Abstract
Background
Atrial fibrillation (AF) has been associated with body size and central obesity, but the impact of different anthropometric measures in this relationship has been inadequately investigated.
Hypothesis
In this study, we examined the association between baseline anthropometric parameters with the incidence of AF in older people, hypothesizing that body size could impact the onset of AF more than fat distribution.
Methods
Our study included 1764 participants with a mean age of 74.3 ± 6.9 years and no AF at baseline. Body mass index (BMI), body height, body surface area (BSA), waist and hip circumference, waist‐to‐stature ratio, waist‐to‐hip ratio, and mid–upper arm circumference (MUAC) were measured by trained physicians. AF was assessed after a 4.4‐year follow‐up.
Results
There were 115 new cases of AF observed after the follow‐up. Taking lower values of these measures for reference, the adjusted AF risk was 2.42 (95% confidence interval [CI]:1.88‐3.12) for the highest stature quartile, 1.36 (95% CI:1.15‐1.62) for BMI ≥30 kg/m2, 2.12 (95% CI:1.73–2.59) for the highest BSA quartile, 1.38 (95% CI: 1.21‐1.56) for higher MUAC, and 1.39 (95% CI: 1.23‐1.58, P < 0.0001) for higher hip circumference values. Central obesity did not seem to relevantly predict the onset of AF in our sample. Stature revealed the strongest impact on the onset of AF (5% higher risk of developing AF per 1 cm increase in height).
Conclusions
Body size, particularly tall stature and obesity, but not fat distribution, seems to be associated with the risk of AF in the elderly.
Keywords: anthropometry, atrial fibrillation, elderly
1. INTRODUCTION
Atrial fibrillation (AF) is one of the most common cardiovascular diseases in adults and the elderly, with a prevalence of around 4% among those 60 to 70 years old, which rises to 10% to 17% in people over 80 years old.1, 2 Among the possible risk factors for the onset of this arrhythmia, the impact of body size, in particular excess weight or tall stature, and central obesity are attracting increasing interest.3
Obesity and overweight, as measured in terms of body mass index (BMI) or, for abdominal obesity, waist circumference and waist‐to‐stature ratio (WSR), seem to be significantly associated with a higher risk of AF in large cohort studies,4, 5, 6, 7, 8, 9 albeit with some contrasting results.4, 10, 11, 12 Stature and body surface area (BSA), as well as hip circumference, have also shown a significant relationship with AF,5, 6, 13, 14, 15 probably due to taller people having larger left atrial dimensions,12, 13, 14 which predisposes to AF according to the multiple wavelet theory.16, 17 Conversely, to our knowledge, only scarce information regarding the relationship between other body measures, such as mid–upper arm circumference (MUAC), have been found in the current literature.
Although the association between AF and body size has been demonstrated in several studies, the relevance of the various anthropometric features in predisposing to AF and, in particular, the impact of fat distribution on the development of AF, has yet to be fully clarified in elderly people.12 Assessing the body measurements more strongly associated with AF may help in the early identification of individuals at higher risk of AF, thereby preventing the thromboembolic complications of the disease.18
In this setting, the aim of our study was to analyze and compare the potential association between BMI, stature, BSA, MUAC, and waist and hip circumference with the onset of AF in a sample of elderly men and women during a 4.4‐year follow‐up. Previous literature suggested a stronger impact of body size than adiposity distribution indices in predicting AF; therefore, we hypothesized that the onset of atrial fibrillation in the elderly may be more influenced by body size than fat distribution.
2. METHODS
2.1. Data source and subjects
The sample considered in our study consisted of individuals enrolled in the Progetto Veneto Anziani (Pro.V.A.), an observational, population‐based cohort study in the Italian population age 65 years or over. This project initially involved 3099 age‐ and sex‐stratified community‐dwelling Caucasian adults (1245 men and 1854 women), randomly selected between 1995 and 1997 using a multistage stratified method.19 Participants were assessed by trained physicians and nurses at clinics, or at home if they were unable to attend a clinic.
Of the 3099 individuals enrolled in the study, 125 subjects were excluded because they already reported AF or a history of paroxysmal AF at baseline, 438 because this information was lacking, 165 due to a lack of anthropometric measurements, 138 who did not complete the follow‐up, and 469 who died during the study period.
The ethical committees of Padua University and the Local Health Units (USSL) No. 15 and No. 18 of the Veneto Region approved the study protocol, and participants gave their written informed consent.
2.2. Exposures and outcomes
Body weight and height were measured with participants wearing light indoor clothing and no shoes, using scales accurate to the nearest 0.1 kg and a stadiometer accurate to the nearest 0.01 m, respectively. BMI was calculated as weight in kilograms over height in meters squared (kg/m2). For the purpose of our study, BMI thresholds were used to define conditions of underweight (BMI <20 kg/m2),20 normal weight (20 < BMI < 25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2), and obesity (BMI ≥ 30 kg/m2). BSA was calculated with the Mosteller formula (BSA = [height (cm) × weight (kg)/3600]).21 Waist circumference was measured midway between the lowest rib and the iliac crest (with participants standing) and expressed in centimeters.22 WSR was calculated as the ratio between waist circumference and stature (both in centimeters). Hip circumference was measured around the widest portion of the buttocks and expressed in centimeters.23 Waist‐to‐hip ratio (WHR) was defined as the ratio between waist and hip circumference. Finally, mid–upper arm circumference (MUAC) was measured in the right upper arm at the midpoint between the tip of the shoulder and the tip of the olecranon process.24
AF (the main outcome of interest in this study) was assessed at baseline and follow‐up based on an overall evaluation of medical history, physical examination (including heart rate measurement at radial and central pulse), medications use (particularly use of anticoagulant drugs), and medical and hospital records using the International Classification of Diseases, Ninth Revision (ICD‐9) code 427.31 for identification of AF.
2.3. Demographic and medical data
Sociodemographic information of the study participants was collected regarding educational level (<5 vs ≥5 years of schooling), monthly income (<500€ vs ≥500€), smoking habits (classifying participants as “never,” “former” if they had stopped at least a year earlier, or “current” smokers), alcohol drinking (yes/no), and regular physical activity in the previous month (considering ≥4 h/wk of at least moderate physical activities, such as brisk walking, cycling, gardening, dancing, or physical exercising). Medical conditions were assessed by board‐certified physicians based on physical examination, medical history, questionnaires, and biochemical analyses. The cardiovascular diseases (CVD) considered were: congestive heart failure, coronary artery diseases (angina and myocardial infarction), stroke, or peripheral artery disease. Hypertension and diabetes were assessed on the basis of the current guidelines to define these diseases in the adult population.25, 26
2.4. Statistical analyses
The sample in the Pro.V.A. study was generalized to the population at large in the 2 geographical areas considered by applying a set of weights according to the gender and age distribution in the reference population (Italy, 1991 census), and the sample fraction.
Normal distributions were tested using the Shapiro‐Wilk test for continuous variables. Quantitative measures were expressed as mean ± standard deviation for continuous variables if normality was satisfied, and as frequency percentages for discrete variables. To compare differences between the means of the covariates by onset of AF at follow‐up, age‐ and sex‐adjusted P values were calculated using a general linear model for continuous variables and logistic regression for discrete variables. Levene's test was used to test the homoscedasticity of the variances and, if its assumption was violated, then Welch's analysis of variance (ANOVA) was used. To test the robustness of our results, we conducted several sensitivity analyses stratifying for clinical factors independently associated with the onset of AF in the fully adjusted model to see if the association between anthropometric parameters and new onset of AF would depend on any medical or other conditions. Particularly, gender seemed to affect our results (P for interaction < 0.0001); therefore, we used gender‐specific cutoffs to categorize anthropometric parameters. The proportional hazards assumption was checked by plotting the Schoenfeld residuals vs time. However, because this test showed a P < 0.0001, a multivariate logistic regression analysis instead of Cox proportional hazard models was used to assess the associations between baseline anthropometric measures and incident AF.
For the purposes of the present study, we classified BMI as underweight (BMI <20 kg/m2), normal weight (20 < BMI < 25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2), and obese (BMI ≥ 30 kg/m2); and stature on the basis of gender‐specific quartiles (for men: <162, 162–166.9, 167–170.9, ≥171 cm; and for women: <149, 149–152.9, 153–156.9, ≥157 cm). As for BSA, we divided our sample into quartiles with the cutoffs: 2.59, 3.00, 3.50 m2. Waist circumference was dichotomized on the basis of the cutoffs used to define metabolic syndrome in women (88 cm) and men (102 cm),27 and using gender‐specific tertiles (cutoffs for men 94 and 100 cm, for women 91 and 100 cm). WSR was categorized considering the median value of the sample (0.61) as a cutoff. Hip circumference was categorized using the gender‐specific median value (for men 99 cm, for women 100.8 cm) as a cutoff. WHR was categorized by the World Health Organization cutoffs to define central obesity (for men 0.90, for women 0.85).23 MUAC was dichotomized using the median value of our sample (30.3 cm) as a cutoff. The exposure variables were put in the fully adjusted model 1 at a time. Factors commonly known to be associated with AF were examined in the univariate analysis for inclusion as covariates; those with a P value <0.05 were included in the fully adjusted model. No variables demonstrated a variance inflation factor greater than 2 on collinearity analysis, so they were all included in the final model. Odds ratio (OR) and 95% confidence interval (CI) were calculated to examine the association between different anthropometric parameters and the onset of AF at follow‐up.
Receiver operator characteristic (ROC) curves were analyzed to compare the sensitivity and specificity of the different anthropometric parameters as a risk factor for AF, measuring the area under the curve (AUC).
All analyses were performed using the SPSS 23.0 for Windows (IBM, Armonk, NY). All statistical tests were 2‐tailed and statistical significance was assumed for a P value <0.05.
3. RESULTS
Compared with the final sample, subjects excluded from our analyses were significantly older (78.4 ± 8.5 years, P < 0.0001) and had lower values of BMI (26.88 ± 4.33 kg/m2, P < 0.0001), MUAC (29.03 ± 3.78 cm, P < 0.0001), waist (95.84 ± 11.40 cm, P < 0.0001), and hip circumference (100.07 ± 9.68 cm, P < 0.0001). Regarding sociodemographic factors and comorbidities, participants excluded from the study were less likely to be physically active (P < 0.0001), smokers (P = 0.008) and drinkers (P = 0.02), they reported significant higher prevalence of CVDs (P < 0.0001), diabetes (P = 0.001) but lower rate of hypertension (P < 0.0001), and fewer medications continually taken (P < 0.0001). No significant differences were observed considering the rate of men and women between subjects included and excluded from our study, as well as educational level and monthly income (data not shown).
The final sample included in our study consisted of 1764 community‐dwelling elderly people (638 male and 1126 female), with a mean age of 74.3 ± 6.9 years (range, 65–96 years) and a mean BMI of 28.0 ± 4.6 kg/m2 (data not weighted). Regarding BMI values, at baseline 426 subjects (24.1%) had normal weight, 39 (2.2%) were underweight, 802 (45.5%) were overweight, and 497 (28.2%) were obese. The sample's baseline characteristics by onset of AF at follow‐up are shown in Table 1. Subjects who developed AF were significantly older than those who did not, they had higher body height, BMI, and BSA values, but lower WSR. The AF group showed a higher prevalence of diabetes, hypertension, and CVDs, and a higher use of medications at the baseline evaluation. Moreover, these subjects reported higher frequency of smoking habits, and lower educational and physical activity levels (Table 1). To minimize the possible effect of competing mortality on our data, a comparison of baseline characteristics between participants who died during the follow‐up with those included in the study and developing or not developing AF was performed (see Supporting Table A1 in the online version of this article). Persons who died during the follow‐up were significantly older, had the highest prevalence of diabetes and CVDs, the highest number of medications used daily, and were less physically active. Regarding anthropometrics, they demonstrated lower values of body height and BSA compared with subjects who developed or did not develop AF during the follow‐up period, but no further significant differences in other anthropometric measures.
Table 1.
Baseline characteristics of the sample (n = 1764) divided by onset of atrial fibrillation at follow‐up
| Variable | Atrial Fibrillation at Follow‐up (n = 115) | No Atrial Fibrillation at Follow‐up (n = 1649) | P Value1 |
|---|---|---|---|
| Age, y | 75.2 ± 6.1 | 74.2 ± 6.9 | <0.0002 |
| Gender, female, % | 59.1 | 64.2 | <0.0001 |
| Medical conditions, % | |||
| Hypertension | 84.3 | 76.4 | <0.0001 |
| Diabetes | 19.1 | 14.6 | <0.0001 |
| Cardiovascular diseases | 25.2 | 16.8 | <0.0001 |
| ≥3 drugs taken daily | 74.5 | 58.5 | <0.0001 |
| Demographic information, % | |||
| Education ≥5 years | 11.9 | 14.8 | 0.001 |
| Current/former smokers | 43.5 | 36.1 | <0.0001 |
| Monthly income ≥500 euros | 37.7 | 38.7 | 0.38 |
| Drinking habits | 71.3 | 67.6 | 0.07 |
| Physical activity >4h/wk | 21.7 | 26.7 | <0.0001 |
| Anthropometric parameters | |||
| Body mass index, kg/m2 | 28.20 ± 4.26 | 27.94 ± 4.64 | 0.03 |
| Height, cm | 160.3 ± 9.0 | 157.9 ± 9.1 | <0.0001 |
| Body surface area, m2 | 3.24 ± 0.69 | 3.07 ± 0.68 | <0.0001 |
| Waist, cm | 96.87 ± 9.97 | 96.87 ± 11.80 | 0.80 |
| Waist‐to‐stature ratio | 0.61 ± 0.06 | 0.62 ± 0.08 | 0.002 |
| Hip circumference, cm | 101.90 ± 8.47 | 101.02 ± 9.65 | 0.97 |
| Waist‐to‐hip ratio | 0.95 ± 0.08 | 0.96 ± 0.08 | 0.57 |
| Mid–upper arm circumference | 30.33 ± 3.11 | 30.30 ± 3.42 | 0.85 |
Numbers are mean values ± standard deviations or percentages (%), as appropriate (weighted data).
Unless otherwise specified, P values are adjusted for age and gender using a general linear model or logistic regression, as appropriate.
Not adjusted for age.
After a mean follow‐up of 4.4 years, 115 new cases of AF (6.5%) were identified (47 male and 68 female), with an age‐ and gender‐specific incidence rate of 13/1000 person‐years (95% CI: 0‐28). Among these, 54 cases (47%) were detected by analyzing medical and hospital records collected during the study period, 44 (38%) by evaluating the new introduction of AF‐related medications, in particular anticoagulant therapy, and 17 (15%) through the medical and physical examination at the follow‐up assessment. The cumulative incidence of AF in our sample divided into categories according to participants’ different anthropometric features is shown in Figure 1. The incidence of AF was significantly increased in subjects with higher values of BMI, stature, BSA, MUAC, and hip circumference, and in those who had intermediate waist circumference. Logistic regression analysis demonstrated a significantly higher risk of AF development in taller individuals (for the fourth height quartile: OR = 2.42, 95% CI: 1.88‐3.12; P < 0.0001), those with a higher BSA (for the fourth quartile: OR = 2.12, 95% CI: 1.73‐2.59, P < 0.0001), those who were obese (OR = 1.36, 95% CI: 1.15‐1.62, P < 0.0001), and in subjects with higher MUAC (OR = 1.38, 95% CI: 1.21‐1.56, P < 0.0001) and hip circumference values (OR = 1.39, 95% CI: 1.23‐1.58, P < 0.0001), even after adjusting for potential confounders (Table 2). Regarding central obesity, intermediate values of waist circumference were associated with an increased risk of AF (OR = 1.60, 95% CI: 1.37‐1.86, P < 0.0001), whereas having higher WSR (OR = 0.72, 95% CI: 0.56‐0.92, P = 0.01) and WHR (OR = 0.68, 95% CI: 0.56‐0.83, P < 0.0001) was significantly associated with a lower risk of developing AF (Table 2). As sensitivity analysis, we repeated logistic regression considering the onset of AF only detected by ICD‐9 code, as dependent variable (see Supporting Table A2 in the online version of this article). The significance of these association was not confirmed for obesity, intermediate waist tertile, and for MUAC, WSR, and WHR only when dichotomized. Conversely, the ORs for the other anthropometric parameters were corroborated at sensitivity analysis.
Figure 1.
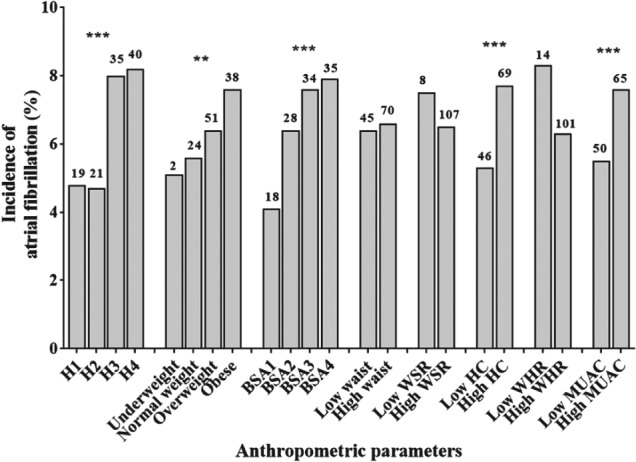
Cumulative incidence of atrial fibrillation in study participants divided by different anthropometric parameters. Absolute values are reported on top of the graph bars. *P < 0.05. **P < 0.01. ***P < 0.0001. Abbreviations: BMI, body mass index; BSA, body surface area; H, height; HC, hip circumference; MUAC, mid–upper arm circumference; WHR, waist‐to‐hip ratio; WSR, waist‐to‐stature ratio. Underweight (BMI < 20 kg/m2), normal weight (20 < BMI < 25 kg/m2), overweight (25 < BMI < 30 kg/m2), obese (BMI > 30 kg/m2). H1 (<162 cm for men; <149 cm for women), H2 (162–166 cm for men; 149–152 cm for women), H3 (167–170 cm for men; 153–156 cm for women), H4 (≥171 cm for men; ≥157 cm for women). BSA1 (<2.59 m2), BSA2 (2.59–2.99 m2), BSA3 (3.00–3.49 m2), BSA4 (≥3.50 m2). Low waist circumference (<102 cm for men, <88 cm for women), high waist circumference (≥102 cm for men, ≥88 cm for women). Low WSR (≤0.61), high WSR (>0.61). Low HC (<99 cm for men, <100.8 cm for women), high HC (≥99 cm for men, ≥100.8 cm for women). Low WHR (<0.90 for men, <0.85 for women), high WHR (≥0.90 for men, ≥0.85 for women). Low MUAC (<30.3 cm), high MUAC (≥30.3 cm).
Table 2.
Association between nutritional–anthropometric parameters and the onset of atrial fibrillation during the follow‐up period in the PRO.V.A. study (weighted data)
| Age‐ and Sex‐Adjusted Model | Fully Adjusted Model1 | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Height, cm | ||||
| Per 1 cm increase | 1.05 (1.04‐1.05) | <0.0001 | 1.05 (1.04‐1.06) | <0.00012 |
| Per 5 cm increase | 1.26 (1.20‐1.31) | <0.0001 | 1.24 (1.18‐1.30) | <0.00012 |
| <162 (m); <149 (w) | 1 [Ref.] | <0.00013 | 1 [Ref.] | <0.00012, 3 |
| 162‐166 (m); 149‐152 (w) | 1.12 (0.91‐1.37) | 0.29 | 1.04 (0.85‐1.28) | 0.702 |
| 167‐170 (m); 153‐156 (w) | 2.50 (2.06‐3.04) | <0.0001 | 2.31 (1.89‐2.82) | <0.00012 |
| ≥171 (m); ≥157 (w) | 2.58 (2.04‐3.26) | <0.0001 | 2.42 (1.88‐3.12) | <0.0001† 2 |
| Body mass index, kg/m2 | ||||
| Per 1 kg/m2 increase | 1.02 (1.00‐1.03) | 0.02 | 1.08 (0.99‐1.02) | 0.33 |
| Normal weight (20 ≤ BMI < 25) | 1 [Ref.] | <0.00013 | 1 [Ref.] | 0.0033 |
| Underweight (BMI <20) | 0.90 (0.56‐1.44) | 0.66 | 1.00 (0.62‐1.61) | 0.99 |
| Overweight (25 ≤ BMI < 30) | 1.18 (1.01‐1.38) | 0.04 | 1.13 (0.97‐1.33) | 0.13 |
| Obese (BMI ≥30) | 1.47 (1.25‐1.74) | <0.0001 | 1.36 (1.15‐1.62) | <0.0001 |
| Body surface area, m2 | ||||
| Per 0.1 m2 increase | 1.04 (1.03‐1.05) | <0.0001 | 1.04 (1.03‐1.05) | <0.0001 |
| <2.59 | 1 [Ref] | <0.00013 | 1 [Ref] | <0.00013 |
| 2.59–2.99 | 1.50 (1.24‐1.81) | <0.0001 | 1.58 (1.30‐1.91) | <0.0001 |
| 3.00–3.49 | 1.86 (1.54‐2.25) | <0.0001 | 1.77 (1.46‐2.14) | <0.0001 |
| ≥3.50 | 2.22 (1.82‐2.70) | <0.0001 | 2.12 (1.73‐2.59) | <0.0001 |
| Waist, cm | ||||
| Per 1 cm increase | 1.00 (1.00‐1.01) | 0.92 | 1.00 (0.99‐1.00) | 0.16 |
| ≥102 vs <102 (m), ≥88 vs <88 (w) | 1.16 (1.01‐1.32) | 0.03 | 1.05 (0.92‐1.21) | 0.47 |
| <94 cm (m), <91 cm (w) | 1 [Ref] | <0.00013 | 1 [Ref] | <0.00013 |
| 94–100 cm (m), 91–100 cm (w) | 1.67 (1.44‐1.94) | <0.0001 | 1.60 (1.37‐1.86) | <0.0001 |
| ≥101 cm (m, w) | 1.20 (1.03‐1.40) | 0.02 | 1.12 (0.96‐1.31) | 0.16 |
| Waist‐to‐stature ratio | ||||
| Per 1 unit increase | 0.27 (0.12‐0.59) | 0.001 | 0.12 (0.05‐0.28) | <0.0001 |
| >0.61 vs ≤0.61 | 0.88 (0.70‐1.11) | 0.80 | 0.72 (0.56‐0.92) | 0.01 |
| Hip circumference (cm) | ||||
| Per 1 cm increase | 1.01 (1.00‐1.02) | 0.003 | 1.01 (1.00‐1.01) | 0.01 |
| >99 vs <99 cm (m); >100.8 vs <100.8 cm (w) | 1.42 (1.26‐1.60) | <0.0001 | 1.39 (1.23‐1.58) | <0.0001 |
| Waist‐to‐hip ratio | ||||
| Per 1 unit increase | 0.30 (0.14‐0.67) | 0.003 | 0.12 (0.05‐0.28) | <0.0001 |
| ≥0.90 vs <0.90 (m), ≥0.85 vs <0.85 (w) | 0.82 (0.68‐0.99) | 0.04 | 0.68 (0.56‐0.83) | <0.0001 |
| Mid–upper arm circumference | ||||
| Per 1 cm increase | 1.01 (0.99‐1.03) | 0.38 | 1.00 (0.98‐1.01) | 0.60 |
| ≥30.3 vs <30.3 cm | 1.48 (1.32‐1.68) | <0.0001 | 1.38 (1.21‐1.56) | <0.0001 |
Abbreviations: BMI, body mass index; BSA, body surface area; CI, confidence interval; m, men; OR, odds ratio; PRO.V.A, Progetto Veneto Anziani; w, women.
The fully adjusted model includes: age (as continuous variable), gender (men/women), baseline presence of: diabetes (yes/no); hypertension (yes/no); cardiovascular diseases (yes/no); educational level (education ≥ 5 vs <5 years); physical activity (≥4 vs < 4 h/week); smoking habits (current/former vs never); number of drugs taken (≥3 vs <3/day).
Weight (as continuous variable) was included in the fully adjusted model.
P values in the first quartile represent P for trend calculated using the Wald test based on a score with the median value of each baseline tertile or quartile for the anthropometric parameter considered.
ROC analyses demonstrated that stature (AUC = 0.57; 95% CI: 0.52‐0.63, P = 0.007) and BSA (AUC = 0.58; 95% CI: 0.52‐0.63, P = 0.007) had the largest AUC for the risk of developing AF (Figure 2). No significant results emerged regarding BMI, waist circumference, WSR, WHR, and hip and arm circumference (Table 3).
Figure 2.
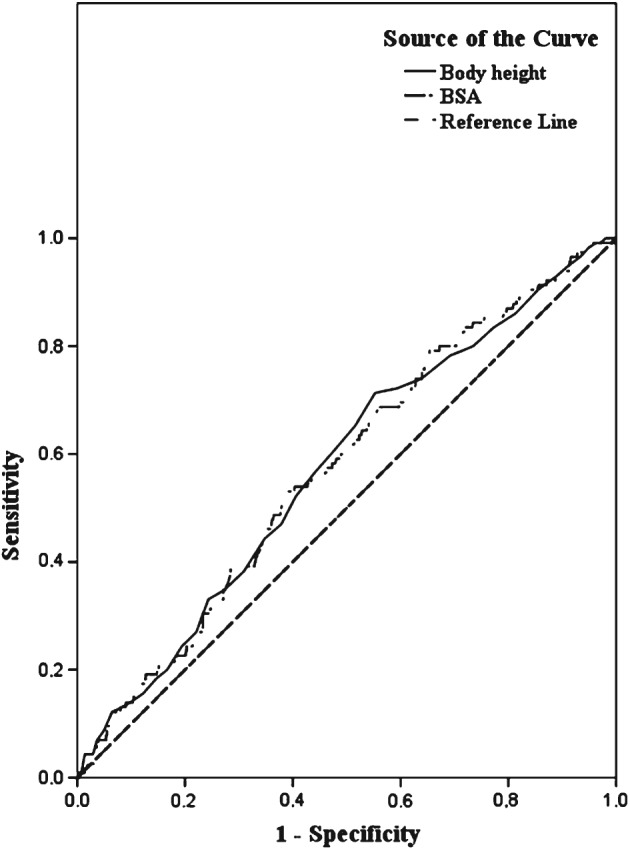
Receiver operating characteristic curve for body height and BSA. Abbreviations: BSA, body surface area.
Table 3.
Area under the receiver operating characteristic curve for anthropometric parameters as a predictor of atrial fibrillation onset during the 4.4‐year follow‐up
| Anthropometric Parameter | AUC | 95% Confidence Interval | P Value1 | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Body height | 0.57 | 0.52 | 0.63 | 0.007 |
| Body surface area | 0.58 | 0.52 | 0.63 | 0.007 |
| Body mass index | 0.54 | 0.48 | 0.59 | 0.19 |
| Waist circumference | 0.51 | 0.46 | 0.56 | 0.82 |
| Waist‐to‐stature ratio | 0.48 | 0.43 | 0.53 | 0.43 |
| Hip | 0.55 | 0.49 | 0.60 | 0.11 |
| Waist to hip | 0.47 | 0.42 | 0.53 | 0.36 |
| Arm | 0.51 | 0.46 | 0.57 | 0.66 |
Abbreviations: AUC, area under the curve.
Asymptotic significance (null hypothesis: true area = 0.5).
4. DISCUSSION
This longitudinal study demonstrated that body size is more strongly associated than fat distribution with the onset of atrial fibrillation in older people. In particular, significant predictors of AF were tall stature, high BSA, obesity, high hip and mid–upper arm circumference, whereas waist, WHR, or WSR demonstrated no relevant associations with AF.
The incidence of AF in our sample of elderly subjects (13/1000 person‐years) was similar to that reported by Heeringa et al, namely 14.7/1000 person‐years, for subjects in the age group 75 to 79 years.28
Tall stature and a high BSA have been associated with AF in several studies on both healthy individuals6, 9, 12, 14, 15 and patients with left ventricular dysfunction.13 The most accredited hypothesis to explain this relationship concerns the link between body size and left atrial dimension.14, 29, 30 As suggested by the multiple wavelet theory proposed by Moe,16 and supported by Allessie et al,17 a larger atrial area promotes the persistent random and simultaneous propagation of multiple independent wavelets. In accordance with this theory, a large prospective study found the relative risk of AF 60% higher for every additional 1 cm in left atrial size beyond 3 cm.30 However, left atrial dimension does not seem to be the only mediator in the relationship between body size and AF, because, as demonstrated by the Cardiovascular Health Study, the adjustment for echocardiographic parameters only slightly attenuated the risk of AF attributable to height.31 This finding suggests that other pathophysiologic mechanisms, such as the influence of growth‐related genes,32 could be involved, thus further investigation is needed to better analyze this issue. The results of our study support the association between body size and AF. In particular, we found that tall stature seems more strictly associated with incident AF than BSA or BMI (ie, an increase in height of 1 cm would increase the risk of AF by 5%). In our sample, this association appeared not linear and was slightly stronger than in the report from Hanna et al, who evaluated a cohort of patients with left ventricular dysfunction.13 Despite our study confirmed that body height was significantly associated with new onset AF, the use of stature cutoffs remain indices with poor reliability in the elderly population because of the age‐related height decline that ranges between 1.5 and 2.1 mm per year over 70 years and is due to multiple causes, such as senile kyphosis and severe osteoporosis.33
As regards obesity, the thickening myocardium frequently reported in overweight individuals may also give rise to a diastolic dysfunction and consequent atrial dilation.34 The excessive levels of cardiac lipids in obese people also seem to be associated with higher levels of myocardial lipid peroxidation and lipoapoptotic processes, both of which predispose to structural changes in the myocardium.35 Our results confirm this association, since we found a 36% higher risk of AF in the obese, consistent with a similar longitudinal study demonstrating that this relationship was stronger in men than in women.6 Unlike Frost et al,6 instead, we found no significant differences per unit increase in BMI, and the ROC curve showed a poor association with AF.
Considering fat distribution, we observed no relevant and unanimous associations between central obesity indices and AF after adjusting for potential confounders. In particular, waist circumference demonstrated only a weak and no linear influence in increasing the risk of AF, whereas WSR and WHR showed an inverse relationship with the onset of such arrhythmia. Our study, therefore, suggests that stature and body size more than adiposity could predict the development of AF, ascribing the results of WSR and WHR to the strong associations demonstrated for both stature and hip circumference with AF onset. Although central adiposity represents one of the most relevant components of the metabolic phenotype, fat distribution per se seemed, in fact, not play an independent role in the development of AF in our sample of older persons. These findings differ from those of similar cohort studies on Asian people, which identified a significant association between AF and central obesity measured in terms of waist circumference,4, 8 but not when the WSR was considered.4 The significant relationships between high mid–upper arm and hip circumference with AF, instead, strengthen the hypothesis that not only fat mass, but also fat‐free mass may be a relevant predictor of AF risk.12, 36, 37, 38 In particular, whereas some authors investigated the relationships between body composition and hip circumference with AF, to our knowledge, no data regarding mid–upper arm circumference have been previously reported. The mechanisms underlying the association between lean mass and AF are not yet fully clarified, although modifications of left ventricular mass related to fat‐free mass may be involved.12, 39 However, these findings highlight the need for further investigations on this topic, particularly for the elderly population, due to the high prevalence of this disease in advanced age and the well‐known changes in body composition of older people.
Our work has several limitations that need to be considered. First of all, the unavailability of electrocardiographic data and the design of our study, with only a 4.4‐year follow‐up evaluation, mean that we may have failed to detect some asymptomatic cases of AF. Second, having considered clinical evidence of CVDs, but not echocardiographic parameters of ventricular dysfunction, may represent a bias of our study, because the latter is a potential determinant of atrial dilation. Third, as mentioned above, the measure of body height in elderly subjects could be affected by biases due to a number of vertebral deformities whose prevalence increases with aging. On the other hand, a strength of our work lies in that we were able to compare different anthropometric measurements. The inclusion in our analysis of information on socioeconomic status, physical activity, and health‐risk behavior as covariates probably strengthens our results, because these factors are potential confounders in the association between body size and AF.
5. CONCLUSION
Our study found that stature and body size may have a greater influence than fat distribution on the development of AF in the elderly population.
Supporting information
APPENDIX A (supporting information)
Table A1. Baseline characteristics of subjects who dead during the follow‐up, compared with those developing and not developing atrial fibrillation after a mean of 4.4 years.
Table A2. Sensitivity analysis: association between nutritional‐anthropometric parameters and the onset of atrial fibrillation (detected by ICD code) during the follow‐up period in the PRO.V.A. study (weighted data)
ACKNOWLEDGMENTS
The authors are grateful to all interviewers, nurses, and physicians who took part in the study.
Author Contributions
All of the authors contributed to the manuscript, read it, approved the final version, and gave permission for their names to be included as coauthors. In particular: C.T., G.S., G.C., and E.M. participated in the conception and design of the study; M.N., C.T., S.M., S.Z., L.S., and E.P. participated in the acquisition, analysis, and interpretation of data; and C.T., G.N., C.C., M.D.R., and G.S. drafted and revised the article.
Conflicts of interest
The authors report no conflicts of interest.
Trevisan C, Maggi S, Curreri C, Nante G, Noale M, De Rui M, Perissinotto E, Sartori L, Zambon S, Crepaldi G, Manzato E and Sergi G. Anthropometric parameters and the incidence of atrial fibrillation in older people: the PRO.V.A study, Clin Cardiol, 2017;40:461–468. 10.1002/clc.22677
Funding information The data collection phase of the PRO.V.A; Fondazione Cassa di Risparmio di Padova e Rovigo; University of Padova; Azienda Unità Locale Socio Sanitaria 15 and 18 of the Veneto Region; Veneto Regional Authority, Grant/Award number: Ricerca Sanitaria Finalizzata n.156/03; University of Padova (Population Aging–Economics, Health, Retirement and the Welfare State–POPA_EHR).
REFERENCES
- 1. Zoni‐Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Desai NR, Giugliano RP. Can we predict outcomes in atrial fibrillation? Clin Cardiol. 2012;35:S10–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of a meta‐analysis. Am Heart J. 2008;155:310–315. [DOI] [PubMed] [Google Scholar]
- 4. Long MJ, Jiang CQ, Lam TH, et al. Atrial fibrillation and obesity among older Chinese: the Guangzhou Biobank Cohort Study. Int J Cardiol. 2011;148:48–52. [DOI] [PubMed] [Google Scholar]
- 5. Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001;250:382–389. [DOI] [PubMed] [Google Scholar]
- 6. Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–495. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe H, Tanabe N, Watanabe T, et al. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baek Y, Yang P‐S, Kim T‐H, et al. Associations of abdominal obesity and new onset atrial fibrillation in general population: a nationwide cohort study in Korea. J Am Coll Cardiol. 2016;67:694. [Google Scholar]
- 9. Nyström PK, Carlsson AC, Leander K, de Faire U, Hellenius M‐L, Gigante B. Obesity, metabolic syndrome and risk of atrial fibrillation: a Swedish, prospective cohort study. PLoS One. 2015;10:e0127111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 11. Ho S‐Y, Lam T‐H, Janus ED. Waist to stature ratio is more strongly associated with cardiovascular risk factors than other simple anthropometric indices. Ann Epidemiol. 2003;13:683–691. [DOI] [PubMed] [Google Scholar]
- 12. Karas MG, Yee LM, Biggs ML, et al. Measures of body size and composition and risk of incident atrial fibrillation in older people: the Cardiovascular Health Study. Am J Epidemiol. 2016;183:998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanna IR, Heeke B, Bush H, et al. The relationship between stature and the prevalence of atrial fibrillation in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:1683–1688. [DOI] [PubMed] [Google Scholar]
- 14. Rosengren A, Hauptman PJ, Lappas G, Olsson L, Wilhelmsen L, Swedberg K. Big men and atrial fibrillation: effects of body size and weight gain on risk of atrial fibrillation in men. Eur Heart J. 2009;30:1113–1120. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt M, Bøtker HE, Pedersen L, Sørensen HT. Adult height and risk of ischemic heart disease, atrial fibrillation, stroke, venous thromboembolism, and premature death: a population based 36‐year follow‐up study. Eur J Epidemiol 2014;29:111–118. [DOI] [PubMed] [Google Scholar]
- 16. Moe G. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn Ther 1962;140:183–188. [Google Scholar]
- 17. Allessie MA, Lammers WJEP, Bonke FIM, Hollen J. Experimental evaluation of Moe's multiple wavelet hypothesis of atrial fibrillation In: Zipes DP, Jalife J, eds. Cardiac Electrophysiology And Arrhythmias. Orlando, Florida: Grune and Straton, Inc; 1985:265–275. [Google Scholar]
- 18. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998;82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 19. Corti M‐C, Guralnik JM, Sartori L, et al. The effect of cardiovascular and osteoarticular diseases on disability in older Italian men and women: rationale, design, and sample characteristics of the Progetto Veneto Anziani (PRO.V.A.) study. J Am Geriatr Soc . 2002;50:1535–1540. [DOI] [PubMed] [Google Scholar]
- 20. Veronese N, De Rui M, Toffanello ED, et al. Body mass index as a predictor of all‐cause mortality in nursing home residents during a 5‐year follow‐up. J Am Med Dir Assoc. 2013;14:53–57. [DOI] [PubMed] [Google Scholar]
- 21. Mosteller RD. Simplified calculation of body‐surface area. N Engl J Med. 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- 22. Seidell JC, Kahn HS, Williamson DF, Lissner L, Valdez R. Report from a Centers for Disease Control and Prevention Workshop on use of adult anthropometry for public health and primary health care. Am J Clin Nutr. 2001;73:123–6. [DOI] [PubMed] [Google Scholar]
- 23. Waist Circumference and Waist‐Hip Ratio . Report of a WHO Expert Consultation, Geneva, 8–11 December 2008. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 24. Tang AM, Dong K, Deitcher M, et al; Food and Nutrition Technical Assistance III Project (FANTA) . Use of Cutoffs for Mid‐Upper Arm Circumference (MUAC) as an Indicator or Predictor of Nutritional and Health‐Related Outcomes in Adolescents and Adults: A Systematic Review. Washington, DC: United States Agency for International Development; 2013:1–39. [Google Scholar]
- 25. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 26. Drouin P, Blickle JF, Charbonnel B, et al. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009;32:S62–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA . 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 28. Heeringa J, van der Kuip DAM, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 29. Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 30. Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 31. Rosenberg MA, Patton KK, Sotoodehnia N, et al. The impact of height on the risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012;33:2709–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gudbjartsson DF, Arnar DO, Helgadottir A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. [DOI] [PubMed] [Google Scholar]
- 33. Dey DK, Rothenberg E, Sundh V, Bosaeus I, Steen B. Height and body weight in the elderly. I. A 25‐year longitudinal study of a population aged 70 to 95 years. Eur J Clin Nutr . 1999;53:905–914. [DOI] [PubMed] [Google Scholar]
- 34. Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–236. [PubMed] [Google Scholar]
- 35. Vincent HK, Powers SK, Stewart DJ, Shanely RA, Demirel H, Naito H. Obesity is associated with increased myocardial oxidative stress. Int J Obes Relat Metab Disord. 1999;23:67–74. [DOI] [PubMed] [Google Scholar]
- 36. Frost L, Benjamin EJ, Fenger‐Grøn M, Pedersen A, Tjønneland A, Overvad K. Body fat, body fat distribution, lean body mass and atrial fibrillation and flutter. A Danish cohort study. Obesity . 2014;22:1546–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nyström PK, Carlsson AC, Leander K, de Faire U, Hellenius M‐L, Gigante B. Obesity, metabolic syndrome and risk of atrial fibrillation: a Swedish, prospective cohort study. PLoS One. 2015;10:e0127111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verdecchia P, Dagenais G, Healey J, et al. Blood pressure and other determinants of new‐onset atrial fibrillation in patients at high cardiovascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtoleran. J Hypertens. 2012;30:1004–1014. [DOI] [PubMed] [Google Scholar]
- 39. Bella JN, Devereux RB, Roman MJ, et al. Relations of left ventricular mass to fat‐free and adipose body mass: the strong heart study. The Strong Heart Study Investigators. Circulation. 1998;98:2538–2544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX A (supporting information)
Table A1. Baseline characteristics of subjects who dead during the follow‐up, compared with those developing and not developing atrial fibrillation after a mean of 4.4 years.
Table A2. Sensitivity analysis: association between nutritional‐anthropometric parameters and the onset of atrial fibrillation (detected by ICD code) during the follow‐up period in the PRO.V.A. study (weighted data)


