Abstract
Background
Apnea diving has gained worldwide popularity, even though the pathophysiological consequences of this challenging sport on the human body are poorly investigated and understood. This study aims to assess the influence of sustained apnea in healthy volunteers on circulating microparticles (MPs) and microRNAs (miRs), which are established biomarkers reflecting vascular function.
Hypothesis
Short intermittent hypoxia due to voluntary breath‐holding affects circulating levels of endothelial cell‐derived MPs (EMPs) and endothelial cell‐derived miRs.
Methods
Under dry laboratory conditions, 10 trained apneic divers performed maximal breath‐hold. Venous blood samples were taken, once before and at 4 defined points in time after apnea. Samples were analyzed for circulating EMPs and endothelial miRs.
Results
Average apnea time was 329 seconds (±103), and SpO2 at the end of apnea was 79% (±12). Apnea was associated with a time‐dependent increase of circulating endothelial cell‐derived EMPs and endothelial miRs. Levels of circulating EMPs in the bloodstream reached a peak 4 hours after the apnea period and returned to baseline levels after 24 hours. Circulating miR‐126 levels were elevated at all time points after a single voluntary maximal apnea, whereas miR‐26 levels were elevated significantly only after 30 minutes and 4 hours. Also miR‐21 and miR‐92 levels increased, but did not reach the level of significance.
Conclusions
Even a single maximal breath‐hold induces acute endothelial activation and should be performed with great caution by subjects with preexisting vascular diseases. Voluntary apnea might be used as a model to simulate changes in endothelial function caused by hypoxia in humans.
Keywords: Breath‐hold, Apnea, Circulating Microparticles, Vascular Function
1. INTRODUCTION
Throughout the last years, apneic diving has become increasingly popular, and apneic divers have been pushing their limits of performance beyond expected boundaries. Prolonged apnea leads to the so‐called diving response, which includes vasoconstriction,1 elevated mean arterial blood pressure,2 bradycardia,3 and increased cerebral blood flow.4 In the case of apnea, these compensatory mechanisms secure an adequate oxygen supply of the brain due to blood redistribution.5
Breath‐holding burdens the cardiovascular system due to extreme peripheral vasoconstriction6 and catecholamine release.7 But apart from scientific interest in and public fascination with the sport, cardiac arrhythmias are commonly seen in people performing apnea.8 Studies focusing on cardiac function under apnea show progressive hindrance to diastolic filling.9 In parallel, dynamic hypoxia (ie, increase of hypercapnia and hypoxia) is seen in patients with airway obstruction such as laryngospasm or bronchospasm, or intermittent in patients with obstructive sleep apnea.
Apneic divers are able to lower their oxygen storage in their lungs, reaching alveolar levels of <30 mm Hg oxygen and high alveolar partial pressures of >50 mm Hg CO2. It is known that endothelial dysfunction is mainly caused by hypoxia. There is evidence that cognitive deficiencies in attention, vigilance, memory, and learning could be explained by endothelial dysfunction caused by short intermittent hypoxia.10, 11
So far, it has not been investigated whether sustained apnea might acutely influence vascular function. We hypothesize that short intermittent hypoxia due to voluntary breath‐hold affects circulating levels of endothelial microparticles (EMPs) and endothelial cell‐derived microRNAs (miRs), which represent surrogate parameters for vascular endothelial function.
2. METHODS
The study was approved by the local research ethics committees (Ethikkommission an der Medizinischen Fakultät der Rheinischen Friedrich‐Wilhelms‐Universität Bonn; Lfd. Nr. 373/13) in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Each subject received an information sheet 14 days before the investigation and provided written informed consent. A static dry apnea setup in a horizontal position was chosen to distinguish effects induced by apnea (ie, hypoxia and hypercapnia) from stimuli caused by face immersion12 or body immersion.9 For this study, 10 apneic divers performed a single maximal breath‐hold at maximal inspiration in a supine position on a padded table in a fully climate‐controlled room. To assess the influence of sustained apnea on vascular health, circulating vascular miRs and EMPs, platelet‐derived microparticles (PMPs), and monocyte‐derived microparticles (MMPs) within the bloodstream of the subject were quantified at different time points after sustained apnea.
2.1. Physical data
Heart rate and peripheral oxygen saturation (SpO2) were measured by a patient‐monitoring device (Expression MR400; Invivo, Gainsville, FL). Lung capacity was measured with a pulmonary function analyzer (Spirometer Model SP10; Contec Medical Systems Co., Ltd., Qinhuangdao, China).
2.2. Preparation of blood samples
Under sterile conditions, venous blood was drawn from the cubital vein 30 minutes before (baseline), directly after (post), 30 minutes, and 4 hours after a maximal single breath‐hold. For long‐term microparticle (MP) analysis, blood was taken after 24 hours. Collected blood was buffered using sodium citrate for MP quantification or ethylenediaminetetraacetic acid for miRs analysis. For MP and miRs analysis, blood was centrifuged at 1500 g for 15 minutes, followed by centrifugation at 13,0000 g for 2 minutes to generate platelet‐deficient plasma. MP levels were measured immediately after collection, using flow cytometry. The platelet‐deficient plasma was stored at −80°C until the miRs levels were analyzed.
2.3. Flow cytometric quantification of MPs
Circulating MPs were measured by flow cytometry from citrate‐buffered blood. One hundred microliters of platelet‐deficient plasma were incubated with monoclonal antibodies against 1) CD144 (PE = Phycoerythrin mouse anti‐human CD144; BD Pharmingen™, 4 μL), 2) CD14 (PE mouse anti‐human CD14, BD Pharmingen, 4 μL) and CD16 (Alexa Fluor 647 Mouse Anti‐Human CD16; BD Pharmingen, 4 μL), or 3) CD31 (PE mouse anti‐human CD31; BD Pharmingen, 4 μL) and CD42b (Allophycocyanin (APC) mouse anti‐human CD42b; BD Pharmingen, 4 μL) for 45 minutes at room temperature.
Afterward, the sample was incubated with fluorescein isothiocyanate (FITC)‐conjugated Annexin V (FITC annexin V; BD Pharmingen, 4 μL) for 15 minutes at room temperature to label phosphatidylserine expressed on MPs. The staining/incubation was followed by the addition of 200 μL sterile phosphate buffered saline (10 mM (4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid) pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). During these processes, the samples were protected from light. EMPs were defined as positively labeled for annexin V and CD144+, MMPs as positively labeled for annexin V and CD14+and CD16+, and PMPs were defined as positively labeled for annexin V, CD31+, as well as CD42b+. Fluorescence‐activated cell sorting (FACS) analysis was performed immediately after FITC annexin V staining, using a FACS‐Calibur flow cytometer (Becton Dickinson Biosciences, San Jose, CA). MP concentration was assessed by direct comparison to flow cytometer counting calibrator beads from TruCOUNT tubes (Becton Dickinson Biosciences). The data were analyzed using Cellquest software (Becton Dickinson Biosciences).
2.4. Quantification of miRs by quantitative polymerase chain reaction
Expression analysis of circulating miRs was performed as previously described by Jansen et al.13 Briefly, RNA was quantified using Nanodrop spectrophotometer (Nanodrop Technologies Inc., Wilmington, DE). There were 10 ng of the total RNA reversely transcribed using the commercially available TaqMan® miRs reverse transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. MiR‐126, miR‐21, miR‐92a, and miR‐26 were detected in circulating MPs using TaqMan miRs assays (Applied Biosystems) on a 7500 HT real‐time polymerase chain reaction machine (Applied Biosystems). Cel‐miR‐39 was used as an endogenous control.14 For all miRs, a threshold cycle (Ct) value over 40 was defined as undetectable. The delta Ct method was used to quantify relative miRs expression. Values were normalized to cel‐miR‐39 and are expressed as 2−ddct .
2.5. Statistics
Baseline data (pre) were compared to different time points after maximal breath‐hold. Blood samples were called after their time when blood was taken (ie, 30 minutes, 4 hours, and 24 hours post breath‐hold). Samples (post) were taken immediately after ending of apnea. Statistical analyses were performed using SPSS 24 software (IBM, Armonk, NY). Median differences were investigated using Friedman's test followed by Dunn's multiple comparison post test. The post test was used to compare each postapnea time point with the corresponding preapnea values. Figures were created by using GraphPad Prism 6.07 (GraphPad Software, Inc., La Jolla, CA). Values were given as mean and standard error of the mean.
2.6. Ethical statement
The design of the study was approved by the local ethics committee of the University of Bonn, Germany (Lfd. Nr. 373/13). The study was performed in healthy awake volunteers during voluntary nonobstructive apnea. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments.
3. RESULTS
All data were acquired from a group of 10 experienced apneic divers in a static, dry laboratory environment (Table 1). Subjects held their breath for 329 seconds (±103; range 168–587 seconds) and dropped to SpO2 levels of 79% (±11) at the end of apnea. Even after the end of breath‐hold, SpO2 decreased further to 71.7 % (±11.95) before resaturation set in response to breathing. The heart rate declined from 86 bpm (±12) at the beginning of apnea to 51 bpm (±11) at the end of apnea. This change was significant using the 2‐tailed t test. Figure 1 shows the original raw data of a typical time course of SpO2 and heart rate during 374 seconds of apnea. After a steady state period of 218 seconds, during which the oxygen stores of the lungs were being consumed, SpO2 started to decrease. At the end of apnea, SpO2 decreased to 79% and started to increase again, with a time delay of 24 seconds after the first inhalation. SpO2 in this patient dropped to an absolute minimum of 68% after apnea.
Table 1.
Data from 10 experienced apneic divers
| Subject No. | Age, y | Height, cm | Weight,kg | BMI | Total Apnea Time, s | SpO2 at the Start of Apnea | SpO2 at the End of Apnea | Minimal SpO2 | Heart Frequency at the Onset of Apnea, bpm | Heart Frequency at the End of Apnea, bpm | Gender |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | 193 | 86 | 23.09 | 305 | 100 | 69 | 59 | 115 | 46 | Male |
| 2 | 37 | 185 | 97 | 28.34 | 309 | 99 | 56 | 49 | 80 | 49 | Male |
| 3 | 47 | 183 | 91 | 27.17 | 244 | 99 | 91 | 86 | 99 | 57 | Male |
| 4 | 30 | 185 | 84 | 24.54 | 587 | 99 | 67 | 67 | 80 | 77 | Male |
| 5 | 51 | 189 | 86 | 24.08 | 326 | 100 | 75 | 67 | 85 | 46 | Male |
| 6 | 25 | 200 | 110 | 27.50 | 374 | 100 | 79 | 68 | 95 | 48 | Male |
| 7 | 30 | 180 | 73 | 22.53 | 168 | 100 | 96 | 91 | 83 | 51 | Male |
| 8 | 47 | 166 | 76 | 27.58 | 338 | 100 | 83 | 73 | 79 | 32 | Male |
| 9 | 54 | 176 | 70 | 22.60 | 365 | 100 | 85 | 75 | 70 | 50 | Female |
| 10 | 39 | 176 | 68 | 21.95 | 278 | 100 | 89 | 82 | 77 | 56 | Female |
| Mean | 41 | 183 | 84 | 25 | 329 | 99.7 | 79 | 71.7 | 86.3 | 51.2 | |
| SD | 9.90 | 9.10 | 12.41 | 2.34 | 103.29 | 0.46 | 11.72 | 11.95 | 12.48 | 10.78 |
Abbreviations: BMI, body mass index; SD, standard deviation, SpO2, peripheral oxygen saturation.
Figure 1.

Raw data of heart rate (HR) and peripheral oxygen saturation (SpO2) measured during 374 seconds of apnea.
To assess whether apnea‐induced hypoxia might affect circulating MPs and miR levels, PMPs, MMPs, and EMPs were quantified. Differences of rang sums were significantly different in Friedman's test for EMPs (Friedman statistic 10.72, P = 0.0299). The post‐test revealed significant differences between preapnea and 4 hours postapnea for EMPs (P < 0.01). These data suggested that apnea induces distinct endothelial activation. Levels of PMPs remained unaffected, and levels of MMPs did not reach level of significance (Figure 2).
Figure 2.
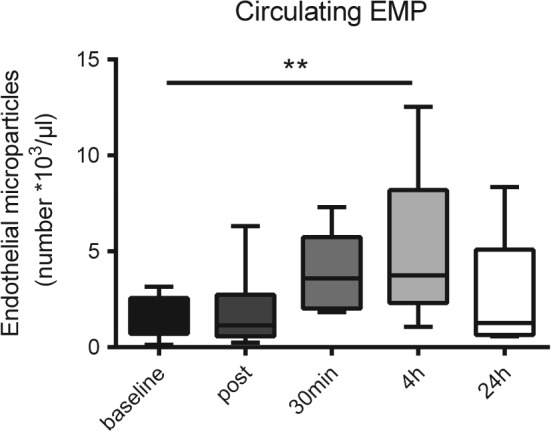
Levels of circulating endothelial cell‐derived microparticles (EMPs) before and at 4 time points after apnea. **P < 0.01.
To explore whether activation of the endothelium might also be associated with the release of endothelial miRs into the circulation, we quantified levels of circulating endothelial miRs (miR‐21, miR‐26, miR‐126, miR‐92) at 4 defined points in time after apnea. Differences of rang sums were significantly different in Friedman's test for miR‐26 (Friedman statistic 12.429, P = 0.0061) and for miR‐126 (Friedman statistic 11.000, P = 0.0057).
Preapnea and 30 minutes postapnea (P < 0.05) as well as 4 hours postapnea (p < 0.01) were significantly different for miR‐26. For miR‐126, all investigated time points (postapnea, 30 minutes postapnea, and 4 hours postapnea) were significantly different compared to preapnea values (P < 0.05). Levels of miR‐21 and miR‐92a were elevated, but did not reach a level of significance because of the high interindividual variability (Figure 3).
Figure 3.

Levels of circulating levels of miR‐21 (A), miR‐26 (B), miR‐92a (C), and miR‐126 (D). *P < 0.05. **P < 0.01.
4. DISCUSSION
The apneic diving sport has gained popularity worldwide throughout the last years, but the acute pathophysiological consequences on the vascular system have been insufficiently investigated to date. In our study, we explored the effects of prolonged breath‐hold and associated hypoxia to the vascular system. We found that a significant decrease in SpO2 during apnea is associated with an increase in circulating EMPs and vascular miRs.
The mean SpO2 levels of the study subjects dropped to 79% at the end of apnea and to even lower levels within the first seconds after onset of respiration. This delay in desaturation is a result of the physiological diving response and has previously been described for apneic divers.5, 7 Peripheral chemoreflex regulation, sympathetic nerve activity,1 and a norepinephrine increase7 leads to peripheral vasoconstriction and hypertension.1, 4 The increase in partial pressure of carbon dioxide (pCO2) leads to an elevated cerebral blood flow, which ensures adequate oxygen supply to the brain, compared to the periphery.5 These vascular redistribution mechanisms help protect the brain during hypoxic events.
EMPs are small membrane vesicles released from endothelial cells, in response to apoptosis and/or activation. Accordingly, patients suffering from diseases associated with systemic endothelial cell damage, such as coronary artery disease or hypertension, show increased plasma levels in circulating EMPs.15
EMPs have various physiological functions, including activation of inflammation and coagulation. By carrying and transferring membrane components and bioactive molecules, they play essential roles in cell–cell crosstalk. EMPs have been involved in a series of gain‐of‐function phenomena by transferring transcription factors that induce expression of target genes, nucleic acids that are translated into biological active proteins, or mature proteins that promptly exert biological activities in recipient cells. The functional effect of EMPs in cardiovascular disease is extremely complex. It depends on the cellular origin, the functional state of the releasing cells, the biological content, the distinct recipient cell, and transfer capacity of intravesicular functional bioactive molecules.16
We detected a significant increase in EMPs 4 hours after a single maximal breath‐hold. Hypoxia is a known trigger for EMP release,17 most probably mediating the increase of circulating EMPs after apnea in our study. Our data are in line with previous findings from another group, who showed that temporary hypoxic conditions in an air‐conditioned chamber can trigger the release of CD31+/ Annexin + EMP in healthy volunteers.18
In patients with coronary artery disease, the levels of circulating EMPs correspond to the degree of endothelial dysfunction.19 This suggests that circulating EMPs function as surrogate parameters for endothelial damage. Furthermore, higher levels of circulating EMPs were associated with a poorer cardiovascular outcome in patients with coronary artery disease in a 6‐year follow‐up study.20 Taking all of these data into account, we can assume that prolonged apnea induces acute endothelial injury, which could have severe consequences in patients with diagnosed and underlying vascular diseases.
In addition to circulating MPs, we analyzed circulating miRNAs released from endothelial cells. Nevertheless, analyzed miRNAs are not specific for endothelial cells. Previous studies have shown that circulating miRNA‐126 is mainly of endothelial cell21 and platelet origin.22 MiRNA‐21 can be released from endothelial cells and from fibroblasts.23, 24 MiRNA‐26 and miRNA‐92 are mainly of endothelial cell origin and can be packed into endothelial microparticles.13
MiRs are powerful regulators of cellular processes. An increasing number of studies demonstrate that miRs can be detected in circulating blood, and that these circulating miRs might be useful biomarkers in patients with cardiovascular or oncological diseases. The present study found that miR‐21, miR‐26, and miR‐126 were released in response to apnea with peak levels after 30 minutes. It is known that miR‐21 and miR‐126 represent 2 of the most prominent miRs within endothelial cells.25
In light of increased levels of EMPs and endothelial miRs in response to apnea, one can speculate that apnea‐associated hypoxia induces endothelial cell activation. This triggers the release of cell fragments and detritus into the circulation. The miR release seems to be a regulated process, because circulating miR‐92 levels were not significantly altered following apnea.
Intermitted hypoxia and hypercapnia is not only seen in divers, but also in clinical situations such as laryngospasm, bronchospasm, or “cannot ventilate cannot intubate” situations. Also obstructive sleep apnea causes intermitted situations of hypoxia and hypercapnia. Corresponding to apneic divers, hypoxia in patients with obstructive sleep apnea results in elevated sympathetic activity,26 elevated arterial blood pressure,27 and, due to hypercapnia, in elevated norepinephrine levels.28 Obstructive sleep apnea patients show an increase in cerebral blood flow, peripheral vasoconstriction, elevated left and right ventricular afterload and cardiac arrhythmia.29 Interestingly, a progressive limitations to diastolic filling and arrhythmias are also seen in healthy apneic divers.8, 9 The respiratory effort during the obstruction of the upper airways may generate substantial intrathoracic pressure fluctuations. The resulting overload of the right heart volume might lead to a secretion of natriuretic peptides.30 These changes in intrathoracic pressure caused by involuntary breathing movements are also seen during voluntary breath‐hold.31 A recent study found a significantly higher prevalence of chronic kidney disease in divers, similar to patients with obstructive sleep apnea.32
One of the main limitations in the field of obstructive sleep apnea syndrome research is the lack of applicable obstructive sleep apnea syndrome models.33 Obstructive sleep apnea syndrome is a risk factor for cardiovascular diseases such as arteriosclerosis, hypertension, and coronary heart disease.34 It is believed that cognitive deficiencies in attention, vigilance, memory, and learning are effected by endothelial dysfunction.10, 11 Patients with obstructive sleep apnea show elevated levels of platelet‐derived microparticles.35 Circulating microparticles of obstructive sleep apnea patients seems to induce endothelin‐mediated angiogenesis.36 Furthermore, an overproduction of EMPs could be inversely correlated to the severity of endothelial dysfunction in children37 and adults38 with obstructive sleep apnea.
Although we found significant elevated EMPs and miRs after a single maximal apnea, we did not find any further correlation between performance, body mass index, or SpO2 values. This might be explained by the high interindividual variety of the divers and/or due to the limited number of athletes. Nevertheless, even usually used cardiac biomarkers for obstructive sleep apnea (ie, N‐terminal pro‐brain natriuretic peptide and brain natriuretic peptide) show weak or no correlation with severity.39, 40 Further studies with bigger cohorts are needed.
To date, hypoxia in humans has only been simulated through the use of hypoxic gas mixtures, due to ethical concerns of other hypoxia‐inducing methods. However, these induced conditions are more representative of high altitude environments due to the resulting hypocapnia. Additionally, the transferability from animal models is limited. In contrast, apneic divers are healthy athletes. They train to depress their breathing impulse, elevate their pCO2 and decrease their partial pressure of oxygen. Considering the amount of similarities (ie, compensatory mechanisms, endothelial dysfunction), apneic divers might function as a clinically relevant model for obstructive sleep apnea.
5. CONCLUSION
A single maximal breath‐hold can induce acute endothelial activation and should be performed with great caution by subjects with preexisting vascular diseases. Voluntary apnea performed by trained apneic divers might be used to simulate clinically relevant hypoxia in humans.
Author Contributions
Lars Eichhorn, MD, and Ramona Dolscheid‐Pommerich, MD, contributed equally to this work.
Conflicts of interest
The authors declare no potential conflicts of interest.
Acknowledgments
The authors thank all of the volunteers who participated in the study, and Anja Reckendorf for critical reading and linguistic assistance.
Eichhorn L, Dolscheid‐Pommerich R, Erdfelder F, Ayub MA, Schmitz T, Werner N and Jansen F. Sustained apnea induces endothelial activation. Clin Cardiol. 2017;40:704–709. 10.1002/clc.22720
Funding Information L.E. was supported by a scholarship of Else‐Kröner‐Fresenius Stiftung. F.J. was supported by the Medical Faculty of the Rheinische Friedrich Wilhelms University Bonn (BONFOR), the “Familie Schambach” foundation, and the German Society of Cardiology.
REFERENCES
- 1. Heusser K, Dzamonja G, Tank J, et al. Cardiovascular regulation during apnea in elite divers. Hypertension. 2009;53:719–724. [DOI] [PubMed] [Google Scholar]
- 2. Perini R, Tironi A, Gheza A, Butti F, Moia C, Ferretti G. Heart rate and blood pressure time courses during prolonged dry apnoea in breath‐hold divers. Eur J Appl Physiol. 2008;104:1–7. [DOI] [PubMed] [Google Scholar]
- 3. Costalat G, Pichon A, Joulia F, Lemaître F. Modeling the diving bradycardia: toward an “oxygen‐conserving breaking point”? Eur J Appl Physiol. 2015;115:1475–1484. [DOI] [PubMed] [Google Scholar]
- 4. Palada I, Obad A, Bakovic D, Valic Z, Ivancev V, Dujic Z. Cerebral and peripheral hemodynamics and oxygenation during maximal dry breath‐holds. Respir Physiol Neurobiol. 2007;157:374–381. [DOI] [PubMed] [Google Scholar]
- 5. Eichhorn L, Erdfelder F, Kessler F, et al. Evaluation of near‐infrared spectroscopy under apnea‐dependent hypoxia in humans. J Clin Monit Comput. 2015;29:749–757. [DOI] [PubMed] [Google Scholar]
- 6. Ferretti G, Costa M. Diversity in and adaptation to breath‐hold diving in humans. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:205–213. [DOI] [PubMed] [Google Scholar]
- 7. Eichhorn L, Erdfelder F, Kessler F, et al. Influence of apnea‐induced hypoxia on catecholamine release and cardiovascular dynamics. Int J Sports Med. 2017;38:85–91. [DOI] [PubMed] [Google Scholar]
- 8. Hansel J, Solleder I, Gfroerer W, et al. Hypoxia and cardiac arrhythmias in breath‐hold divers during voluntary immersed breath‐holds. Eur J Appl Physiol. 2009;105:673–678. [DOI] [PubMed] [Google Scholar]
- 9. Marabotti C, Piaggi P, Menicucci D, et al. Cardiac function and oxygen saturation during maximal breath‐holding in air and during whole‐body surface immersion. Diving Hyperb Med. 2013;43:131–137. [PubMed] [Google Scholar]
- 10. Wang Q, Wu Q, Feng J, Sun X. Obstructive sleep apnea and endothelial progenitor cells. Patient Prefer Adherence. 2013;7:1077–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng J, Wu Q, Zhang D, Chen B. Hippocampal impairments are associated with intermittent hypoxia of obstructive sleep apnea. Chin Med J (Engl) . 2012;125:696–701. [PubMed] [Google Scholar]
- 12. Kjeld T, Pott FC, Secher NH. Facial immersion in cold water enhances cerebral blood velocity during breath‐hold exercise in humans. J Appl Physiol (1985) . 2009;106:1243–1248. [DOI] [PubMed] [Google Scholar]
- 13. Jansen F, Wang H, Przybilla D, et al. Vascular endothelial microparticles‐incorporated microRNAs are altered in patients with diabetes mellitus. Cardiovasc Diabetol. 2016;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood‐based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dignat‐George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. [DOI] [PubMed] [Google Scholar]
- 16. Gupta SK, Bang C, Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet. 2010;3:484–488. [DOI] [PubMed] [Google Scholar]
- 17. Vince RV, Chrismas B, Midgley AW, McNaughton LR, Madden LA. Hypoxia mediated release of endothelial microparticles and increased association of S100A12 with circulating neutrophils. Oxid Med Cell Longev. 2009;2:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lichtenauer M, Goebel B, Fritzenwanger M, et al. Simulated temporary hypoxia triggers the release of CD31+/Annexin + endothelial microparticles: a prospective pilot study in humans. Clin Hemorheol Microcirc. 2015;61:83–90. [DOI] [PubMed] [Google Scholar]
- 19. Werner N, Wassmann S, Ahlers P, Kosiol S, Nickenig G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:112–116. [DOI] [PubMed] [Google Scholar]
- 20. Sinning J‐M, Losch J, Walenta K, Böhm M, Nickenig G, Werner N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J. 2011;32:2034–2041. [DOI] [PubMed] [Google Scholar]
- 21. Jansen F, Yang X, Proebsting S, et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc. 2014;3:e001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zampetaki A, Willeit P, Tilling L, et al. Prospective study on circulating MicroRNAs and risk of myocardial infarction. J Am Coll Cardiol. 2012;60:290–299. [DOI] [PubMed] [Google Scholar]
- 23. Bang C, Batkai S, Dangwal S, et al. Cardiac fibroblast‐derived microRNA passenger strand‐enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wahl P, Wehmeier UF, Jansen FJ, et al. Acute effects of different exercise protocols on the circulating vascular microRNAs −16, −21, and −126 in trained subjects. Front Physiol. 2016;7:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Voellenkle C, Rooij J van, Guffanti A, et al. Deep‐sequencing of endothelial cells exposed to hypoxia reveals the complexity of known and novel microRNAs. RNA. 2012;18:472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gilmartin GS, Tamisier R, Curley M, Weiss JW. Ventilatory, hemodynamic, sympathetic nervous system, and vascular reactivity changes after recurrent nocturnal sustained hypoxia in humans. Am J Physiol Heart Circ Physiol. 2008;295:H778–H785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mohsenin V. Obstructive sleep apnea and hypertension: a critical review. Curr Hypertens Rep. 2014;16:482. [DOI] [PubMed] [Google Scholar]
- 28. Bisogni V, Pengo MF, Maiolino G, Rossi GP. The sympathetic nervous system and catecholamines metabolism in obstructive sleep apnoea. J Thorac Dis. 2016;8:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–127. [DOI] [PubMed] [Google Scholar]
- 30. Valo M, Wons A, Moeller A, Teupe C. Markers of myocardial ischemia in patients with coronary artery disease and obstructive sleep apnea: effect of continuous positive airway pressure therapy. Clin Cardiol. 2015;38:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dujic Z, Uglesic L, Breskovic T, et al. Involuntary breathing movements improve cerebral oxygenation during apnea struggle phase in elite divers. J Appl Physiol. 2009;107:1840–1846. [DOI] [PubMed] [Google Scholar]
- 32. Oh YJ, Jung JY, Kim SS, Chae K‐S, Rhu J, Lee C. The association of kidney function with repetitive breath‐hold diving activities of female divers from Korea, Haenyeo. BMC Nephrol. 2017;18:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drager LF, Polotsky VY, O'Donnell CP, Cravo SL, Lorenzi‐Filho G, Machado BH. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2015;309:H1101–H1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 35. Maruyama K, Morishita E, Sekiya A, et al. Plasma levels of platelet‐derived microparticles in patients with obstructive sleep apnea syndrome. J Atheroscler Thromb. 2012;19:98–104. [DOI] [PubMed] [Google Scholar]
- 36. Tual‐Chalot S, Gagnadoux F, Trzepizur W, Priou P, Andriantsitohaina R, Martinez MC. Circulating microparticles from obstructive sleep apnea syndrome patients induce endothelin‐mediated angiogenesis. Biochim Biophys Acta. 2014;1842:202–207. [DOI] [PubMed] [Google Scholar]
- 37. Kheirandish‐Gozal L, Bhattacharjee R, Kim J, Clair HB, Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yun C‐H, Jung K‐H, Chu K, et al. Increased circulating endothelial microparticles and carotid atherosclerosis in obstructive sleep apnea. J Clin Neurol. 2010;6:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cherniack NS. N‐terminal pro‐B‐type naturetic peptide (NTBNP): so much promise and such a disappointment. Sleep Breath. 2008;12:3–5. [DOI] [PubMed] [Google Scholar]
- 40. Maeder MT, Schoch OD, Rickli H. A clinical approach to obstructive sleep apnea as a risk factor for cardiovascular disease. Vasc Health Risk Manag. 2016;12:85–103. [DOI] [PMC free article] [PubMed] [Google Scholar]


