐hospital CFRs in ACS patients with acute HF despite recent advances in pharmacological therapies.
Although less than 10% of patients with an ACS present with HF, this group of patients contributes to a large part to the in‐hospital mortality rates in ACS patients. Our data demonstrate that ACS patients presenting with either de novo AHF or ADCHF exhibit an up to 10‐fold increase in in‐hospital mortality and MACCE compared to patients without HF, without any change over the observational period. Although rates for specific ACS therapy increased in patients with HF over the years, rates for specific HF therapy remained unchanged. Importantly, the high‐risk group of patients with either de novo AHF or ADCHF receives the lowest rates of pharmacological and interventional therapies.
Despite recent advances in therapy, outcome in HF still is dismal. In patients with stable CHF, pharmacological therapy including ACE inhibitors, angiotensin receptor blockers, β‐blockers, mineralocorticoid‐receptor antagonists, and just recently angiotensin‐receptor‐neprilysin inhibitors, as well as the implementation of devices such as cardiac resynchronization therapy and the use of implantable cardiac defibrillators, seem to have improved survival during the last decades.1, 5, 20 Although historical 5‐year mortality rates in CHF were as high as 60%, modern therapy was able to decrease these rates by approximately 50%.1 Similar advances have been reported for ACS21 and for cardiogenic shock,16 where the use of more intense drug treatment and percutaneous coronary interventions was associated with better outcomes. In AHF, drugs such as diuretics, nitrates, dobutamine,22 nesiritide,23 milrinone,3, 24 and levosimendan25 are largely used to improve symptoms but did not improve survival. Therefore, effective treatment options are still lacking and the prognosis remains poor, especially in combination with ACS.
The present analysis of over 36 000 patients admitted to acute cardiac care hospitals in Switzerland corroborates these findings by showing high in‐hospital morbidity and mortality rates in patients presenting in a real‐world population with both ACS and HF. In line with other registries, almost two‐thirds of patients with ACS and HF presented with de novo AHF.26, 27 According to the EuroHeart Failure Survey II, ACS is the predominant cause of de novo AHF, contributing to 42% of de novo AHF.28 Specifically, in the case of large myocardial infarction, extensive ischemia with myocardial stunning or the presence of ischemic mitral regurgitation, ACS may be a trigger of de novo AHF or ADCHF. In these patients, in‐hospital mortality is substantially higher than in ACS patients lacking acute HF symptoms.26, 29 In contrast to the SWEDEHEART (Swedish Web‐system for Enhancement and Development of Evidence‐based care in Heart disease Evaluated According to Recommended Therapies) Registry reporting a decrease in the incidence of HF complicating acute myocardial infarction between 1996 and 2008, no decrease in the incidence of concomitant ACS and HF was found in the AMIS‐Plus Registry between 2000 and 2014. This discrepancy might be due to differences in inclusion criteria and definitions of concomitant HF used in the 2 registries. In the AMIS‐Plus Registry, patients with any ACS were included, and a more stringent definition of HF was applied (ie, Killip class ≥ III resolving of symptoms following HF therapies), whereas in the SWEDEHEART Registry, only patients with acute myocardial infarction were included and the diagnosis of HF required the presence of Killip class ≥ II or the use of diuretics.29 These differences might account for the lower incidence of HF in the AMIS‐Plus Registry compared to the SWEDEHEART Registry (9% vs 46%, respectively).
Importantly, patients presenting with acute HF received lower rates of potentially life‐saving therapies. In these patients, intensity of drug treatment and rates of percutaneous coronary revascularization were lower than in patients without acute HF. The reasons for this finding most probably are the missing evidence for the prognostic benefit of drug treatment in this patient population, the difficult clinical or hemodynamic situation on admission that obviates optimal HF treatment, and the transfer to a catheterization laboratory, as shown in previous registries, where there was an impaired prognosis with an underuse of therapies in patients at high risk.9, 10, 11, 12, 13, 30
Of special concern is the fact that acutely ill patients with the highest risk (ie, patients with de novo AHF or ADCHF) showed a decreasing rate of specific HF therapy and an unchanged high mortality over the years, whereas more stable patients with CHF were treated more adequately and had decreasing mortality rates. In the present cohort, ACE‐inhibitor/angiotensin receptor blocker and β‐blocker use was lowest in the groups with the highest in‐hospital mortality (de novo AHF and ADCHF). In patients with de novo AHF, β‐blocker use even further decreased over the observational period. There is growing evidence that lack of β‐blocker therapy or β‐blocker withdrawal during an episode of AHF is associated with a significant increase in in‐hospital mortality and short‐term mortality in HF patients with reduced LVEF.31 In addition, the extent of heart rate decrease in response to β‐blockers during hospitalization for AHF correlates with survival, suggesting that heart rate control by β‐blockers may be important in AHF.32 Previous randomized trials in high‐risk patients with cardiogenic shock showed better survival in patients who were treated more intensely with percutaneous coronary intervention.33 It remains, however, unclear if a more aggressive invasive treatment approach would lead to similar results in patients with ACS presenting with HF or rather to more complications and higher mortality.
Based on the present results, new concepts of HF treatment in ACS patients should be established, particularly in the subset of patients presenting with AHF. This may include the rapid onset of combined invasive and pharmacological treatment measures directed to cardio and end‐organ protection. Aggressive treatment with afterload‐decreasing and cardioprotective drugs,34 as well as invasive strategies including percutaneous coronary interventions and temporary mechanical circulatory support (for selected patients),35 may be performed as soon as possible in ACS patients with acute HF. Such strategies of intensified HF therapy should be tested in future clinical trials to assess their benefit and complications in this high‐risk patient group.
As a retrospective analysis of a large registry, the current analysis is subject to inherent limitations. Patients were enrolled based on their final diagnosis of ACS and not HF, and therefore represent rather an ACS than a HF population. Accordingly, HF was defined differently than in specific HF guidelines. In some patients, the information about HF was missing completely, whereas there was no indication of the type of HF (ie, HF with reduced ejection fraction or HF with preserved ejection fraction) in most patients. In addition, there was no central adjudication of events. However, the large database, the clear definitions for HF, and the endpoints used make this analysis sound. Finally, due to the advent of high‐sensitivity troponin during the observational period, patient characteristics and diagnosis in the different groups might have changed over the years, and treatments such as P2Y12 inhibitors and percutaneous interventions may actually have been harmful in some patient groups who would be under‐represented or excluded from clinical trials.
5. CONCLUSION
Approximately 10% of ACS patients have either chronic or acute HF. Patients with acute HF have both the highest mortality and the lowest treatment rates. Although in recent years, advances in pharmacological and interventional therapies improved in‐hospital mortality of patients without HF and CHF patients, mortality of ACS patients with acute HF remains unchanged over the last decade. Acute HF with or without hemodynamic compromise is an independent predictor of in‐hospital outcome in patients with ACS. Strategies for the improvement of outcomes in these patients should be established and tested in larger, randomized trials.
Conflicts of interest
The authors declare no potential conflicts of interest.
Jeger RV, Pfister O, Radovanovic D, et al. Heart failure in patients admitted for acute coronary syndromes: A report from a large national registry. Clin Cardiol. 2017;40:907–913. 10.1002/clc.22745
Raban V. Jeger, MD, and Otmar Pfister, MD, are joint first authors.
Funding information The registry received unrestricted grants from the Swiss Heart Foundation and from Abbott AG, Amgen AG, AstraZeneca AG, Bayer AG, B. Braun Medical AG, Biotronik AG, Daiichi‐Sankyo/Lilly AG, Johnson & Johnson AG–Cordis Division, A. Menarini AG, Mepha Pharma AG, Merck Sharp & Dohme–Chibret AG, Novartis Pharma AG, Pfizer AG, Servier SA, SIS Medical AG, St. Jude Medical, Takeda Pharma AG, and Vascular Medical AG, which are all in Switzerland. The sponsors did not have any role in the study design; in the collection, analysis, and interpretation of data; in writing the report; or in the decision to submit the article for publication.
REFERENCES
- 1. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J . 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 2. Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW; ADHERE Scientific Advisory Committee and Investigators . Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J . 2007;153:1021–1028. [DOI] [PubMed] [Google Scholar]
- 3. Cuffe MS, Califf RM, Adams KF, et al. Short‐term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. [DOI] [PubMed] [Google Scholar]
- 4. Steg PG, Dabbous OH, Feldman LJ, et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation. 2004;109:494–499. [DOI] [PubMed] [Google Scholar]
- 5. Sacks CA, Jarcho JA, Curfman GD. Paradigm shifts in heart‐failure therapy—a timeline. N Engl J Med. 2014;371:989–991. [DOI] [PubMed] [Google Scholar]
- 6. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization and long‐term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006;295:2511–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phillips HR, O'Connor CM, Rogers J. Revascularization for heart failure. Am Heart J . 2007;153(4 suppl):65–73. [DOI] [PubMed] [Google Scholar]
- 8. Velazquez EJ, Lee KL, Jones RH, et al. Coronary‐artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roe MT, Chen AY, Riba AL, et al. Impact of congestive heart failure in patients with non‐ST‐segment elevation acute coronary syndromes. Am J Cardiol. 2006;97:1707–1712. [DOI] [PubMed] [Google Scholar]
- 10. Wu AH, Parsons L, Every NR, Bates ER; Second National Registry of Myocardial Infarction . Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI‐2). J Am Coll Cardiol . 2002;40:1389–1394. [DOI] [PubMed] [Google Scholar]
- 11. Segev A, Strauss BH, Tan M, et al. Prognostic significance of admission heart failure in patients with non‐ST‐elevation acute coronary syndromes (from the Canadian Acute Coronary Syndrome Registries). Am J Cardiol. 2006;98:470–473. [DOI] [PubMed] [Google Scholar]
- 12. Bahit MC, Lopes RD, Clare RM, et al. Heart failure complicating non‐ST‐segment elevation acute coronary syndrome: timing, predictors, and clinical outcomes. JACC Heart Fail. 2013;1:223–229. [DOI] [PubMed] [Google Scholar]
- 13. Steg PG, Kerner A, Van de Werf F, et al. Impact of in‐hospital revascularization on survival in patients with non‐ST‐elevation acute coronary syndrome and congestive heart failure. Circulation. 2008;118:1163–1171. [DOI] [PubMed] [Google Scholar]
- 14. Kaul P, Ezekowitz JA, Armstrong PW, et al. Incidence of heart failure and mortality after acute coronary syndromes. Am Heart J . 2013;165:379–385.e372. [DOI] [PubMed] [Google Scholar]
- 15. Radovanovic D, Erne P. AMIS Plus: Swiss registry of acute coronary syndrome. Heart. 2010;96:917–921. [DOI] [PubMed] [Google Scholar]
- 16. Jeger RV, Radovanovic D, Hunziker PR, et al. Ten‐year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med. 2008;149:618–626. [DOI] [PubMed] [Google Scholar]
- 17. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. [DOI] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 19. Killip T III, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol . 1967;20:457–464. [DOI] [PubMed] [Google Scholar]
- 20. McMurray JJ, Packer M, Desai AS, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 21. Chan MY, Sun JL, Newby LK, et al. Trends in clinical trials of non‐ST‐segment elevation acute coronary syndromes over 15 years. Int J Cardiol. 2013;167:548–554. [DOI] [PubMed] [Google Scholar]
- 22. Colucci WS, Wright RF, Braunwald E. New positive inotropic agents in the treatment of congestive heart failure. Mechanisms of action and recent clinical developments. 1. N Engl J Med . 1986;314:290–299. [DOI] [PubMed] [Google Scholar]
- 23. O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 24. Colucci WS, Wright RF, Braunwald E. New positive inotropic agents in the treatment of congestive heart failure. Mechanisms of action and recent clinical developments. 2. N Engl J Med . 1986;314:349–358. [DOI] [PubMed] [Google Scholar]
- 25. Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297:1883–1891. [DOI] [PubMed] [Google Scholar]
- 26. Tarvasmäki T, Harjola VP, Nieminen MS, et al. Acute heart failure with and without concomitant acute coronary syndromes: patient characteristics, management, and survival. J Card Fail. 2014;20:723–730. [DOI] [PubMed] [Google Scholar]
- 27. AlFaleh H, Elasfar AA, Ullah A, et al. Acute heart failure with and without acute coronary syndrome: clinical correlates and prognostic impact (From the HEARTS registry). BMC Cardiovasc Disord. 2016;16:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. [DOI] [PubMed] [Google Scholar]
- 29. Desta L, Jernberg T, Löfman I, et al. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail . 2015;3:234–242. [DOI] [PubMed] [Google Scholar]
- 30. Jeger RV, Harkness SM, Ramanathan K, et al. Emergency revascularization in patients with cardiogenic shock on admission: a report from the SHOCK trial and registry. Eur Heart J. 2006;27:664–670. [DOI] [PubMed] [Google Scholar]
- 31. Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of beta‐blocker withdrawal in acute decompensated heart failure: a systematic review and meta‐analysis. JACC Heart Fail. 2015;3:647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeVore AD, Mi X, Mentz RJ, et al. Discharge heart rate and β‐blocker dose in patients hospitalized with heart failure: findings from the OPTIMIZE‐HF registry. Am Heart J. 2016;173:172–178. [DOI] [PubMed] [Google Scholar]
- 33. Jeger RV, Urban P, Harkness SM, et al. Early revascularization is beneficial across all ages and a wide spectrum of cardiogenic shock severity: a pooled analysis of trials. Acute Card Care. 2011;13:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin‐2, for treatment of acute heart failure (RELAX‐AHF): a randomised, placebo‐controlled trial. Lancet. 2013;381:29–39. [DOI] [PubMed] [Google Scholar]
- 35. Brown JL, Estep JD. Temporary percutaneous mechanical circulatory support in advanced heart failure. Heart Fail Clin. 2016;12:385–398. [DOI] [PubMed] [Google Scholar]


