Abstract
The American and European expert documents recommend transcatheter aortic valve replacement (TAVR) for inoperable or high‐surgical‐risk patients with severe aortic stenosis. In comparison, efficacy of TAVR is relatively less studied in low‐ to intermediate‐surgical‐risk patients. We sought to discover whether TAVR can be as effective as surgical aortic valve replacement (SAVR) in low‐ to intermediate‐surgical‐risk candidates. Four randomized clinical trials (RCTs) and 8 prospective matched studies were selected using PubMed/MEDLINE, Embase, and Cochrane Library (inception: March 2017). Results were reported as random‐effects odds ratio (OR) with 95% confidence interval (CI). Among 9851 patients, analyses of RCTs showed that all‐cause mortality was comparable between TAVR and SAVR (short term, OR: 1.19, 95% CI: 0.86‐1.64, P = 0.30; mid‐term, OR: 0.97, 95% CI: 0.75‐1.26, P = 0.84; and long term, OR: 0.97, 95% CI: 0.81‐1.16, P = 0.76). The analysis restricted to matched studies showed similar outcomes. In the analysis stratified by study design, no significant differences were noted in the RCTs for stroke, whereas TAVR was better than SAVR in matched studies at short term only (OR: 0.46, 95% CI: 0.33‐0.65, P < 0.001). TAVR is associated with reduced risk of acute kidney injury and new‐onset atrial fibrillation (P < 0.05). However, increased incidence of permanent pacemaker implantation and paravalvular leaks was observed with TAVR. TAVR can provide similar mortality outcome compared with SAVR in low‐ to intermediate‐surgical‐risk patients with critical aortic stenosis. However, both procedures are associated with their own array of adverse events.
Keywords: Meta‐analysis, Surgical Aortic Valve Replacement, Transcatheter Aortic Valve Replacement
1. INTRODUCTION
Both the American and the European expert documents recommend transcatheter aortic valve replacement (TAVR) for inoperable or high‐surgical‐risk patients with severe aortic stenosis (AS).1, 2 In comparison, efficacy of TAVR is relatively less studied in low‐ to intermediate‐surgical‐risk patients. As per the current guidelines, surgery remains the procedure of choice in the subset of patients with severe symptomatic AS. There have been emerging data suggestive of comparable efficacy of TAVR with surgical aortic valve replacement (SAVR). For instance, the Placement of Aortic Transcatheter Valves (PARTNER) 2 cohort, a randomized trial, showed comparable primary endpoints of death and disabling stroke, between TAVR and surgery, among intermediate‐risk patients.3 These results were further validated in meta‐analyses showing equivalent mortality outcomes between both approaches at 30 days and mid‐term.4, 5 With the arrival of the Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial, an updated pooled analysis comparing both interventions in such subset of population was warranted.6 Thus, we present a meta‐analysis incorporating the data from all randomized clinical trials (RCTs) and prospective cohort studies to assess relative efficacy and safety of TAVR compared with SAVR among low‐ to intermediate‐surgical‐risk patients.
2. METHODS
This meta‐analysis was reported according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. In total, 216 articles were retrieved using PubMed/MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials (inception: March 2017). The following search terms were employed: “transcatheter aortic valve replacement,” “TAVR,” “surgical aortic valve replacement,” “SAVR,” “TAVI,” “SAVI,” “low to moderate surgical risk,” and “severe aortic stenosis.” Search was restricted to human RCTs and prospective cohort studies (see Supporting Information, Search Algorithm, in the online version of this article). Figure 1 explains the search strategy finalizing 12 studies after independent scrutiny by 2 authors (SUK and MAS).
Figure 1.

Search strategy. Study‐selection process through Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA)
Eligible studies were published RCTs and prospective matched studies comparing TAVR and SAVR and reporting outcomes of interest in severe AS patients at low surgical risk (Society of Thoracic Surgeons Predicted Risks of Mortality [STS‐PROM] score < 4% or logistic European System for Cardiac Operative Risk Evaluation [LES] <10%) or intermediate risk (STS‐PROM 4%–8% or LES 10%–20%). As STS‐PROM was demonstrated to be superior to LES, priority was given to the STS‐PROM score7, 8; LES was used only if the STS‐PROM score was not available. Matched studies were included if both interventional groups were matched for preoperative factors or propensity score to reduce the impact of inherent confounding variables (see Supporting Information, Table 4, in the online version of this article). Data extraction was done using a standardized collection form, and risk of bias was assessed at the study level. Quality assessment of RCTs and matched studies was appraised by Cochrane methods and Newcastle‐Ottawa Scale criteria, respectively (see Supporting Information, Tables 1 and 2, in the online version of this article).9, 10
The primary outcome was all‐cause mortality. The secondary outcomes assessed were myocardial infarction (MI), (disabling) stroke, and cardiac mortality. Procedural complications investigated were need for pacemaker (PM) implantation, vascular access complications, acute kidney injury (AKI), new‐onset atrial fibrillation (NOAF), major bleeding, infection, and paravalvular leak. For major bleeding we accepted the outcome definition as reported by the original studies, although there were slight differences across the studies. Comparison of the length of hospital stay and difference in New York Heart Association (NYHA) functional class following the index procedure were assessed as well. Primary outcome and secondary outcomes were assessed at the short term (≤30 days), mid‐term (1 year), and long term (>1 year) follow‐up. PM implantation, vascular access complication, AKI, NOAF, major bleeding, and infection were checked at short term.
The number of events with respective sample sizes were extracted for each variable to construct odds ratios (ORs) and absolute difference for dichotomous outcomes, and mean difference for continuous variables with 95% confidence interval (CI). The SURTAVI study has reported event rates according to Bayesian analysis; therefore, we used crude event rates and corresponding sample sizes from the study for the analyses.6 P < 0.05 was set as significant, and heterogeneity was assessed using Q statistics, with I 2 > 50% considered consistent with high degree of heterogeneity (see Supporting Information, Table 7, in the online version of this article).11 To reduce the risk of bias, we have stratified the analyses based on study design, demonstrating separate analyses for randomized and matched studies. Sensitivity analysis with serial exclusion of studies was performed to evaluate inconsistency in outcomes. Method‐of‐moments meta‐regression analysis was executed to evaluate the effects of baseline characteristics on primary outcome (see Supporting Information, Table 6, in the online version of this article). Publication bias was investigated using funnel plots and Egger tests (see Supporting Information, Figures 6–8, in the online version of this article). Comprehensive Meta‐Analysis software version 3.0 (Biostat, Englewood, NJ) was used for all analyses.
3. RESULTS
A total of 9851 subjects from 4 RCTs and 8 observational matched studies were included in the final analysis3, 6, 12, 13, 14, 20, 21, 22, 23, 24, 25, 26, 27. Mean age of the total population ranged from 78 to 83 years, and mean LES was 13.3. The predominant approach was transfemoral (TF), and the majority of patients belonged to NYHA classes III and IV. For baseline characteristics of study participants, see Supporting Information, Table 9, in the online version of this article. The procedural characteristics of studies, and descriptions of matching in prospective observational studies, are described in the Supporting Information, Tables 4 and 5, respectively, in the online version of this article.
Among RCTs, 4 studies contributed data for short term and 3 studies reported data for total mortality at mid‐term and long term. Eight observational matched studies reported outcome at short term, and 5 articles provided information at mid‐term. In RCTs pooled analysis, there was no significant difference in outcome among both the interventions (short term, OR: 1.19, 95% CI: 0.86‐1.64, P = 0.30; mid‐term, OR: 0.97, 95% CI: 0.75‐1.26, P = 0.84; and long term, OR: 0.97, 95% CI: 0.81‐1.16, P = 0.76). Identical results were observed for matched studies analysis (Figure 2). Sensitivity analysis performed by serial exclusion of 1 study at a time did not affect the outcome in RCTs restricted analysis. However, all‐cause mortality was significantly reduced at short term with TAVR after removal of each study in analysis of observational studies. This trend was consistent at mid‐term after removal of Tamburino et al.12 Funnel plots and Egger test could not establish publication bias (P > 0.05).
Figure 2.
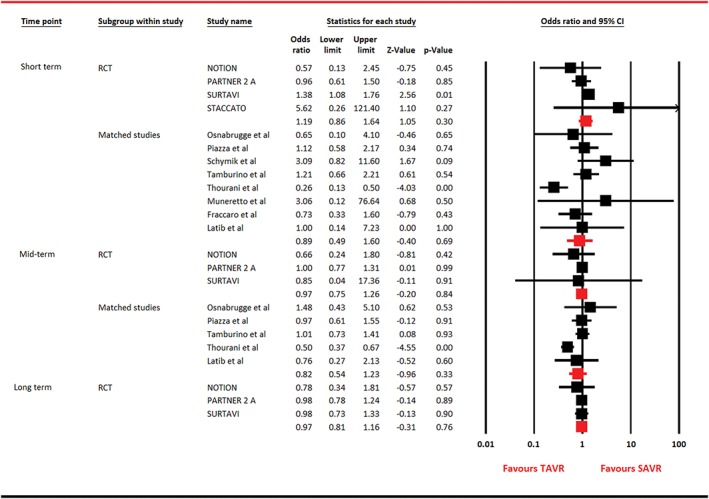
Forest plot for all‐cause mortality. Abbreviations: CI, confidence interval; NOTION, Nordic Aortic Valve Intervention; PARTNER, Placement of Aortic Transcatheter Valves; RCT, randomized clinical trial; SAVR, surgical aortic valve replacement; STACCATO, A Prospective, Randomized Trial of Transapical Transcatheter Aortic Valve Implantation vs Surgical Aortic Valve Replacement in Operable Elderly Patients with Aortic Stenosis; SURTAVI, Surgical Replacement and Transcatheter Aortic Valve Implantation; TAVR, transcatheter aortic valve replacement
For cardiac mortality, 3 RCTs reported outcome, whereas 4 observational studies provided data at short term and 2 studies contributed information at mid‐term. In RCTs restricted analyses, comparable outcomes were noticed between the 2 arms at short term (OR: 1.16, 95% CI: 0.93‐1.45, P = 0.18) and long term (OR: 0.91, 95% CI: 0.69‐1.20, P = 0.50). However, at mid‐term, TAVR was associated with 22% risk reduction in cardiac mortality in RCTs‐based analysis and a 48% risk reduction in matched studies restricted analysis (Figure 3). For MI, analysis restricted to RCTs was composed of 3 trials, whereas analysis on observational studies was performed on 7 and 3 studies at short term and mid‐term, respectively. There was no difference in outcomes between both the arms with regard to MI (Figure 4). For stroke, 4 RCTs participated at short term, with 3 trials contributing data at mid‐term and long term. Seven observational studies reported stroke at short term, and 3 articles reported outcome at mid‐term. Except for analysis restricted to observational studies, which showed a 54% risk reduction in stroke with TAVR at short term, the rest of the analyses were unable to generate this statistical advantage (Figure 5).
Figure 3.
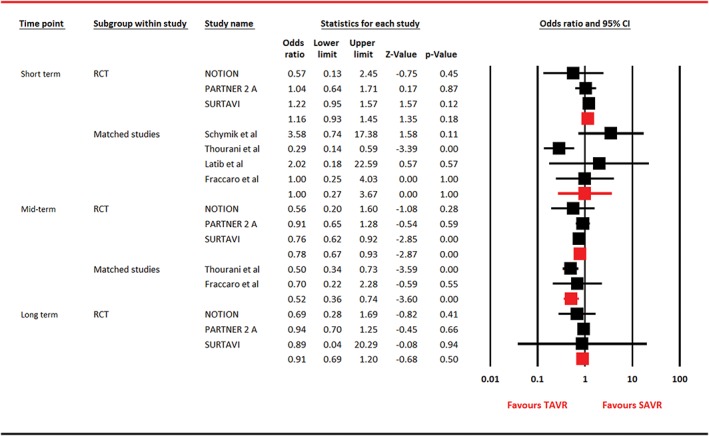
Forest plot for cardiac mortality. Abbreviations: CI, confidence interval; NOTION, Nordic Aortic Valve Intervention; PARTNER, Placement of Aortic Transcatheter Valves; RCT, randomized clinical trial; SAVR, surgical aortic valve replacement; SURTAVI, Surgical Replacement and Transcatheter Aortic Valve Implantation; TAVR, transcatheter aortic valve replacement
Figure 4.
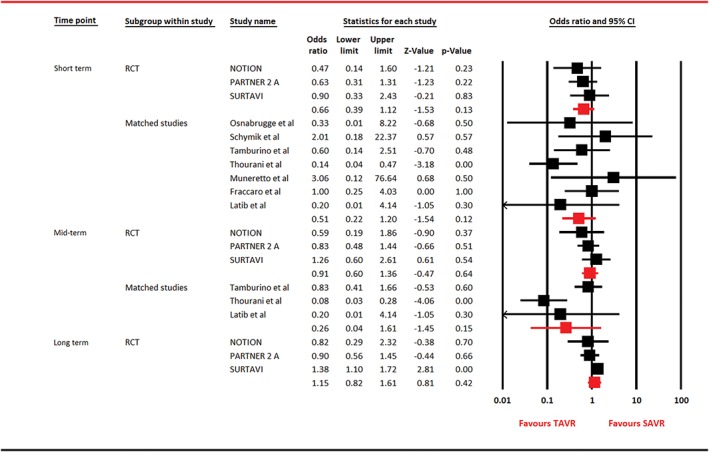
Forest plot for myocardial infarction. Abbreviations: CI, confidence interval; NOTION, Nordic Aortic Valve Intervention; PARTNER, Placement of Aortic Transcatheter Valves; RCT, randomized clinical trial; SAVR, surgical aortic valve replacement; SURTAVI, Surgical Replacement and Transcatheter Aortic Valve Implantation; TAVR, transcatheter aortic valve replacement
Figure 5.

Forest plot for disabling stroke. Abbreviations: CI, confidence interval; NOTION, Nordic Aortic Valve Intervention; PARTNER, Placement of Aortic Transcatheter Valves; RCT, randomized clinical trial; SAVR, surgical aortic valve replacement; STACCATO, A Prospective, Randomized Trial of Transapical Transcatheter Aortic Valve Implantation vs Surgical Aortic Valve Replacement in Operable Elderly Patients with Aortic Stenosis; SURTAVI, Surgical Replacement and Transcatheter Aortic Valve Implantation; TAVR, transcatheter aortic valve replacement
At short term, TAVR was associated with increased risk of vascular access complications (RCTs, OR: 3.12, 95% CI: 1.17‐8.34, P = 0.02; matched studies, OR: 9.49, 95% CI: 1.62‐55.62, P = 0.01), permanent PM implantation (RCTs, OR: 4.86, 95% CI: 1.37‐17.23, P = 0.01; matched studies, OR: 2.74, 95% CI: 1.20‐6.22, P = 0.02), and paravalvular leak (P < 0.05; see Supporting Information, Figure 4, in the online version of this article). Conversely, SAVR had higher risk of AKI (RCTs, OR: 0.38, 95% CI: 0.24‐0.63, P < 0.001; matched studies, OR: 0.44, 95% CI: 0.28‐0.69, P < 0.001) and NOAF (RCTs, OR: 0.21, 95% CI: 0.16‐0.30, P < 0.001; matched studies, OR: 0.36, 95% CI: 0.02‐5.28, P = 0.45). There was no difference in outcome in terms of major bleeding (RCTs, OR: 0.47, 95% CI: 0.10‐2.27, P = 0.34; matched studies, OR: 0.25, 95% CI: 0.04‐1.48, P = 0.13) and infection (RCTs, OR: 1.30, 95% CI: 0.46‐3.65, P = 0.62; matched studies, OR: 0.88, 95% CI: 0.63‐1.23, P = 0.45). Compared with SAVR, TAVR was clearly associated with reduced duration of hospital stay (mean difference: –3.98, 95% CI: –5.17 to –4.83, P < 0.001).
For NYHA functional class, at short term, outcome was contributed by PARTNER 2 and SURTAVI, whereas for the mid‐term and long term, data were provided by Nordic Aortic Valve Intervention (NOTION), PARTNER 2, and SURTAVI.3, 6, 13, 14 Overall, there were no substantial differences noticed between both interventions at all the time points (see Supporting Information, Figure 5, in the online version of this article).
Meta‐regression analysis was conducted to assess the impact of age, male sex, coronary artery disease, year, chronic obstructive pulmonary disease, hypertension, liver disease, diabetes mellitus, LES, STS score, CoreValve, SAPIEN valve, left ventricular ejection fraction, transpical approach, TF approach, NYHA class III–IV, concomitant TAVR percutaneous coronary intervention, and concomitant SAVR coronary artery bypass grafting on primary outcome (see Supporting Information, Table 6, in the online version of this article). Both diabetes mellitus and LES had functional impact on the odds of short‐term mortality with TAVR (see Supporting Information, Figure 1, in the online version of this article).
4. DISCUSSION
The previous reports on this issue were largely based on nonrandomized studies.15, 16 This generates a higher degree of selection and attrition bias; drawing conclusions from such reports could be incorrect, as outcomes are affected by various confounding factors. To overcome this issue, we performed the analysis restricted to RCTs and prospective matched studies separately. In this, the largest meta‐analysis to our knowledge, we have tried to explore a few cardinal issues. This meta‐analysis attempts to determine whether TAVR is as efficacious and safe as SAVR in subjects with critical symptomatic AS who are considered to be at low to intermediate surgical risk. We also asked, how does the aortic valve replacement strategy affect the incidence of all‐cause mortality, cardiac mortality, MI, and stroke?
Our meta‐analysis suggests that both procedures carry comparable outcomes with regard to all‐cause mortality. These results were consistently observed in both randomized and nonrandomized studies‐based analyses and are in line with previous pooled analyses.5, 16 In the SURTAVI trial, event rate for all‐cause mortality at 30 days was 2.2% for TAVR and 1.7% for SAVR, with comparable incidence rates at 1 year (6.7% vs 6.8%) and 2 years (11.4% vs 11.6%).6 Similarly, in the PARTNER 2 study, identical all‐cause mortality outcomes were noticed at 30 days (3.9% vs 4.1%), 1 year (12.3% vs 12.9%), and 2 years (16.7% vs 18%).3 Survival benefit with TAVR is predominantly derived by improvement in cardiovascular mortality secondary to fewer periprocedural adverse events. Therefore, in establishing TAVR efficacy in low to intermediate risk, the index procedure should to be able to demonstrate cardiovascular survival benefit. This important parameter has been constantly missing in prior meta‐analyses. Our meta‐analyses for both RCTs and observational studies were able to demonstrate significant cardiovascular survival benefit at mid‐term, with equivalent outcomes at the short term and long term, compared with SAVR.
In the SURTAVI trial, TAVR was associated with reduced stroke risk throughout the follow‐up duration: at 30 days (3.4% vs 5.6%), 1 year (5.4% vs 6.9%), and 2 years (6.2% vs 8.4%).6 However, this trend was not observed in other RCTs. Our analysis of RCTs also showed that stroke rate was identical between both groups at all of the follow‐ups. However, at the short term, a 54% risk reduction was noticed with TAVR in analysis restricted to matched studies. These results reaffirm the previous meta‐analysis predominantly based on nonrandomized studies (OR: 0.72, 95% CI: 0.56‐0.92, P = 0.01).4 This hints toward a possible preventive role of TAVR with regard to stroke; however, well‐designed RCTs are required to further assess these findings.
With regard to MI, we could not establish statistical superiority for either of the arms, in both randomized‐ and nonrandomized‐derived analyses. These findings are similar to those of Arora et al, who presented a similar nonsignificant result at 30 days (OR: 0.55, 95% CI: 0.24‐1.21), but contradict the results of Zhou et al, who were able to report 58% significant risk reduction at 30 days.4, 16
TAVR has been associated with reduced incidence of AKI and NOAF, and it was clearly superior in terms of length of hospital stay. This benefit has been consistently noticed in prior studies.4, 5 Conversely, the higher need for PM implantation, paravalvular leak, and excess rate of vascular access with TAVR is also consistent with other meta‐analyses.5, 15, 16 Paravalvular leak is a critical complication and has been consistently observed with TAVR. The PARTNER trial reported that patients with postprocedural paravalvular leak had higher mortality rates than did patients without this complication.17
It is important to mention here that all the included studies in this review report using TAVR devices that are not contemporary and reflect absence of sealing skirts for balloon‐expandable or self‐expandable valves, inability to reposition or remove self‐expanding valves, and larger sheath size for both balloon‐expandable and self‐expandable TAVR devices. Therefore, it is possible that, with the contemporary devices and deployment strategies, the rates of PM implantations, conduction abnormalities, and paravalvular leaks will be reduced, and the results might turn in favor of TAVR. However, well‐designed RCTs using modern devices are required to further assess this assumption.
The data are inconsistent in generating relative superiority of either of the interventions in terms of NYHA functional class. In a comprehensive review by Kim et al., a mean improvement in NYHA class was noticed after TAVR (6–11 months: range, –0.8 to –2.1 classes; 12–23 months: range, –0.8 to –2.1 classes; 24–35 months: range, –1.2 to –2.6 classes; and ≥36 months: range, –1.2 to –1.6 classes).18 However, the same publication reported failure of various studies to show improvement in NYHA class. Our results predominantly represent equivalent outcomes among both interventions across NYHA I–IV functional classes.
4.1. Study limitations
This study is limited by certain issues. First, there are noticeable variations among the studies with regard to the definition of surgical risk and outcomes, valve type, and delivery system. As most of the studies were done with TAVR devices that are not contemporary, this review is limited to showing the effects of old TAVR devices. Furthermore, the majority of the studies included in this review have used the TF approach, and the use of the transapical and trans‐subclavian approach is extremely limited (see Supporting Information, Table 3, in the online version of this article). Therefore, this review predominantly generates evidence for the patients who had TAVR through the TF approach. Second, we did not discuss the medical economics of the index procedures and health benefits measured as the number of added life‐years or quality‐adjusted life years. It is important to mention that the data regarding cost‐effectiveness of TAVR (assessed by incremental cost‐effective ratio for life‐years or quality‐adjusted life years) are more convincing for inoperable or high‐risk candidates and predominantly favor affluent countries.19 For instance, Osnabrugge et al. reported that in patients with intermediate risk, despite the shorter hospital stay with TAVR, the cumulative cost of the TAVR procedure and subsequent patient follow‐up resulted in comparatively higher patient costs (€40 802 TAVR vs €33 354 SAVR, P = 0.01) and total costs (€46 217 TAVR vs €35 511 SAVR, P = 0.009).20 Hence, it remains to be determined, for countries with low to moderate income, if TAVR can be economically acceptable in this subset of population. Third, the data for long‐term duration are relatively sparse, and only 3 studies reported outcomes at >1 year. This is a major limitation, especially in younger or low‐risk candidates, in whom making the choice for the TAVR becomes difficult due to lack of long‐term data about the durability of the valve. Finally, this meta‐analysis demonstrates the results in an older population (average age ≥ 80 years); thus, it remains to be proven whether TAVR is noninferior to SAVR in a relatively young population.
5. CONCLUSION
In patients with symptomatic severe AS who carry low to intermediate surgical risk, SAVR and TAVR can provide similar mortality outcomes. Both interventions are associated with their own array of adverse events. However, the authors emphasize that because this meta‐analysis does not demonstrate the current practice, which employs advanced devices with better mechanical options, it is very likely that the observed findings may change with the use of modern devices. Finally, as the majority of the studies reported short‐term and mid‐term outcomes, more studies with adequately long follow‐up duration are required to assess the clinical effects of both interventions for endorsing the expansion of TAVR in this subset of patients.
Supporting information
Appendix S1. Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Osama Ismail, MD, for the grammatical review of this article.
Conflicts of interest
The authors declare no potential conflicts of interest.
Khan SU, Lone AN, Saleem MA, Kaluski E. Transcatheter vs surgical aortic‐valve replacement in low‐ to intermediate‐surgical‐risk candidates: A meta‐analysis and systematic review. Clin Cardiol. 2017;40:974–981. 10.1002/clc.22807
REFERENCES
- 1. Nishimura RA, Otto CM, Bonow RO, et al. 2017. AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 2. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 4. Zhou Y, Wang Y, Wu Y, et al. Transcatheter versus surgical aortic valve replacement in low to intermediate risk patients: a meta‐analysis of randomized and observational studies. Int J Cardiol. 2017;228:723–728. [DOI] [PubMed] [Google Scholar]
- 5. Gargiulo G, Sannino A, Capodanno D, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement: a systematic review and meta‐analysis. Ann Intern Med. 2016;165:334–344. [DOI] [PubMed] [Google Scholar]
- 6. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 7. Hemmann K, Sirotina M, De Rosa S, et al. The STS score is the strongest predictor of long‐term survival following transcatheter aortic valve implantation, whereas access route (transapical versus transfemoral) has no predictive value beyond the periprocedural phase. Interact Cardiovasc Thorac Surg. 2013;17:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piazza N, Wenaweser P, van Gameren M, et al. Relationship between the logistic EuroSCORE and the Society of Thoracic Surgeons Predicted Risk of Mortality score in patients implanted with the CoreValve ReValving system—a Bern‐Rotterdam Study. Am Heart J. 2010;159:323–329. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Altman DG, Gøtzsche PC, et al; Cochrane Bias Methods Group, Cochrane Statistical Methods Group . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 11. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tamburino C, Barbanti M, D'Errigo P, et al. 1‐Year outcomes after transfemoral transcatheter or surgical aortic valve replacement. J Am Coll Cardiol. 2015;66:804–812. [DOI] [PubMed] [Google Scholar]
- 13. Thyregod HG, Steinbrüchel DA, Ihlemann N, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1‐year results from the all‐comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015;65:2184–2194. [DOI] [PubMed] [Google Scholar]
- 14. Søndergaard L, Steinbrüchel DA, Ihlemann N, et al. Two‐year outcomes in patients with severe aortic valve stenosis randomized to transcatheter versus surgical aortic valve replacement: the all‐comers Nordic Aortic Valve Intervention randomized clinical trial. Circ Cardiovasc Interv. 2016. 10.1161/CIRCINTERVENTIONS.115.003665. [DOI] [PubMed] [Google Scholar]
- 15. Khan AR, Khan S, Riaz H, et al. Efficacy and safety of transcatheter aortic valve replacement in intermediate surgical risk patients: a systematic review and meta‐analysis. Catheter Cardiovasc Interv. 2016;88:934–944. [DOI] [PubMed] [Google Scholar]
- 16. Arora S, Misenheimer JA, Jones W, et al. Transcatheter versus surgical aortic valve replacement in intermediate‐risk patients: a meta‐analysis. Cardiovasc Diagn Ther. 2016;6:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators . Two‐year outcomes after transcatheter or surgical aortic‐valve replacement. N Engl J Med. 2012;366:1686–1695. [DOI] [PubMed] [Google Scholar]
- 18. Kim CA, Rasania SP, Afilalo J, et al. Functional status and quality of life after transcatheter aortic valve replacement: a systematic review. Ann Intern Med. 2014;160:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fanari Z, Weintraub WS. Cost‐effectiveness of transcatheter versus surgical management of structural heart disease. Cardiovasc Revasc Med. 2016;17:44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osnabrugge RL, Head SJ, Genders TS, et al. Costs of transcatheter versus surgical aortic valve replacement in intermediate‐risk patients. Ann Thorac Surg. 2012;94:1954–1960. [DOI] [PubMed] [Google Scholar]
- 21. Nielsen HH, Klaaborg KE, Nissen H, et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention. 2012;8:383–389. [DOI] [PubMed] [Google Scholar]
- 22. Piazza N, Kalesan B, van Mieghem N, et al. A 3‐center comparison of 1‐year mortality outcomes between transcatheter aortic valve implantation and surgical aortic valve replacement on the basis of propensity score matching among intermediate‐risk surgical patients. JACC Cardiovasc Interv. 2013;6:443–451. [DOI] [PubMed] [Google Scholar]
- 23. Latib A, Maisano F, Bertoldi L, et al. Transcatheter vs surgical aortic valve replacement in intermediate‐surgical‐risk patients with aortic stenosis: a propensity score–matched case‐control study. Am Heart J. 2012;164:910–917. [DOI] [PubMed] [Google Scholar]
- 24. Muneretto C, Bisleri G, Moggi A, et al. Treating the patients in the ‘grey‐zone’ with aortic valve disease: a comparison among conventional surgery, sutureless valves and transcatheter aortic valve replacement. Interact Cardiovasc Thorac Surg. 2015;20:90–95. [DOI] [PubMed] [Google Scholar]
- 25. Schymik G, Heimeshoff M, Bramlage P, et al. A comparison of transcatheter aortic valve implantation and surgical aortic valve replacement in 1,141 patients with severe symptomatic aortic stenosis and less than high risk. Catheter Cardiovasc Interv. 2015;86:738–744. [DOI] [PubMed] [Google Scholar]
- 26. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate‐risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. [DOI] [PubMed] [Google Scholar]
- 27. Fraccaro C, Tarantini G, Rosato S, et al; OBSERVANT Research Group. Early and midterm outcome of propensity‐matched intermediate‐risk patients aged ≥80 years with aortic stenosis undergoing surgical or transcatheter aortic valve replacement (from the Italian Multicenter OBSERVANT Study). Am J Cardiol. 2016;117:1494–1501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary Material


