Abstract
Background
It is unclear whether more severe coronary atherosclerosis is a prerequisite to an initial acute coronary event in women vs men.
Hypothesis
Women may have more severe coronary atherosclerosis than men in patients with acute coronary event.
Methods
We used intravascular optical coherence tomography (OCT) to evaluate gender differences in culprit‐plaque morphology in patients with a first ST‐segment elevation myocardial infarction (STEMI).We retrospectively enrolled 211 consecutive patients who experienced a first STEMI and underwent an OCT examination of their infarct‐related artery before primary percutaneous coronary intervention.
Results
Of the 211 patients enrolled, 162 (76.7%) were men and 49 (23.2%) were women. The women were significantly older than the men (mean age, 60.2 ± 8.2 vs 55.7 ± 11.2 years; P = 0.01) and less likely to be current smokers (P = 0.02). Moreover, the delay from symptom onset to reperfusion was longer in women than in men (7.6 ± 6.1 vs 5.5 ± 4.4 hours; P = 0.01). The OCT data indicated that there were no gender differences in culprit‐plaque morphology, including lipid length, lipid arc, minimum fibrous cap thickness, or minimum lumen area. Additionally, no gender differences were found in the prevalence of plaque rupture, thin‐cap fibroatheroma, residual thrombus, microvessels, macrophages, cholesterol crystals, or calcification.
Conclusions
Among patients presenting with a first STEMI, there were no differences in culprit plaque features between women and men.
Keywords: Acute Myocardial Infarction, Gender, Optical Coherence Tomography, Plaque Characteristics
1. INTRODUCTION
A generally increased focus on modifying cardiovascular risks and beginning prompt coronary reperfusion has improved the prognosis in patients with ST‐segment elevation myocardial infarction (STEMI) over the past 2 decades.1, 2 Notably, a gender difference still exists in the presentation, pathophysiology, treatment, and outcomes of STEMI.2 Women presenting with STEMI are usually older and have more comorbidities and higher mortality than do men.2, 3, 4, 5, 6 Some studies have suggested that compared with men, women are relatively protected from coronary artery disease (CAD) and atherosclerosis7, 8 and may sustain more severe coronary atherosclerosis before experiencing their first acute coronary event. Pathological studies suggest that gender may confer differences in coronary‐plaque characteristics.9 These findings suggest the existence of a potential distinct form of atherosclerotic pathophysiology that is specific to women. However, few available studies have explored the in vivo association between gender and culprit‐plaque characteristics in patients with STEMI.
Optical coherence tomography (OCT) is a feasible intravascular imaging modality. This novel approach has a high resolution of approximately 10 μm, which permits accurate in vivo evaluation of plaque characteristics that are closely correlated with the pathology.10
The purpose of the present study was to evaluate differences in OCT‐estimated culprit‐plaque characteristics between women and men with a first STEMI.
2. METHODS
2.1. Study population
We retrospectively enrolled 211 consecutive patients who were admitted to the Second Affiliated Hospital of Harbin Medical University for a first STEMI between January 2015 and December 2015. All patients underwent primary percutaneous coronary intervention (PCI) and a pre‐intervention OCT examination within 12 hours of symptom onset. STEMI was diagnosed in cases in which typical ischemic chest pain was sustained for ≥30 minutes in combination with a ST‐segment elevation ≥1 mm in ≥2 contiguous electrocardiography leads and in which myocardial necrosis markers (creatine kinase‐MB and troponin I) were elevated. The exclusion criteria included left main disease, cardiogenic shock, serious hepatic or renal dysfunction, a massive thrombus, and poor OCT image quality. Patients with prior history of MI, PCI, or coronary artery bypass grafting, or severe left ventricular dysfunction, were also excluded because it would be potentially difficult to acquire and interpret OCT‐obtained images from patients with these conditions.
This study was conducted with the approval of the Ethics Review Committee of the Second Affiliated Hospital of Harbin Medical University, Harbin, China. All patients provided written informed consent for this study.
2.2. OCT image acquisition
All patients received standard antiplatelet and antithrombotic therapy with a loading dose of oral aspirin (300 mg) and ticagrelor (180 mg) and an intravenous bolus injection of unfractionated heparin (100 IU/kg) prior to the catheterization procedure. At the discretion of the interventional cardiologist, aspiration thrombectomy was manually performed using an aspiration catheter (Export Aspiration Catheter; Medtronic CardioVascular, Santa Rosa, CA) prior to intracoronary imaging. After antegrade coronary flow was restored, OCT pullback of the infarct‐related artery was performed using a frequency‐domain OCT system (C7‐XR OCT Intravascular Imaging System; St. Jude Medical, St. Paul, MN) before stent deployment.
2.3. Angiographic analysis
An angiographic analysis was performed using a validated automated edge‐detection algorithm by an independent investigator who was blinded to the OCT findings. The culprit lesion was identified by combining analyses of the electrocardiographic findings, left ventricular wall motion abnormalities, and angiographic lesion morphology. The quantitative coronary angiography included assessments of the minimum lumen diameter, reference diameter, stenosis diameter, and lesion length.
2.4. OCT image analysis
The acquired OCT images were analyzed by 2 experienced investigators who were blinded to clinical information. Any disagreement was resolved by consensus. Representative OCT images are shown in the Figure 1. The culprit lesion was categorized as a plaque rupture (PR), plaque erosion (PE), or calcified nodule (CN).11 PR was defined as the presence of a cavity in the plaque that was discontinuous with the fibrous cap (Figure 1A). PE was defined as the presence of a thrombus on an irregular luminal surface with no evidence of fibrous‐cap disruption (Figure 1B). CN was classified as fibrous‐cap disruption over a calcified plaque characterized by superficial calcium, protruding calcification, and the presence of substantive calcium distal and/or proximal to the lesion (Figure 1C). Plaque characteristics were defined using previously validated criteria.12, 13, 14 The plaques were classified as lipid plaques, fibrous plaques, and calcific plaques. A lipid plaque was defined as a signal‐rich fibrous cap with a signal‐poor region and a diffuse border. For each lipid plaque, the minimum fibrous‐cap thickness and maximum lipid arc were measured. A thin‐cap fibroatheroma (TCFA) was defined as a lipid‐rich plaque with a lipid arc ≥90° and a thin fibrous cap thickness < 65 μm (Figure 1D). A fibrous plaque was defined as a plaque that appeared as a homogeneous and highly backscattered region with low attenuation. A calcified plaque was defined as the presence of a low‐scattering region with a sharp border (Figure 1E). Microvessels were defined as signal tubular or vesicular structures that were viewed in ≥3 consecutive frames. Cholesterol crystals were identified as linear, signal‐rich structures adjacent to the plaque (Figure 1F). Macrophage infiltration was characterized by the presence of signal‐rich, distinct, or confluent punctate regions with heterogeneous backward shadows. A residual thrombus was identified as a mass (>250 μm in diameter) that protruded into the vessel lumen from the luminal surface (Figure 1G and H).
Figure 1.
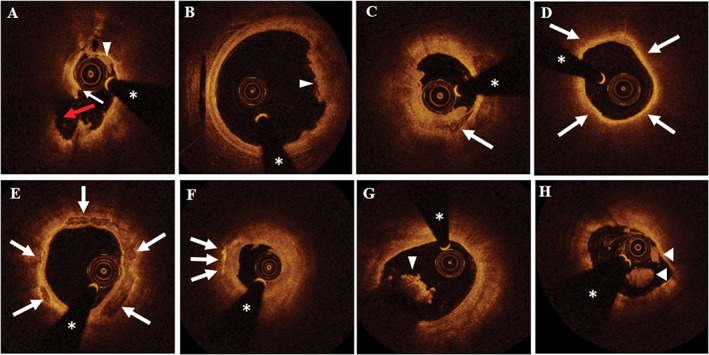
Representative OCT images of culprit‐plaque characteristics. (A) A PR was observed as a luminal thrombus (triangle) that was associated with a fibrous‐cap disruption (white arrow) and cavity formation (red arrow). (B) A PE was imaged as a luminal white thrombus (triangle) over a fibrous plaque with a thick cap without evidence of disruption. (C) A CN was detected as a disrupted fibrous cap over a calcified plaque that was characterized by superficial calcium (arrow). (D) TCFA (arrows delineate a large lipid pool). (E) Calcification (arrows indicate calcium deposits). (F) The arrows indicate cholesterol crystals. (G) A triangle indicates a red thrombus. (H) Triangles indicate a white thrombus. The asterisks indicate a guidewire shadow artifact. Abbreviations: CN, calcified nodule; OCT, optical coherence tomography; PE, plaque erosion; PR, plaque rupture; TCFA, thin‐cap fibroatheroma
2.5. Statistical analysis
All statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY). Categorical variables are presented as frequencies and percentages, and comparisons of these variables were performed using the χ2 or Fisher exact test, as appropriate. Continuous data are presented as the mean ± SD for normally distributed variables or as the median (interquartile range) for non–normally distributed variables. Differences between continuous variables were compared using unpaired Student t test or Mann–Whitney test. A P value <0.05 was regarded as significant.
3. RESULTS
3.1. Patients and angiographic features
The baseline clinical characteristics of the patients are listed in Table 1. Of the 211 patients, 49 (23.2%) were women and 162 (76.7%) were men. Among the women, 42 (86%) were postmenopausal. The women were significantly older than the men (mean age, 60.2 ± 8.2 vs 55.7 ± 11.2 years, respectively; P = 0.01) and had higher levels of high‐density lipoprotein cholesterol than did the men (43.9 ± 21.4 vs 36.8 ± 21.5 mg/dL; P = 0.05). The men were more likely to be current smokers (63% of men vs 26% of women; P = 0.02). Additionally, the delay from symptom onset to reperfusion was longer in women than in men (women, 7.6 ± 6.1 hours vs men, 5.5 ± 4.4 hours; P = 0.008). No significant differences were found in the incidences of hypertension, diabetes mellitus, and drug use prior to admission between women and men.
Table 1.
Baseline clinical characteristics
| Variable | Men, n = 162 | Women, n = 49 | P Value |
|---|---|---|---|
| Age, y | 55.7 ± 11.2 | 60.2 ± 8.2 | 0.01 |
| Risk factors | |||
| HTN | 70 (43.2) | 25 (51.0) | 0.41 |
| Hyperlipidemia | 65 (40.1) | 22 (44.9) | 0.62 |
| DM | 32 (19.8) | 13 (26.5) | 0.32 |
| Current smoker | 87 (62.6) | 11 (26.2) | 0.02 |
| D2B time, min | 15 (25–35) | 17.5 (30–38) | 0.92 |
| Delay from symptom onset to reperfusion, h | 5.5 ± 4.4 | 7.6 ± 6.1 | 0.01 |
| Medications before admission | |||
| ASA | 61 (37.7) | 17 (35.4) | 0.74 |
| Statins | 8 (4.9) | 4 (8.2) | 0.48 |
| Laboratory parameters | |||
| TC, mg/dL | 139.1 ± 78.5 | 161.6 ± 74.9 | 0.08 |
| LDL‐C, mg/dL | 91.8 ± 56.6 | 106.0 ± 53.0 | 0.12 |
| HDL‐C, mg/dL | 36.8 ± 21.5 | 43.9 ± 21.4 | 0.05 |
| TG, mg/dL | 117.5 ± 97.0 | 127.2 ± 86.8 | 0.53 |
| HbA1c, % | 6.5 ± 1.4 | 6.7 ± 1.6 | 0.55 |
| eGFR, mL/min | 99.9 ± 28.8 | 99.3 ± 29.0 | 0.89 |
| hs‐CRP, mg/L | 6.03 ± 5.42 | 7.27 ± 5.52 | 0.16 |
| Peak TnI, μg/L | 32.6 (76.0–144.8) | 22.5 (72.4–135.3) | 0.67 |
Abbreviations: ASA, acetylsalicylic acid (aspirin); D2B, door‐to‐balloon; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; SD, standard deviation; TC, total cholesterol; TG, triglycerides; TnI, troponin I.
Data are presented as n (%), mean ± SD, or median (IQR).
The angiographic characteristics of the patients are summarized in Table 2. There were no differences in the distribution of the infarct‐related artery, Thrombolysis In Myocardial Infarction (TIMI) flow grade, and quantitative coronary angiography findings.
Table 2.
Angiographic findings
| Variable | Men, n = 162 | Women, n = 49 | P Value |
|---|---|---|---|
| IRA | |||
| LAD | 82 (50.6) | 26 (53.1) | 0.08 |
| LCX | 26 (16.0) | 2 (4.1) | |
| RCA | 54 (33.3) | 21 (42.9) | |
| Multivessel disease | 83 (51.2) | 23 (46.9) | 0.63 |
| Aspiration thrombectomy | 150 (92.6) | 49 (100) | 0.07 |
| Baseline TIMI flow grade | |||
| 0/1 | 138 (85.2) | 43 (87.8) | 0.82 |
| 2 | 16 (9.9) | 4 (8.2) | |
| 3 | 8 (4.9) | 2 (4.1) | |
| Final TIMI flow grade | |||
| 2 | 15 (9.3) | 4 (8.2) | 1.00 |
| 3 | 147 (90.7) | 45 (91.8) | |
| QCA findings | |||
| Lesion length, mm | 15.3 ± 13.2 | 13.8 ± 10.2 | 0.48 |
| RVD, mm | 3.11 ± 2.77 | 2.90 ± 1.80 | 0.64 |
| MLD, mm | 0.22 ± 0.48 | 0.20 ± 0.43 | 0.87 |
| Diameter stenosis, % | 92.4 ± 16.7 | 92.8 ± 15.1 | 0.88 |
Abbreviations: IRA, infarct‐related artery; LAD, left anterior descending artery; LCX, left circumflex artery; MLD, minimum lumen diameter; QCA, quantitative coronary analysis; RCA, right coronary artery; RVD, reference vessel diameter; SD, standard deviation; TIMI, Thrombolysis In Myocardial Infarction.
Data are presented as n (%) or mean ± SD.
3.2. OCT findings
The differences in the culprit‐plaque morphologies between women and men are summarized in Table 3. The average reference vessel area was smaller in women than in men (women, 6.31 ± 1.97 mm2 vs men, 7.25 ± 2.57 mm2; P = 0.02). No significant difference was found in the minimum lumen area. Although the women had a numerically greater prevalence of lipid‐rich plaques than men (83.7% in women vs 73.5% in men; P = 0.18) and larger lipid arcs (women, 316.0° ± 60.8° vs men, 295.2° ± 64.7°; P = 0.10), these differences were not significant. Lipid length, minimum fibrous‐cap thickness, and the incidence of PR, TCFA, microvessels, macrophages, cholesterol crystals, calcification, and residual thrombus were also similar between the women and men.
Table 3.
OCT findings
| Variables | Men, n = 162 | Women, n = 49 | P Value |
|---|---|---|---|
| MLA, mm2 | 1.59 ± 1.07 | 1.46 ± 0.58 | 0.41 |
| Mean RVA, mm2 | 7.25 ± 2.57 | 6.31 ± 1.97 | 0.02 |
| Stenosis area, % | 77.0 ± 11.5 | 75.4 ± 10.0 | 0.37 |
| Mechanism underlying ACS | |||
| PR | 91 (56.2) | 32 (65.3) | 0.52 |
| PE | 66 (40.7) | 16 (32.7) | |
| CN | 5 (3.1) | 1 (2.0) | |
| Plaque type | |||
| Lipid‐rich | 119 (73.5) | 41 (83.7) | 0.18 |
| Fibrous | 43 (26.5) | 8 (16.3) | 0.18 |
| Fibrocalcific plaque | 39 (24.1) | 10 (20.4) | 0.70 |
| TCFA | 98 (60.5) | 31 (63.3) | 0.87 |
| Lipid length, mm | 11.4 ± 9.3 | 12.7 ± 7.6 | 0.36 |
| Fibrous‐cap thickness, μm | 59.5 ± 21.8 | 65.5 ± 42.3 | 0.33 |
| Lipid arc, degrees | 295.2 ± 64.7 | 316.0 ± 60.8 | 0.10 |
| Macrophages | 138 (85.2) | 45 (91.8) | 0.34 |
| Microvessels | 77 (47.5) | 25 (51.0) | 0.75 |
| Cholesterol crystals | 42 (26.0) | 15 (30.6) | 0.58 |
| Residual thrombus | 150 (92.6) | 48 (98.0) | 0.31 |
| Residual thrombus length, mm | 4 (6.9–10.0) | 3.2 (5.5–8.7) | 0.29 |
Abbreviations: ACS, acute coronary syndrome; CN, calcified nodule; IQR, interquartile range; MLA, minimum lumen area; PE, plaque erosion; PR, plaque rupture; RVA, reference vessel area; SD, standard deviation; TCFA, thin‐cap fibroatheroma.
Data are presented as n (%), mean ± SD, or median (IQR).
4. DISCUSSION
In this OCT study, we focused on patients with a first STEMI and assessed gender differences in coronary plaque characteristics and the pathogenesis of acute clinical events. No significant difference was found in culprit‐plaque morphology between women and men.
4.1. Gender and mechanism underlying ACS
A prior pathological study suggested that the most common mechanism underlying coronary thrombosis was PR with subsequent PE and that CNs were infrequent.13 A postmortem study of subjects with sudden cardiac death demonstrated that PE was observed more often in women and younger individuals, whereas men were more likely to present with PR, suggesting that the mechanism underlying STEMI might differ between women and men.9 In another study of STEMI pathology, PR (rather than PE) occurred more commonly in women age > 50 years.15 Similarly, Yahagi et al16 found that menopause had an important effect in women, with young women presenting a higher prevalence of PE than older women. However, the current study and previous in vivo imaging studies reported different findings. An intravascular ultrasound (IVUS) study conducted by Schoenenberger et al17 provided evidence showing that plaque morphology in culprit vessels was similar between young women and men. In contrast, in the highest age tertile, culprit vessels were more rupture‐prone in men, and the prevalence of a necrotic core and dense calcium was higher in men than in women. An OCT study performed in a setting of acute coronary syndrome (ACS) demonstrated that there were no significant differences in the frequencies of PR and thrombus between women and men.18 Similarly, Guagliumi et al19 showed that the prevalence of PR was similar between age‐matched women and men who presented with STEMI and underwent primary PCI. In the current study, there were no significant differences in the prevalence of PR, PE, and CN at culprit sites between women and men with a first STEMI. The reasons that the mechanisms underlying AMI result in worse clinical outcomes in women are difficult to explain.
4.2. Gender and plaque characteristics
A previous study reported that angiographic and IVUS evaluations indicate that compared with men, women had less extensive CAD, fewer PRs and necrotic cores, lower calcium levels, smaller lumens, and a similar plaque burden. TCFA also might be a stronger marker of plaque instability in women than in men.20 Qian et al21 also found that women had fewer plaques than men and that the lowest gender differences were observed in the oldest tertile (age > 68 years).
Notably, several subsequent OCT studies have reported inconsistent findings regarding the relationship between gender and plaque features. In patients with stable CAD, a recent study reported that there was no difference in plaque characteristics between men and women who were referred for coronary angiography based on OCT, IVUS, and near‐infrared spectroscopy.22 In 42 patients who had ACS and OCT examinations, women had a numerically greater prevalence of lipid‐rich plaques (79% in men vs 89% in women; P = 0.84) and TCFA (45% in men vs 67% in women; P = 0.45) and a lower minimum fibrous‐cap thickness (53.8 mm in men vs 45.4 mm in women; P = 0.25); but these differences were not significant.18 The number of plaques with disruptions, calcifications, or thrombi was also similar between men and women.18 In patients presenting with STEMI, a previous study found no gender differences in the composition and morphology of the culprit plaque and aspirated thrombus or in immune and inflammatory serum biomarkers between age‐matched men and women.19 However, whether coronary atherosclerosis is more severe in women than in men prior to the first acute coronary event remains unclear. In the present study, we enrolled patients who presented with a first STEMI. In this study, there were no gender‐specific differences in underlying plaque characteristics at the culprit site. The presence of TCFA, cholesterol crystals, and calcification within the culprit plaques was similar between women and men. Gender‐related differences were not observed for the arc and length of the lipid content, the thickness of the fibrous cap, or the minimum lumen area. The findings in this study are consistent with those of previous OCT studies and extend the results of earlier findings related to the first STEMI population. Unsurprisingly, in the present study, the incidence of PR and PE was similar between women and men because the women were significantly older than the men and were thus more likely to have a PR. Furthermore, the majority of the women appeared to be postmenopausal.
4.3. Causes of differences in clinical outcomes between the genders
Female gender is more highly associated than male gender with worse outcomes and higher mortality after STEMI.2, 3, 4, 5, 6 The reasons for these gender‐related differences in clinical outcomes are not known. Generally, female patients with STEMI are older than males and have more frequent comorbidities.2, 3, 4, 5, 6, 23 Consistent with previous investigations, we also found that women were significantly older when they presented with their first STEMI, with the average age being 60.2 years in women and 55.7 years in men. The higher incidence of STEMI in older female patients might be related to the beneficial effects that circulating estrogens exert on the vascular endothelium.24 However, distinguishing between the effects of age and menopause is difficult. Additionally, many studies have shown that women with STEMI are more likely to experience a longer delay from symptom onset to reperfusion.23, 25, 26 As previously reported, the delay from symptom onset to reperfusion was significantly longer in women with a first STEMI than in similarly affected men in the current study. Sederholm Lawesson et al27 found that there was a significant gender‐related difference in the prevalence of renal dysfunction in STEMI, and renal dysfunction appeared to be a more important prognostic marker in women. Additionally, women report higher levels of psychological stress than men, and this partially explains their worse recovery rates.28 In the current in vivo study, we did not identify gender‐related differences in the mechanisms underlying ACS or in culprit‐lesion characteristics. It therefore seems illogical to use the mechanisms underlying STEMI and culprit‐plaque morphology to explain gender differences in long‐term clinical outcomes.
4.4. Study limitations
Several limitations of the present analysis should be acknowledged. First, selection bias may have occurred, because this study is a retrospective observational analysis performed at a single center. Second, although the exclusion criteria were understandable, they may have greatly limited the set of patients included in the analysis. This issue limits the generalizability of the findings to determine gender differences in the pathophysiology of ACS on a broad level. Third, some recent reports have questioned whether OCT assessment of lipids is appropriate, particularly for analyzing macrophages and superficial calcification. Fourth, in most patients with a TIMI flow grade of 0/1 or in the presence of a large thrombus burden at the culprit site, aspiration thrombectomy was performed to reestablish effective vessel patency with a TIMI flow grade of 2/3. Hence, the potential effect of the thrombus aspiration catheter crossing the culprit lesion on plaque etiology (including the lipid content of lipid‐rich plaques) cannot be entirely dismissed. Fifth, a residual thrombus can influence the accurate evaluation of OCT images. Therefore, in the present study, we excluded patients with a significant residual thrombus. Sixth, the number of patients with CNs was small. Seventh, further study is required to determine whether gender differences exist in the morphology of non‐culprit lesions. Finally, the study design appears to have an intrinsic limitation related to the imbalance between women and men in the population (women/men = 0.3). Nonetheless, this imbalance reflects the true distribution of patients with a first STEMI, and significant baseline differences were observed in the age and smoking status of the study groups.
5. CONCLUSION
In patients presenting with a first STEMI, there were no significant differences in culprit‐plaque characteristics between women and men. The underlying mechanisms that contribute to STEMI and culprit‐plaque features do not appear to account for the gender‐associated differences that were found in clinical outcomes after STEMI. Based on the culprit‐plaque features, it might be difficult to develop different treatment strategies for men and women who present with STEMI.
ACKNOWLEDGMENTS
The authors thank all of the investigators and support staff who were involved in the completion of this study.
Conflicts of interest
The authors declare no potential conflicts of interest.
Sun R, Sun L, Fu Y, et al. Culprit plaque characteristics in women vs men with a first ST‐segment elevation myocardial infarction: In vivo optical coherence tomography insights. Clin Cardiol. 2017;40:1285–1290. 10.1002/clc.22825
Funding information This study was supported by grants 81330033 and 81571749 from the National Natural Science Foundation of China.
Contributor Information
Jinwei Tian, Email: tianjinweidr2009@163.com.
Bo Yu, Email: yubodr2009@163.com.
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association Circulation. 2015;131:e29–e322 [published corrections appear in http://circ.ahajournals.org/content/131/4/e29.full]. . [DOI] [PubMed] [Google Scholar]
- 2. Mehta LS, Beckie TM, DeVon HA, et al; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. [DOI] [PubMed] [Google Scholar]
- 3. Berger JS, Elliott L, Gallup D, et al. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pancholy SB, Shantha GP, Patel T, et al. Sex differences in short‐term and long‐term all‐cause mortality among patients with ST‐segment elevation myocardial infarction treated by primary percutaneous intervention: a meta‐analysis. JAMA Intern Med. 2014;174:1822–1830. [DOI] [PubMed] [Google Scholar]
- 5. Bucholz EM, Butala NM, Rathore SS, et al. Sex differences in long‐term mortality after myocardial infarction: a systematic review. Circulation. 2014;130:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kovacic JC, Mehran R, Karajgikar R, et al. Female gender and mortality after percutaneous coronary intervention: results from a large registry. Catheter Cardiovasc Interv. 2012;80:514–521. [DOI] [PubMed] [Google Scholar]
- 7. Dimitrova KR, DeGroot K, Myers AK, et al. Estrogen and homocysteine. Cardiovasc Res. 2002;53:577–588. [DOI] [PubMed] [Google Scholar]
- 8. van Lennep HW, Westerveld HT, Zwinderman AH, et al. Differential effect of female gender on coronary artery disease and peripheral artery disease. Neth Heart J. 2002;10:500–505. [PMC free article] [PubMed] [Google Scholar]
- 9. Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core: a frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. [DOI] [PubMed] [Google Scholar]
- 10. Bezerra HG, Costa MA, Guagliumi G, et al. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv. 2009;2:1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jia H, Abtahian F, Aguirre AD, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jang IK, Tearney GJ, MacNeill B, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111:1551–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 suppl):C13–C18. [DOI] [PubMed] [Google Scholar]
- 14. Tearney GJ, Regar E, Akasaka T, et al; International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation (IWG‐IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. [DOI] [PubMed] [Google Scholar]
- 15. Burke AP, Farb A, Malcom GT, et al. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–2116. [DOI] [PubMed] [Google Scholar]
- 16. Yahagi K, Davis HR, Arbustini E, et al. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239:260–267. [DOI] [PubMed] [Google Scholar]
- 17. Schoenenberger AW, Urbanek N, Toggweiler S, et al. Ultrasound‐assessed non‐culprit and culprit coronary vessels differ by age and gender. World J Cardiol. 2013;5:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chia S, Christopher Raffel O, Takano M, et al. In‐vivo comparison of coronary plaque characteristics using optical coherence tomography in women vs. men with acute coronary syndrome. Coron Artery Dis. 2007;18:423–427. [DOI] [PubMed] [Google Scholar]
- 19. Guagliumi G, Capodanno D, Saia F, et al; OCTAVIA Trial Investigators . Mechanisms of atherothrombosis and vascular response to primary percutaneous coronary intervention in women versus men with acute myocardial infarction: results of the OCTAVIA study. JACC Cardiovasc Interv. 2014;7:958–968. [DOI] [PubMed] [Google Scholar]
- 20. Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5(3 suppl):S62–S72. [DOI] [PubMed] [Google Scholar]
- 21. Qian J, Maehara A, Mintz GS, et al. Impact of gender and age on in vivo virtual histology—intravascular ultrasound imaging plaque characterization (from the global Virtual Histology Intravascular Ultrasound [VH‐IVUS] registry). Am J Cardiol. 2009;103:1210–1214. [DOI] [PubMed] [Google Scholar]
- 22. Bharadwaj AS, Vengrenyuk Y, Yoshimura T, et al. Multimodality intravascular imaging to evaluate sex differences in plaque morphology in stable CAD. JACC Cardiovasc Imaging. 2016;9:400–407. [DOI] [PubMed] [Google Scholar]
- 23. Weissler‐Snir A, Kornowski R, Sagie A, et al. Gender differences in left ventricular function following percutaneous coronary intervention for first anterior wall ST‐segment elevation myocardial infarction. Am J Cardiol. 2014;114:1473–1478. [DOI] [PubMed] [Google Scholar]
- 24. Chakrabarti S, Morton JS, Davidge ST. Mechanisms of estrogen effects on the endothelium: an overview. Can J Cardiol. 2014;30:705–712. [DOI] [PubMed] [Google Scholar]
- 25. Benamer H, Bataille S, Tafflet M, et al. Longer pre‐hospital delays and higher mortality in women with STEMI: the e‐MUST Registry. EuroIntervention. 2016;12:e542–e549. [DOI] [PubMed] [Google Scholar]
- 26. Guerchicoff A, Brener SJ, Maehara A, et al. Impact of delay to reperfusion on reperfusion success, infarct size, and clinical outcomes in patients with ST‐segment elevation myocardial infarction: the INFUSE‐AMI Trial (INFUSE‐Anterior Myocardial Infarction). JACC Cardiovasc Interv. 2014;7:733–740. [DOI] [PubMed] [Google Scholar]
- 27. Sederholm Lawesson S, Tödt T, Alfredsson J, et al. Gender difference in prevalence and prognostic impact of renal insufficiency in patients with ST‐elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2011;97:308–314. [DOI] [PubMed] [Google Scholar]
- 28. Xu X, Bao H, Strait K, et al. Sex differences in perceived stress and early recovery in young and middle‐aged patients with acute myocardial infarction. Circulation. 2015;131:614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]


