Abstract
Background
The epigenetic changes underlying the development of atrial fibrillation (AF) remain incompletely understood. Limited evidence suggests that abnormal DNA methylation might be involved in the pathogenesis of AF. In the present study, we evaluated the methylation status of genomic DNA from myocardial tissue in AF patients and sinus rhythm (SR) patients systematically.
Hypothesis
DNA methylation dysregulations will be associated with valvular AF.
Methods
Right atrial myocardial tissue was obtained from rheumatic valvular patients who had undergone valve replacement surgery (SR group, n = 10; AF group, n = 10). The global DNA methylation level, the promoter methylation level of the natriuretic peptide receptor‐A gene (NPRA), and its correlation with the mRNA expression level of DNA methyltransferase genes were detected.
Results
The global DNA methylation level was significantly higher in the AF group than in the SR group (P < 0.05). The NPRA mRNA expression was decreased and the NPRA gene was hypermethylated in the AF group (P < 0.05). Meanwhile, the NPRA mRNA expression level has a negative correlation with the mean methylation level in the promoter region of the NPRA gene.
Conclusions
DNA methylation dysregulations may be relevant in the pathogenesis of AF. DNA methyltransferase 3B likely plays an essential role in the DNA methylation dysregulations in AF.
Keywords: Atrial Fibrillation, DNA Methylation, Dysregulation
1. INTRODUCTION
Atrial fibrillation (AF), the most common sustained clinical arrhythmia, is associated with high disability and mortality and is presenting a rapidly growing public health and economic burden.1, 2 Experimental and clinical studies of AF indicate that atrial remodeling, such as electrical remodeling and structural remodeling, is very important in arrhythmia maintenance.3 Previous studies also have shown that cardiac fibrosis, endothelial dysfunction, oxidative stress, and inflammation were associated with AF.4, 5 Many research studies focused on unraveling the mechanisms of atrial remodeling have demonstrated that human AF often reflects multiple interacting causative factors; thus, the mechanisms underlying AF susceptibility are multiple and incompletely understood.6
Epigenetic alterations could bring about heritable changes in gene expression without mutating the DNA sequence and regulate key events in cellular homeostasis.7 DNA methylation is one of the most common epigenetic modifications. Preliminary research has shown correlations between DNA methylation and cardiovascular disease (CVD), including atherosclerosis, heart failure (HF), myocardial infarction, and cardiac hypertrophy.8, 9, 10 DNA methylation occurs at both global and specific gene promoter levels. The methylation state can be transmitted through cell division. DNA methylation stabilizes chromatin structure during transcription, which can regulate many downstream transcriptional processes. Aberrant DNA methylation affects the transcription and expression of critical regulatory genes and induces a proatherogenic cellular phenotype, which plays key roles in endothelial‐cell dysfunction, abnormal vascular smooth‐muscle‐cell proliferation, extracellular matrix formation, and inflammation in CVD.11 Increased DNA methylation of related genes could senesce endothelial cells and smooth‐muscle cells in CVD, such as atherosclerosis.11 The dysregulation of epigenetic regulation in gene expression has been implicated in the development of AF12, 13; however, the impact of DNA methylation upon the initiation and/or persistence of valvular AF has not been investigated in detail.
In this study, we detected the dysregulations of DNA methylation in human atrial tissue in 2 groups, sinus rhythm (SR) and AF patients. The abnormal methylation status of the natriuretic peptide receptor‐A gene (NPRA), global DNA methylation, and DNA methyltransferase (DNMT) may present a potential therapy strategy for AF patients.
2. METHODS
2.1. Ethics statement
Human right atrial myocardium was collected according to a protocol approved by the ethics committee of the Second Xiangya Hospital, Central South University, Hunan, China. We obtained written informed consent from each patient.
2.2. Human right atrial myocardium
Right atrial myocardium tissue was obtained from 20 patients undergoing mitral valve replacement. Right atrial segments were cut and frozen‐stored immediately in liquid nitrogen for DNA and RNA isolation. Of these 20 patients, 10 were in SR and another 10 exhibited persistent AF confirmed by electrocardiogram. Individual patient characteristics are listed in the Table. None of the patients in our study received medications that could interfere with DNA methylation, such as angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, or statins.
2.3. Genomic DNA extraction and DNA methylation sequencing
DNA from frozen tissue was isolated as previously described.14 After isolation, genomic DNA was treated with the EpiTect bisulfate kit (Qiagen, Hilden, Germany) according to manufacturer protocol to enable the complete conversion of unmethylated cytosines into uracils. The gene promoter fragments were amplified using nested polymerase chain reaction (PCR) and then cloned into a pGEM‐T vector (Promega, Madison, WI). The 10 independent clones were then sequenced for each of the amplified fragments.15 (Forward primer [F]: 5′‐TGGGGCGTAAGTGGGTTTCGGTTGT‐3′; reverse primer [R]: 5′‐CCGACCGACCCAAATCTCCGACTAT‐3′).
2.4. Measurement of global DNA methylation by an ELISA‐like reaction
Global DNA methylation was measured using the MethylFlash Methylated DNA Quantification Ultra Kit, according to the instructions provided by the manufacturer (Epigentek Group Inc., Farmingdale, NY). The extracted DNA was bound to a strip well specifically treated to have a high affinity for DNA. The methylated fraction of the total DNA was then recognized using an anti‐5‐methylcytosine antibody and quantified through an ELISA‐like reaction. The amount of methylated DNA is proportional to the optical density, and the percentage of methylated DNA can thus be calculated.16
2.5. RNA isolation and real‐time quantitative reverse transcriptase–polymerase chain reaction (RT‐PCR)
Total RNA was isolated using Trizol reagent according to standard protocol (Invitrogen, Carlsbad, CA). cDNA synthesis was performed using the RevertAid First Strand cDNA Synthesis Kit (Fermentas, Burlington, ON, Canada) and 1 µg of total input RNA according to the manufacturer's instructions. Real‐time quantitative PCR was performed using a Rotor‐Gene 3000 (Corbett Research, Mortlake, NSW, Australia), and mRNA levels were quantified using the SYBR Premix Ex Taq real‐time PCR kit (TaKaRa Biotechnology Co. [Dalian], China). β‐actin was also amplified and used as a loading control.16 (NPRA/F: 5′‐GCATTGAGCTGACACGAAAA‐3′; NPRA/R: 5′‐GTCCAGGGTGATGCTCTCAT‐3′; DNA‐methyltransferase (DNMT)‐1/F: 5′‐AAGAACGGCATCCTGTACCGAGTT‐3′; DNMT1/R: 5′‐TGCTGCCTTTGATGTAGTCGGAGT‐3′; DNMT3A/F: 5′‐TTTGAGTTCTACCGCCTCCTGCAT‐3′; DNMT3A/R: 5′‐GTGCAGCTGACACTTCTTTGGCAT‐3′; DNMT3B/F: 5′‐AGTGTGTGAGGAGTCCATTGCTGT‐3′; DNMT3B/R: 5′‐GCTTCCGCCAATCACCAAGTCAAA‐3′; β‐actin/F: 5′‐GCACCACACCTTCTACAATGAGC‐3′; β‐actin/R: 5′‐GGATAGCACAGCCTGGATAGCAAC‐3).
2.6. Statistical analysis
The independent t test analysis was used to test for differences among the 2 groups. The Pearson correlation coefficient was used for the correlation analysis between methylation patterns and the clinical characteristics. P values <0.05 were considered significant. All statistical analyses were carried out using SPSS software version 22.0 (IBM Corp., Armonk, NY). Data are presented as mean ± SD.
3. RESULTS
3.1. Detection of global DNA methylation levels and correlation with age
The global DNA methylation levels were significantly increased in the AF group (SR group vs AF group: 1.63 ± 0.50 vs 2.47 ± 0.99, P = 0.027; power = 0.82; Figure 1A). We also found the average global DNA methylation levels had a positive correlation with age in the AF group (r = 0.779, P = 0.008; Figure 1B).
Figure 1.
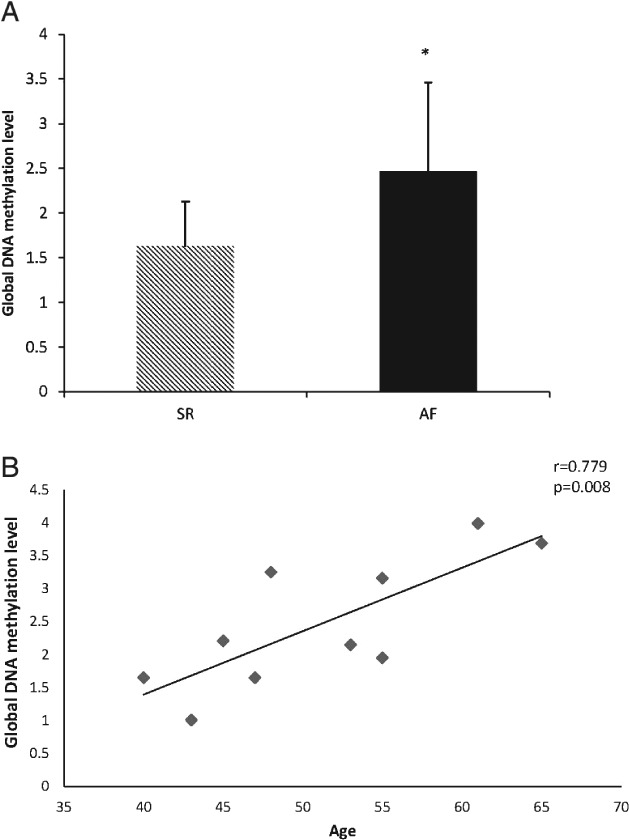
The global DNA methylation level (A) is significantly higher in the AF group and (B) has a positive correlation with age in AF patients (r = 0.779, P = 0.008). Statistical significance: P < 0.05. Abbreviations: AF, atrial fibrillation; SR, sinus rhythm.
3.2. mRNA expression and promoter methylation levels of the NPRA gene
Compared with the SR group, the NPRA mRNA levels were significantly decreased in the AF group (SR group vs AF group: 2.21 ± 0.83 vs 1.35 ± 0.66, P = 0.020; power = 0.83; Figure 2A).
Figure 2.
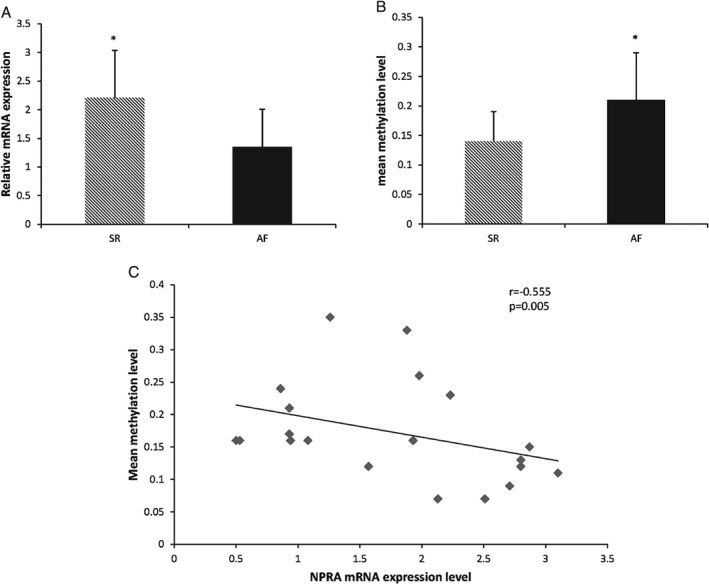
(A) The mRNA expression of the NPRA gene is significantly decreased in the AF group. (B) The methylation level of the NPRA gene is significantly increased in the AF group. (C) The mRNA expression level of the NPRA gene has a negative correlation with the mean methylation level in the promoter region (r = −0.555, P = 0.005). Statistical significance: P < 0.05. Abbreviations: AF, atrial fibrillation; NPRA, natriuretic peptide receptor‐A; SR, sinus rhythm
We measured the methylation levels of CG pairs in 307bp of the NPRA gene (position −967 to −661, 20 CG pairs, CpG‐density 48%). The mean methylation status of most CG pairs was significantly increased in the AF group (SR group vs AF group: 0.14 ± 0.05 vs 0.21 ± 0.08, P = 0.031; power = 0.79; Figure 2B). The mRNA expression level of the NPRA gene had a negative correlation with the mean methylation status in the promoter region (r = −0.555, P = 0.005; Figure 2C).
3.3. The mRNA expression levels of DNMTs and the correlation with NPRA promoter methylation levels
The expression levels of DNMT3B were found to be significantly increased in the AF group (SR group vs AF group: 1.50 ± 0.56 vs 2.29 ± 0.73, P = 0.014; power = 0.87; Figure 3A). No significant difference was found between the 2 groups for DNMT1 and DNMT3A (P > 0.05; Figures not shown). Meanwhile, DNMT3B had significant positive correlation with the NPRA gene promoter methylation levels (r = 0.653, P = 0.001; Figure 3B). This correlation did not occur between NPRA gene promoter methylation levels and DNMT1 or DNMT3A mRNA expression levels (r = 0.171 and P = 0.424 for DNMT1; r = 0.283 and P = 0.180 for DNMT3A; Figures not shown).
Figure 3.
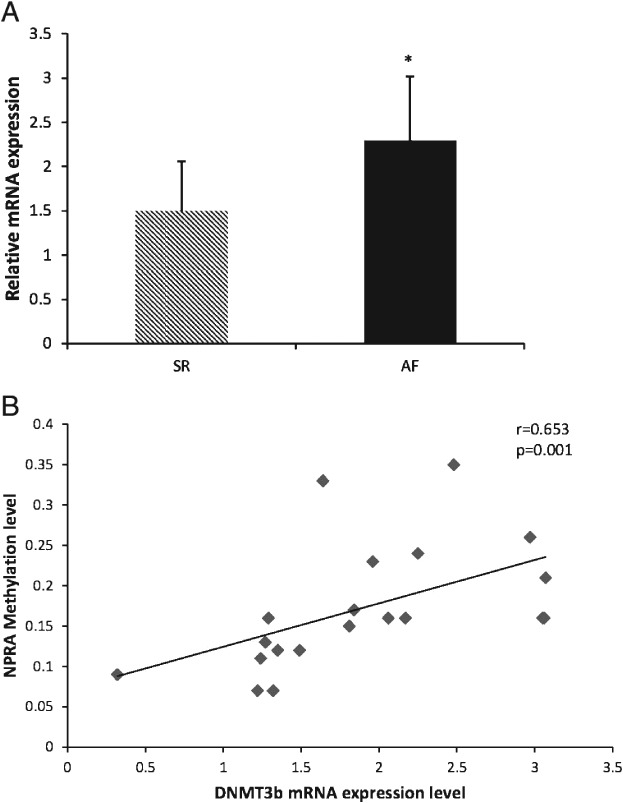
The mRNA expression of the DNMT3B gene (A) is significantly increased in the AF group and (B) has a positive correlation with the methylation level of the NPRA gene (r = 0.653, P = 0.001). Statistical significance: P < 0.05. Abbreviations: AF, atrial fibrillation; DNMT3B, DNA methyltransferase 3B; NPRA, natriuretic peptide receptor‐A; SR, sinus rhythm
4. DISCUSSION
The origin of complex disorders is attributed to a combination of environmental and heritable factors. Epigenetic regulation, which is considered to be an important environmental factor of disease outcome, had been investigated in many research studies.17, 18 Epigenetic means heritable changes in gene expression that will not change the primary DNA sequence. DNA methylation, one of the epigenetic modifications, through the localization of gene silencing at the methylated cytosine‐phosphate‐guanine (CpG) dinucleotides, leads to genomic imprinting, silencing of repetitive DNA elements, chromatin structure regulating, and gene‐expression control.4, 19 A well‐characterized functional effect of DNA methylation is gene‐expression regulation; gene promoter hypomethylation correlates with increased expression of the gene, whereas hypermethylation leads to transcriptional silencing.19
DNA methylation dysregulation has been researched in a number of cardiac diseases19; however, in terms of DNA methylation in AF, the mechanism remains questionable. In recent years, several studies have shown that the DNA methylation regulation mechanism is related to the pathogenesis of AF. Tao et al4 found that DNA methylation plays a central role in the maintenance of cardiac fibrosis, which contributes to the pathogenesis of AF. They also showed that DNMT3A plays an essential role in Ras association domain family 1 isoform A (RASSF1A)‐mediated upregulation of extracellular signal‐regulated kinases 1/2 (ERK1/2) in cardiac fibrosis. Heart failure is a risk factor that can increase AF and induce cardiac hypermethylation. Kao et al20 found that Pitx2c promoter hypermethylation and the level of DNMT1 increased in atrium affected by HF compared with normal atrium. Angiotensin II could increase the level of Pitx2c promoter methylation and decrease the protein level compared with control cells.
In our study, we first investigated whether DNA methylation regulations are part of the pathogenesis of AF. We found that global DNA methylation levels were significantly increased in the AF group compared with the SR group. Our results are consistent with several studies related to global DNA methylation status in cardiovascular diseases. Kao et al20 found that HF can increase AF and induce cardiac hypermethylation. Sharma et al21 showed that global DNA methylation in peripheral blood was significantly higher in coronary artery disease patients than in controls. Watson et al22 proved that hypoxia‐induced profibrotic changes were correlated with global DNA hypermethylation. Zhang et al23 showed that global DNA methylation is related to inflammation in atherosclerosis. Evidence shows that cardiac fibrosis, endothelial dysfunction, oxidative stress, and inflammation contribute to the pathogenesis of AF.4, 5 This means that global DNA hypermethylation–related fibrotic and inflammatory changes may have a tight relationship with AF.
Aging is an important factor that is related to global DNA hypomethylation and promoter hypermethylation. AF is an age‐related disease; previous study shows that AF patients have a higher age compared with non‐AF patients.24 The difference in age in the 2 groups in our results correlated with this study (AF patients are older compared with SR patients). Our results showed that global DNA methylation levels had a positive correlation with age. This result correlated with the research performed by Sharma et al,21 who found a trend of global hypermethylation in coronary artery disease patients with age and observed significant hypermethylation in the higher age group. The exact regulation mechanism is still not known; we had the hypothesis that in AF patients, age‐related global DNA hypomethylation was offset by promoter‐specific hypermethylation and result in increased global DNA methylation.
Natriuretic peptides (NPs) are a family of 3 structurally related hormone and paracrine factors: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C‐natriuretic peptide.25 Of the 3 NPs, ANP and BNP are secreted from the cardiac atria and ventricles, respectively, and are viewed as critical regulators and potential therapeutic targets for the treatment of cardiovascular diseases.25 NPs exert their effects via 3 NP receptors: natriuretic peptide receptor‐A (NPR‐A), NPR‐B, and NPR‐C. ANP and BNP activate the transmembrane guanylyl cyclase, NPR‐A.25 Kurosaki et al26 found that the ANP and BNP concentrations were elevated in AF and that the BNP concentration was reduced after ablation. Zeng et al27 found that ANP and BNP decreased significantly after cardioversion in patients with AF and could be useful predictors of relapsed AF. Egom et al28 found that NPRC knockout mice have an increased susceptibility to AF. However, the effect and mechanism of NPRA in AF is still poorly understood. In our study, we found that the mRNA levels of the NPRA gene were significantly decreased in the AF group and the hypermethylation within the promoter region of the NPRA gene correlated with its decreased expression in AF. NPRA gene knockout mice show cardiac hypertrophy, fibrosis, and inflammation.29 This evidence demonstrated that hypermethylation and dysregulated expression of the NPRA gene may indeed play an important role in AF.
The DNA methylation reaction is catalyzed and maintained by DNMTs.30, 31 DNMT overexpression has been observed in many diseases, and use of DNMT antagonists could inhibit genes’ promoter methylation, which means that DNMTs may be used as targets for treatment.30, 31 We detected the effect of DNMTs in AF patients. These mainly have 3 human DNMTs, named DNMT1, DNMT3A, and DNMT3B.32 DNMT1 ensures that the preexisting methylation patterns are faithfully copied to the newly synthesized DNA strand during DNA replication, whereas DNMT3A and DNMT3B are mainly responsible for introducing new cytosine methylation at previously unmethylated CpG sites.32 Tao et al4 had reported that DNMT3A plays an important role in RASSF1A‐mediated upregulation of ERK1/2 in rat cardiac fibrosis. Kao et al20 showed that DNMT1 increased Pitx2c promoter hypermethylation in HF atrium, compared with normal atrium. In our study, the expression of DNMT3B was significantly increased in AF patients and had a positive correlation with NPRA promoter hypermethylation, which means that the effect of DNMT3B is predominant and more important in the AF disease process.
4.1. Study limitations
This clinical research investigation analyzed samples of right atrial myocardial tissue. The number of patients and patient consent would influence selection and grouping, so patient age is not matched in the 2 groups. AF cases were identified by electrocardiogram, self‐reported history of a physician diagnosis, or hospitalization. Poor awareness of AF among Chinese patients made the duration of AF history difficult to identify. Another limitation is the relatively small sample size, which may have limited the statistical power for analyses. Our preliminary findings in this study suggest that increased DNA methylation and DNMT changes may be factors in AF susceptibility; further functional studies should to be done to validate. We will continue our study in this field with the support of the National Natural Science Foundation of China (no. 81500302).
5. CONCLUSION
Our results suggest that DNA methylation might act as an important bridge to link epigenetic variation and AF susceptibility. Increased DNA methylation, both global and NPRA gene promoter levels, is related to cardiac hypertrophy, fibrosis, and inflammation and causes increased mortality in AF. Meanwhile, DNMT3B is a critical participant in this epigenetic regulation. All of these results provide potential insights into molecular mechanisms of AF with potential therapeutic implications.
Conflicts of interest
The authors declare no conflicts to disclose.
Shen K, Tu T, Yuan Z, et al. DNA methylation dysregulations in valvular atrial fibrillation. Clin Cardiol. 2017;40:686–691. 10.1002/clc.22715
Funding information National Natural Science Foundation of China, Grant/Award numbers: No. 81500302, No. 81470507, No. 81600273, No. 81570310).
REFERENCES
- 1. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high‐risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY‐AF). Clin Cardiol. 2015;38:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heijman J, Voigt N, Nattel S, et al. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. [DOI] [PubMed] [Google Scholar]
- 4. Tao H, Shi KH, Yang JJ, et al. Epigenetic mechanisms in atrial fibrillation: new insights and future directions. Trends Cardiovasc Med. 2016;26:306–318. [DOI] [PubMed] [Google Scholar]
- 5. Li JY, He Y, Ke HH, et al. Plasma oxidative stress and inflammatory biomarkers are associated with the sizes of the left atrium and pulmonary vein in atrial fibrillation patients. Clin Cardiol. 2017;40:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrade J, Khairy P, Dobrev D, et al. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 7. Ragunathan K, Jih G, Moazed D. Epigenetics: epigenetic inheritance uncoupled from sequence‐specific recruitment. Science. 2015;348:1258699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim SY, Morales CR, Gillette TG, et al. Epigenetic regulation in heart failure. Curr Opin Cardiol. 2016;31:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muka T, Koromani F, Portilla E, et al. The role of epigenetic modifications in cardiovascular disease: a systematic review. Int J Cardiol. 2016;212:174–183. [DOI] [PubMed] [Google Scholar]
- 10. Zhong J, Agha G, Baccarelli AA. The role of DNA methylation in cardiovascular risk and disease: methodological aspects, study design, and data analysis for epidemiological studies [published correction appears in Circ Res. 2016;118:e30). Circ Res. 2016;118:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Udali S, Guarini P, Moruzzi S, et al. Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Med. 2013;34:883–901. [DOI] [PubMed] [Google Scholar]
- 12. Choi SY, Ryu Y, Kee HJ, et al. Tubastatin A suppresses renal fibrosis via regulation of epigenetic histone modification and Smad 3‐dependent fibrotic genes. Vasc Pharmacol. 2015;72:130–140. [DOI] [PubMed] [Google Scholar]
- 13. Koutsis G, Siasos G, Spengos K. The emerging role of microRNA in stroke. Curr Top Med Chem. 2013;13:1573–1588. [DOI] [PubMed] [Google Scholar]
- 14. Long C, Yin B, Lu Q, et al. Promoter hypermethylation of the RUNX3 gene in esophageal squamous cell carcinoma. Cancer Invest. 2007;25:685–690. [DOI] [PubMed] [Google Scholar]
- 15. Wang BX, Yin BL, He B, et al. Overexpression of DNA damage‐induced 45 α gene contributes to esophageal squamous cell cancer by promoter hypomethylation. J Exp Clin Cancer Res. 2012;31:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen C, Yin B, Wei Q, et al. Aberrant DNA methylation in thymic epithelial tumors. Cancer Invest. 2009;27:582–591. [DOI] [PubMed] [Google Scholar]
- 17. Wilson AS, Power BE, Molloy PL. DNA hypomethylation and human disease. Biochim Biophys Acta. 2007;1775:138–162. [DOI] [PubMed] [Google Scholar]
- 18. Pogribny IP, Beland FA. DNA hypomethylation in the origin and pathogenesis of human disease. Cell Mol Life Sci. 2009;66:2249–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whayne TF. Epigenetics in the development, modification, and prevention of cardiovascular disease. Mol Biol Rep. 2015;42:765–776. [DOI] [PubMed] [Google Scholar]
- 20. Kao YH, Chen YC, Chung CC, et al. Heart failure and angiotensin II modulate atrial Pitx2c promoter methylation. Clin Exp Pharmacol Physiol. 2013;40:379–384. [DOI] [PubMed] [Google Scholar]
- 21. Sharma P, Kumar J, Garg G, et al. Detection of altered global DNA methylation in coronary artery disease patients. DNA Cell Biol. 2008;27:357–365. [DOI] [PubMed] [Google Scholar]
- 22. Watson CJ, Collier P, Tea I, et al. Hypoxia‐induced epigenetic modifications are associated with cardiac tissue fibrosis and the development of a myofibroblast‐like phenotype. Hum Mol Genet. 2014;23:2176–2188. [DOI] [PubMed] [Google Scholar]
- 23. Hai Z, Zuo W. Aberrant DNA methylation in the pathogenesis of atherosclerosis. Clin Chim Acta. 2016;456:69–74. [DOI] [PubMed] [Google Scholar]
- 24. Yamauchi T, Sakata Y, Miura M, et al; CHART‐2 Investigators. Prognostic impact of atrial fibrillation and new risk score of its onset in patients at high risk of heart failure—a report from the CHART‐2 study. Circ J . 2017;81:185–194. [DOI] [PubMed] [Google Scholar]
- 25. Potter LR, Yoder AR, Flora DR, et al. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;341–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurosaki K, Tada H, Hashimoto T, et al. Plasma natriuretic peptide concentrations as a predictor for successful catheter ablation in patients with drug‐refractory atrial fibrillation. Circ J. 2007;71:313–320. [DOI] [PubMed] [Google Scholar]
- 27. Zeng QX, Wei MF, Zhang W, et al. Level of natriuretic peptide determines outcome in atrial fibrillation. J Atr Fibrillation. 2010;1:559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Egom EE, Vella K, Hua R, et al. Impaired sinoatrial node function and increased susceptibility to atrial fibrillation in mice lacking natriuretic peptide receptor C. J Physiol. 2015;593:1127–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pandey KN. Guanylyl cyclase / atrial natriuretic peptide receptor‐A: role in the pathophysiology of cardiovascular regulation. Can J Physiol Pharmacol. 2011;89:57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin B, Robertson KD. DNA methyltransferases, DNA damage repair, and cancer. Adv Exp Med Biol. 2013;754:3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gros C, Fahy J, Halby L, et al. DNA methylation inhibitors in cancer: recent and future approaches. Biochimie. 2012;94:2280–2296. [DOI] [PubMed] [Google Scholar]
- 32. Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. [DOI] [PubMed] [Google Scholar]


