Abstract
The prevalence of interatrial block (IAB) is high in the elderly, particularly in those with heart disease. Despite this high prevalence—and the association of IAB with the risk of atrial fibrillation (AF), stroke, and cognitive decline—little information exists about the prognosis of older patients with IAB. P‐wave duration and morphology are associated with risk of developing AF, stroke, and cognitive decline in elderly patients with structural heart disease. The aim of the Interatrial Block and Yearly Events (BAYES) registry is to assess the impact of IAB on the risk of AF and stroke during 3 years of follow‐up. A series of 654 ambulatory patients age ≥70 years with heart disease from 35 centers will be included in 3 similar‐size groups of patients. Group A: normal P‐wave duration (<120 ms); Group B: partial IAB (P‐wave duration ≥120 ms without biphasic [plus/minus] morphology in the inferior leads II, III, and aVF); and Group C: advanced IAB (P‐wave duration ≥120 ms with biphasic [plus/minus] morphology in the inferior leads II, III, and aVF). Patients will be managed according to current recommendations. The 2 primary endpoints are defined as (1) AF duration >5 minutes and documented in any form of electrocardiographic recording; and (2) stroke. Results from this study might significantly improve the knowledge of IAB and its impact on the outcome of elderly patients with heart disease and could open the door to the use of anticoagulation therapy in some elderly patients with IAB.
Keywords: interatrial block; elderly; atrial fibrillation; stroke
1. INTRODUCTION
The classification of interatrial block (IAB) was published in 19791 and its electrocardiographic (ECG) criteria a few years later.2 The relevance of this conduction disturbance is now clear due to its association with atrial fibrillation (AF)3 and stroke,4 and its high prevalence.5 In 2012, a consensus paper6 defined the current ECG diagnostic criteria: partial IAB (P‐wave duration ≥120 ms) and advanced IAB (P‐wave duration ≥120 ms plus biphasic [plus/minus] morphology in inferior leads II, III, and aVF). The interest in this topic has increased in recent years, and a large number of studies recently have been published regarding the prevalence of IAB and its associations with the risk of AF, ischemic stroke, and cognitive decline.7, 8, 9, 10, 11, 12, 13, 14, 15 However, most studies have been performed retrospectively in the general population, where, even in the presence of IAB, the incidence of AF and stroke is low. Little information exists about the prognosis of elderly patients with heart disease and IAB. To quantify the independent association of IAB with AF and ischemic stroke, it is necessary to conduct a large registry study including patients with partial and advanced IAB and certain clinical characteristics associated with AF development and to compare them against a similar population without IAB (normal P‐wave duration and morphology).
Therefore, the purpose of the Interatrial Block and Yearly Events (BAYES) registry is to assess the individual and combined associations between 2 P‐wave characteristics, duration and morphology, and the risk of developing AF, stroke, and cognitive decline in elderly patients with structural heart disease.
2. METHODS
2.1. Design and study population
This is a prospective, multicenter, international, and observational registry to be conducted at 35 centers, with a 3‐year follow‐up (for a list of participating countries and investigators, see Supporting Information, Appendix, in the online version of this article). Patients will be managed according to current recommendations. A series of 654 ambulatory patients age >70 years who have heart disease will be included. This initiative has been endorsed by the Geriatric Cardiology Section of the Spanish Society of Cardiology, the International Society of Electrocardiology, and the International Society of Cardiovascular Pharmacotherapy.
The registry will include 3 similar‐size groups of patients (Figure):
Group A: Normal P wave duration (<120 ms).
Group B: Partial IAB (P‐wave duration ≥120 ms without biphasic [plus/minus] morphology in the inferior leads II, III, and aVF).
Group C: Advanced IAB (P‐wave duration ≥120 ms with biphasic [plus/minus] morphology in the inferior leads II, III, and aVF).
Figure 1.
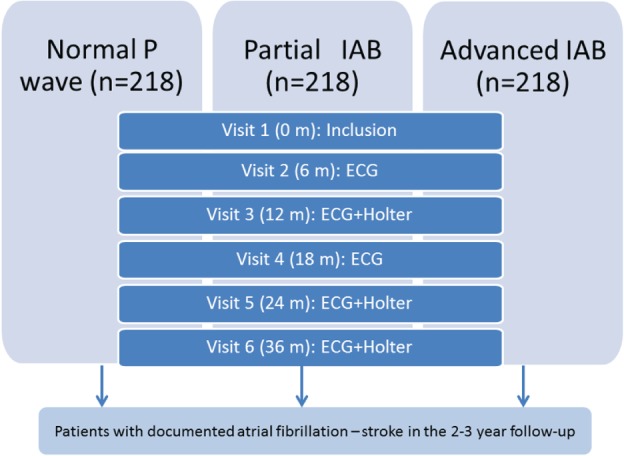
Design of the Interatrial Block and Yearly Events (BAYES) registry. Abbreviations: ECG, electrocardiography; IAB, interatrial block.
2.2. Inclusion criteria
Inclusion criteria are age ≥70 years, sinus rhythm, and structural heart disease, defined as the presence of ≥1 of the following conditions:
Ischemic heart disease (prior coronary artery bypass grafting, prior percutaneous coronary intervention, stable chronic angina, unstable angina, myocardial infarction).
Heart failure of any cause requiring ≥2 drugs for heart failure treatment or New York Heart Association class ≥2.
Arterial hypertension, defined as blood pressure ≥135/85 mm Hg or requiring ≥2 drugs.
Mitral/aortic valve disease labeled at least as “moderate” or “significant” by the treating physician.
And, ≥1 of the following echocardiography parameters:
Left ventricular hypertrophy: interventricular septum >12 mm.
Left ventricular ejection fraction <45%.
Left atrial diameter >45mm.
2.3. Exclusion criteria
The following are exclusion criteria for this study:
Prior AF or other clinical indication for anticoagulation (venous thromboembolism, pulmonary embolism, or other).
Prior stroke.
Intracardiac devices: pacemaker, implantable cardioverter‐defibrillator, or ventricular assist device.
Unable to follow up for ≥3 years.
Unable to provide informed consent.
Hospitalized patients.
2.4. P‐wave duration and morphology
A standard 12‐lead ECG recording will be obtained for each participant at the baseline visit (Table) and will be digitalized (scanned at 300 DPI minimum). A standardized protocol and settings (25 mm/sec and 10 mm = 1 mV) will be used to record all the ECGs. P‐wave duration and the presence of IAB will be manually measured and assessed as follows.
Table 1.
Chart of BAYES registry with number of visits and tests performed in each visit
| Months | Clinical Information | Echocardiography | ECG | 24‐Hour Holter Monitoring | ||
|---|---|---|---|---|---|---|
| Inclusion | Exclusion | |||||
| Baseline inclusion/exclusion criteria | 0 | IHD or HF or HTN or mitral‐aortic valve disease; informed consent | Prior AF, clinical indication for anticoagulation, intracardiac device; Pfeiffer test | LVH, septum thickness >12 mm, or LVEF <45%, or left atrium >45 mm | Sinus rhythm | Optional |
| Follow‐up | 6, 12, 18, 24, 36 | AF/stroke assessment, Pfeiffer test | ECG | Optional | ||
Abbreviations: AF, atrial fibrillation; BAYES, Interatrial Block and Yearly Events; ECG, electrocardiography; HF, heart failure; HTN, hypertension; IAB, interatrial block; IHD, ischemic heart disease; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy.
2.4.1. P‐wave duration
To measure P‐wave duration, we will analyze the digitalized ECG images using GeoGebra software, version 4.2 (https://www.geogebra.org). The ECG image will be amplified up to 20× its original size to define the interval between the earliest and the latest detection of atrial depolarization in the frontal leads, defined as a positive or negative deflection, respectively, that deviates from the baseline before the QRS complex.14 The software allows to manually draw lines on the ECG and provides the distance between 2 points, which will be converted into milliseconds (ms).
2.4.2. P‐wave morphology
Partial IAB is defined as a P‐wave duration ≥120 ms, and advanced IAB as P‐wave duration ≥120 ms plus biphasic P‐wave morphology in the inferior leads (in lead II, a P‐wave positive with an isodiphasic final part is also accepted). All the ECGs will be analyzed by an expert observer in the ECG Core Lab (Fundació Investigació Cardiovascular, ICCC, Hospital de Sant Pau, Barcelona, Spain) with a very high intraindividual concordance of the P‐wave duration measurement, assessed by reanalyzing 30 ECGs (intraclass correlation coefficient = 0.98 [95% confidence interval: 0.96‐0.99]). QRS complex morphology, ST‐ and T‐wave abnormalities, and the presence of premature beats (atrial or ventricular) in the baseline ECG also will be recorded.
2.5. Study endpoints
Clinical follow‐up will be carried out by local investigators. Visits are planned at 6, 12, 18, 24, and 36 months. Two primary endpoints are defined: (1) an AF episode longer than 5 minutes and documented in any ECG recording (1‐lead ECG, monitor strip, conventional ECG, or 24‐hour Holter monitoring); and (2) ischemic stroke, characterized as a neurological deficit attributed to an acute focal injury of the central nervous system by a vascular cause producing cerebral infarction, not caused by hemorrhage. Other secondary endpoints also will be considered: (1) initiation of anticoagulation during the follow‐up period; (2) cognitive decline or increase in Pfeiffer test >1 point; and (3) all‐cause mortality.
Clinical endpoints will be adjudicated by a central committee after reviewing all the available medical records provided by the local investigators. This committee will be blinded to the ECG characteristics of the patient.
2.6. Sample‐size calculation
The sample‐size estimation is based on the association between IAB and AF and the following assumptions:
Incidence of AF in the nonexposed population. According to recent worldwide data, the incidence of new AF in the population age >70 years in Western Europe and North America, in a 2‐year follow‐up, is estimated to be approximately 4% (for individuals with a normal P‐wave duration and the clinical characteristics defined in the inclusion criteria).9
Effect size of the association between the presence of IAB and AF. Little information exists about this association, thus lacking enough data to allow precise measurement of the sample size. Based on recent and classical published studies on patients belonging to group B (partial IAB), they may present a 3× higher incidence of AF than the group of individuals with a normal P wave, and those with advanced IAB may present even a higher risk.
Accepting an α risk of 0.05 and a β risk of 0.2 in a 2‐sided test, the inclusion of 218 subjects in each group will provide enough power to detect statistically significant relative risk ≥3 between the groups of partial IAB and normal P wave, and a relative risk ≥2 between the groups of advanced and partial IAB. The incidence rate in the follow‐up period in the normal P‐wave group has been estimated to be 4%, in the partial‐IAB group 12%, and in the advanced‐IAB group 24%. A dropout rate of 10% has been anticipated.
As a secondary analysis, we have also estimated the required sample size to compare the incidence of AF between the advanced‐IAB group and the rest, with enough power to detect a statistically significant relative risk ≥3 between the groups of advanced IAB and the rest. The incidence rate during the follow‐up period in the advanced‐IAB group has been estimated to be 18% and in the other groups to be 6%. A dropout rate of 10% has been anticipated.
2.7. Statistical analysis
Standard parametric and nonparametric methods will be used to compare the characteristics of the patients included in each group, and also of the patients with and without confirmed AF or stroke during the follow‐up. The association between ECG characteristics and time to AF or stroke will be assessed using Cox regression. We will also explore the linear and nonlinear association between P‐wave duration and AF using smoothing spline methods. All analyses will be performed using R Language and Environment for Statistical Computing, version 3.1.0 (R Foundation, Vienna, Austria).
2.8. Ethical considerations
All participants will be informed about the aims and procedures of the project and will sign written informed consent. The project will be conducted in accordance with the World Medical Association Declaration of Helsinki related to Ethical Principles for Medical Research Involving Human Subjects, the Convention on Human Rights and Biomedicine of the Council of Europe (1997), and the Additional Protocol to the Convention on Human Rights and Biomedicine, concerning Biomedical Research (2005).
2.9. Study timeline
The study begins in November 2016. The estimated end of the inclusion period is April 2017, and the estimated completion date for the study is April 2020.
2.10. Holter substudy
A Holter substudy has been planned and will include 24‐Holter recording at baseline and at follow‐up visits. We will record AF episodes and also the occurrence of >100 premature atrial beats/24 hours or 1 run of 10 consecutive beats. All sites will be recommended to include their patients in this substudy, but Holter monitoring will not be mandatory, due to financial issues.
3. DISCUSSION
The results of this registry could confirm the hypothesis that IAB is an important risk factor for developing AF and stroke. If this is the case, it may well open the door to the use of anticoagulation therapy in elderly patients with IAB,15 particularly those with Bayés’ syndrome (advanced IAB associated with AF).9 Of course, a randomized controlled trial in this subselected population would be necessary, but this registry might permit the proof of concept needed to justify such a randomized controlled trial.
4. CONCLUSION
The results of the BAYES registry might contribute to a better understanding of the impact of IAB on the prognosis of elderly patients with heart disease.
4.1. Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
APPENDIX S1
Martínez‐Sellés M, Baranchuk A, Elosua R and de Luna AB. Rationale and design of the BAYES (Interatrial Block and Yearly Events) registry, Clin Cardiol, 2016. doi: 10.1002/clc.22647
REFERENCES
- 1. de Luna AJ Bayés. Block at the auricular level [article in Spanish]. Rev Esp Cardiol . 1979;32:5–10. [PubMed] [Google Scholar]
- 2. Bayés de Luna A, Fort de Ribot R, Trilla E, et al. Electrocardiographic and vectorcardiographic study of interatrial conduction disturbances with left atrial retrograde activation. J Electrocardiol . 1985;18:1–13. [DOI] [PubMed] [Google Scholar]
- 3. Bayés de Luna A, Cladellas M, Oter R, et al. Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia. Eur Heart J . 1988;9:1112–1118. [DOI] [PubMed] [Google Scholar]
- 4. Ariyarajah V, Puri P, Apiyasawat S, et al. Interatrial block: a novel risk factor for embolic stroke? Ann Noninvasive Electrocardiol. 2007;12:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spodick DH, Ariyarajah V. Interatrial block: a prevalent, widely neglected, and portentous abnormality. J Electrocardiol. 2008;41:61–62. [DOI] [PubMed] [Google Scholar]
- 6. Bayés de Luna A, Platonov P, García‐Cosio F, et al. Interatrial blocks: a separate entity from left atrial enlargement: a consensus report. J Electrocardiol . 2012;45:445–451. [DOI] [PubMed] [Google Scholar]
- 7. Enriquez A, Sarrias A, Villuendas R, et al. New‐onset atrial fibrillation after cavotricuspid isthmus ablation: identification of advanced interatrial block is key. Europace. 2015;17:1289–1293. [DOI] [PubMed] [Google Scholar]
- 8. Martínez‐Sellés M, Massó‐van Roessel A, Álvarez‐García J, et al. Interatrial block and atrial arrhythmias in centenarians: prevalence, associations, and clinical implications. Heart Rhythm. 2016;13:645–651. [DOI] [PubMed] [Google Scholar]
- 9. Conde D, Seoane L, Gysel M, et al. Bayés’ syndrome: the association between interatrial block and supraventricular arrhythmias. Expert Rev Cardiovasc Ther. 2015;13:541–550. [DOI] [PubMed] [Google Scholar]
- 10. O'Neal WT, Kamel H, Zhang ZM, et al. Advanced interatrial block and ischemic stroke: the Atherosclerosis Risk in Communities Study. Neurology. 2016;87:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu JT, Wang SL, Chu YJ, et al. CHADS2 and CHA2DS2‐VASc scores predict the risk of ischemic stroke outcome in patients with interatrial block without atrial fibrillation. J Atheroscler Thromb . 2016. doi: 10.5551/jat.34900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Neal WT, Zhang ZM, Loehr LR, et al. Electrocardiographic advanced inter‐atrial block and atrial fibrillation risk in the general population. Am J Cardiol. 2016;117:1755–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nielsen JB, Kühl JT, Pietersen A, et al. P‐wave duration and the risk of atrial fibrillation: results from the Copenhagen ECG Study. Heart Rhythm. 2015;12:1887–1895. [DOI] [PubMed] [Google Scholar]
- 14. Martínez‐Sellés M, Robledo LA, Baranchuk A. Interatrial block and the risk of ischemic stroke. J Atheroscler Thromb . 2016. doi: 10.5551/jat.37242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martínez‐Sellés M, Fernandez Lozano I, Baranchuk A, et al. Should we anticoagulate patients at high risk of atrial fibrillation? Rev Esp Cardiol (Engl Ed). 2016;69:374–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1


