Abstract
Background
About a decade past the first transcatheter aortic valve implantation (TAVI), data are limited regarding temporal trends accompanying its evolution from novel technology to mainstream therapy. We evaluated these trends in a large multicenter TAVI registry.
Hypothesis
TAVI is changing and improving with time.
Methods
Patients who underwent TAVI between January 2008 and December 2014 at 3 high‐volume Israeli centers were divided into 5 time quintiles according to procedure date. Outcomes were analyzed and reported according to Valve Academic Research Consortium‐2.
Results
A total of 1285 patients were studied (43% male; mean age, 83 ± 3 years; mean Society of Thoracic Surgeons [STS] score, 5.5 ± 3.6). Over time, there was a shift toward treating patients at lower STS score, increased use of conscious sedation and transfemoral approach, and decreased use of balloon predilatation. The balloon‐expandable to self‐expandable valve utilization ratio decreased, the valve‐in‐valve experience increased from 4% to 17% of all TAVI volume, and length of hospital stay was halved (P = 0.006). Kaplan‐Meier survival curves showed gradual decrease in mortality risk (P = 0.031), but there was no significant 1‐year mortality decrease by multivariable analysis. Each year increment was associated with an adjusted 20%, 15%, and 12% decrease in new pacemaker obligation (P = 0.004), new pacemaker obligation or left bundle branch block (P = 0.008), and in‐hospital infections (P = 0.082), respectively.
Conclusions
Temporal trends accompanying TAVI evolution include its utilization in lower‐risk patients, procedural simplification, improved overall survival, decreased pacemaker obligation, and shorter hospital stay.
Keywords: Valvular heart disease, Cardiac, catheterization/diagnostic interventional, Aortic disease
1. INTRODUCTION
More than a decade past the first transcatheter aortic valve implantation (TAVI), the procedure is widely accepted by the medical community and is endorsed by European and American guidelines, as well as the US Food and Drug Administration, indicating TAVI as the intervention of choice in “inoperable” severe aortic stenosis patients and as an alternative to surgery in high‐risk patients.1, 2 With >150 000 worldwide procedures performed and growing at a rate of 40% annually,3 little is known about the temporal trends for TAVI regarding patient characteristics, technical issues, and outcomes accompanying its evolution from novel technology to mainstream therapy. Therefore, the present study aimed to evaluate TAVI temporal trends in a large multicenter Israeli registry.
2. METHODS
Consecutive patients with severe symptomatic aortic stenosis who underwent TAVI between January 2008 and December 2014 at 1 of 3 tertiary medical centers in Israel were included in the analysis (Rabin Medical Center, Petah Tikva: n = 328 [25.5%]; Sheba Medical Center, Tel Hashomer: n = 375 [29.2%]; and Sourasky Medical Center, Tel Aviv: n = 582 [45.3%]). The study was approved by the institutional review board at each of the participating centers.
Severe aortic stenosis was defined by echocardiography as a valvular orifice area <1.0 cm2 or <0.6 cm2/m2 and/or a mean transaortic valvular gradient >40 mm Hg and/or jet velocity >4.0 m/s. All patients underwent a rigorous assessment process that included history‐taking, physical examination, physical performance measures, cognitive assessments, laboratory tests, and calculation of the Society of Thoracic Surgeons (STS) score predicted risk of mortality (STS‐PROM). Eligibility for TAVI was established based on the consensus of a multidisciplinary heart team and even if the calculated STS score was low, if patients were deemed as high surgical risk based on other factors and comorbidities or frailty measures not included in the standardized risk scores. The number of operators per center remained stable throughout the study period. All endpoints were defined and collected using the Valve Academic Research Consortium‐2 (VARC‐2) consensus definitions and pooled into a dedicated multicenter database.4
2.1. Study population
For the purpose of this study, patients were divided into 5 time quintiles according to their procedural date (Q1: 2008–2010, 260 patients; Q2: 2011, 251 patients; Q3: 2012, 266 patients; Q4: 2013, 261 patients; and Q5: 2014, 248 patients).
The selection of the transcatheter valve was at the discretion of the heart team. Patients underwent TAVI via the transfemoral, transapical, transaxillary, or direct aortic access routes. The transfemoral access was the default approach in all centers unless there were anatomic limitations that led to selection of an alternative access. Similarly, the decision on other technical issues, such as whether to perform the procedure under conscious sedation or general anesthesia, was according the planned implantation approach and at the discretion of the interventional team in each center.
Prespecified clinical and laboratory data were collected for all patients at baseline prior to the procedure, immediately postprocedure, during the index hospitalization, and during long‐term follow‐up. Collected data included patient medical history, electrocardiography, echocardiography, catheterization laboratory studies, laboratory tests, and clinical outcomes. In‐hospital outcomes were collected according to the VARC‐2 document.4
2.2. Statistical analysis
Categorical data are reported as frequency and percentages, and comparison between groups was performed using the χ2 test or the Fisher exact test, as appropriate. Continuous variables are presented as mean ± SD or median and interquartile range, and comparisons were performed with the 2‐sample t test or the 2‐sample Wilcoxon rank‐sum (Mann‐Whitney) test. The probability of all‐cause mortality by TAVI procedure year was graphically displayed according to the Kaplan‐Meier method, with comparison of instantaneous risk by the log‐rank test. Univariate Cox regression analysis was used to evaluate the association between calendar year (assessed both as a categorical and as a continuous variable) and mortality with follow‐up censored at 1 year. The best subsets regression procedure was used to identify significant variables to be included in the multivariable regression models. Multivariable Cox regression analysis was used to evaluate the association between calendar year and mortality with follow‐up censored at 1 year, adjusting for the following variables: age, sex, left ventricular ejection fraction, STS‐PROM, transaortic gradients at baseline, and history of valve replacement surgery, diabetes mellitus, chronic obstructive pulmonary disease, percutaneous coronary intervention, and atrial fibrillation. Separate multivariable models were constructed using logistic regression analysis for the following endpoints: new permanent pacemaker (PPM) implantation, new PPM implantation or left bundle branch block (LBBB), in‐hospital infections, and hospital admission days. Data were registered in an electronic file and analyzed using SAS software version 9.4 (SAS Institute, Inc., Cary, North Carolina). A 2‐tailed P value <0.05 was considered statistically significant.
3. RESULTS
Between January 2008 and December 2014, a total of 1285 TAVI procedures were performed in the 3 medical centers collaborating in this study. Annual implantation rate increased during the first years (2008–2010) and remained roughly constant thereafter (251–266 TAVI procedures per year during 2010–2014).
3.1. Patient characteristics
The mean age of the total study population was 82 ± 7 years; 57% were female. Patients’ mean STS‐PROM score was 5.5 ± 3.6, and mean aortic valve area was 0.7 ± 0.16 cm2. Most patients (80%) were severely symptomatic (New York Heart Association class III/IV), 39% had depressed left ventricular function (left ventricular ejection fraction <40%), and 17% were adjudged frail. Over the 5 timeframes, there were no temporal trends regarding patient age, sex, and most of the comorbidities (Table 1). Conversely, throughout the study period, the proportion of high‐risk patients gradually decreased along with an increase in the proportion of intermediate‐ and low‐risk patients based on the STS‐PROM (mean STS: 6.7 ± 4.9 in 2008–2010 and 4.8 ± 2.8 in 2014; P for trend <0.001). The STS score risk‐classification proportions over time are presented in Supporting Information, Figure 1, in the online version of this article. There was also an increase in the prevalence of atrial fibrillation and PPM and a decrease in the prevalence of peripheral vascular disease and chronic obstructive pulmonary disease between the earlier and more recent TAVI periods. There was a clear temporal trend in the direction of higher mean aortic valve area (0.64 ± 0.16 cm2 in 2008–2010 to 0.74 ± 0.19 cm2 in 2014; P < 0.001) and heterogeneity among some other variables, but without any recognized unidirectional temporal pattern (Table 1).
Table 1.
Baseline characteristics of patients stratified according to TAVI time quintile
| Characteristic | Total, N = 1285 | Q1: 2008–2010, n = 260 | Q2: 2011, n = 251 | Q3: 2012, n = 266 | Q4: 2013, n = 261 | Q5: 2014, n = 248 | P Value |
|---|---|---|---|---|---|---|---|
| Age, y | 82 ± 7 | 83 ± 7 | 83 ± 8 | 82 ± 7 | 82 ± 7 | 82 ± 8 | 0.165 |
| Male sex | 43 | 40 | 39 | 40 | 50 | 46 | 0.031 |
| STS score | 5.5 ± 3.6 | 6.7 ± 4.9 | 5.4 ± 3.4 | 5.1 ± 3.5 | 5.4 ± 3.9 | 4.8 ± 2.8 | <0.001 |
| DM | 36 | 36 | 29 | 34 | 37 | 42 | 0.039 |
| HTN | 88 | 83 | 89 | 91 | 89 | 87 | 0.096 |
| Dyslipidemia | 81 | 80 | 79 | 78 | 85 | 82 | 0.380 |
| PVD | 12 | 14 | 16 | 10 | 9 | 10 | 0.079 |
| COPD | 19 | 25 | 20 | 18 | 19 | 14 | 0.049 |
| Current smoker | 6 | 5 | 9 | 5 | 7 | 3 | 0.122 |
| “Porcelain” aorta | 5 | 5 | 6 | 5 | 3 | 6 | 0.821 |
| AF | 31 | 30 | 25 | 32 | 30 | 38 | 0.044 |
| CAD | 51 | 52 | 51 | 48 | 56 | 48 | 0.283 |
| MI | 18 | 17 | 16 | 19 | 24 | 13 | 0.025 |
| PCI | 33 | 31 | 34 | 30 | 41 | 31 | 0.090 |
| CABG | 20 | 26 | 16 | 18 | 21 | 18 | 0.026 |
| PPM | 10 | 7 | 6 | 8 | 14 | 11 | 0.019 |
| ICD | 1 | 2 | 1 | 2 | 2 | 1 | 0.837 |
| CKD | 25 | 27 | 23 | 25 | 27 | 23 | 0.672 |
| Hgb, g/dL | 12 ± 1 | 12 ± 2 | 12 ± 1 | 12 ± 2 | 12 ± 2 | 12 ± 2 | 0.801 |
| Frailty | 17 | 28 | 10 | 11 | 15 | 24 | <0.001 |
| NYHA class | |||||||
| II | 16 | 14 | 17 | 13 | 22 | 18 | 0.002 |
| III | 56 | 62 | 57 | 63 | 46 | 54 | |
| IV | 24 | 20 | 24 | 22 | 31 | 26 | |
| Mean aortic valve area, cm2 | 0.7 ± 0.16 | 0.64 ± 0.16 | 0.69 ± 0.19 | 0.70 ± 0.20 | 0.71 ± 0.19 | 0.74 ± 0.19 | <0.001 |
| Mean aortic valve gradient, mm Hg | 47 ± 16 | 47 ± 16 | 48 ± 15 | 48 ± 17 | 48 ± 16 | 46 ± 18 | 0.311 |
| Peak aortic valve gradient, mm Hg | 76 ± 23 | 77 ± 24 | 78 ± 24 | 76 ± 23 | 77 ± 24 | 72 ± 22 | 0.073 |
| LVEF <50% | 39 | 48 | 36 | 37 | 32 | 39 | 0.065 |
Abbreviations: AF, atrial fibrillation; CABG, coronary artery bypass grafting surgery; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; Hgb, hemoglobin; HTN, hypertension; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PPM, permanent pacemaker; PVD, peripheral vascular disease; Q, quintile; SD, standard deviation; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Categorical variables are presented as % and continuous variables are presented as mean ± SD.
Figure 1.
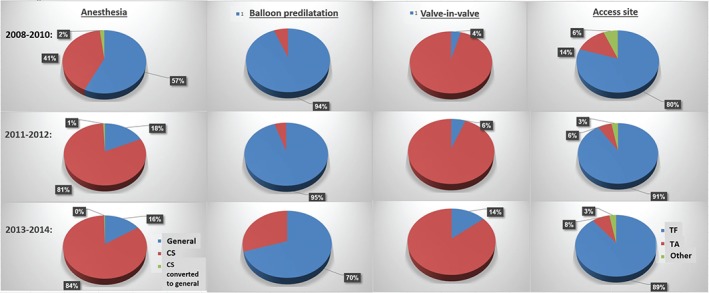
Temporal trends in procedural characteristics. Presented in blue are (left to right) anesthesia, balloon predilatation, valve‐in‐valve TAVI, and access site. For simplification, the 2011–2012 and 2013–2014 calendar‐year cohorts were joined. Abbreviations: CS, conscious sedation; TA, transapical; TAVI, transcatheter aortic valve implantation; TF, transfemoral.
3.2. Procedural characteristics
Regarding procedural characteristics stratified in consort with TAVI calendar year‐group (Figure 1; also see Supporting Information, Table 1, in the online version of this article), there was a clear drift toward more frequent practice of conscious sedation (85% in 2014 vs 41% in 2008–2010; P < 0.001) and transfemoral approach (91.1% in 2014 vs 80% in 2008–2010; P < 0.001), and less implementation of balloon predilatation, which decreased from ~95% to ~55% of cases (P < 0.001). The use of balloon‐expandable devices was lowest in 2008–2010, peaked to 40% in 2012, and decreased again to ~30% in 2014. Additionally, valve‐in‐valve experience increased significantly in the most recent cohort and constituted 17% of all TAVI volume, compared with 4% in early years (P < 0.001).
3.3. Prosthetic valve function
Figure 2 summarizes the in‐hospital procedural complications and outcomes according to procedure calendar year. There were no remarkable temporal trends in prosthetic valve function: TAVI time period did not influence the success rate of device implantation, which overall was excellent and reached 94.5% (complete procedural and in‐hospital outcomes description is presented in Supporting Information, Table 2, in the online version of this article). Notably, device success low tide appeared in 2012, together with the apex in utilization of balloon‐expandable devices. The incidence of postprocedural > moderate paravalvular leak remained quite persistent over the course of the study and stood at approximately 6% to 8% (P = 0.941). Although the differences were without clinically substantial change, postprocedural transaortic gradients significantly decreased over time (10 ± 5 mm Hg in 2008–2010 vs 8.5 ± 3.5 mm Hg in 2014; P = 0.042).
Figure 2.
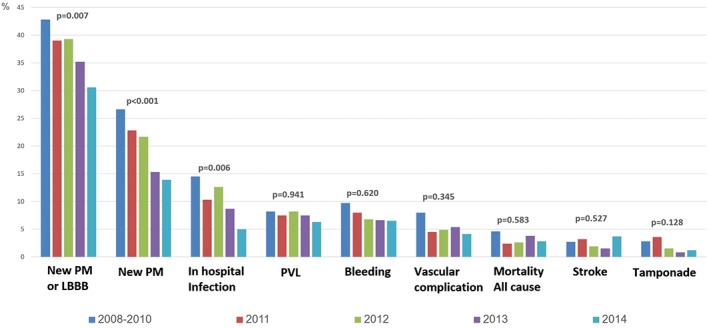
In‐hospital procedural complications by TAVI annual cohort. PVL is defined as ≥ moderate; bleeding is defined as major or life‐threatening; vascular complications are defined as major, all using the VARC‐2 consensus definitions. Abbreviations: LBBB, left bundle branch block; PM, pacemaker; PVL, paravalvular leak; TAVI, transcatheter aortic valve implantation; VARC‐2, Valve Academic Research Consortium‐2.
Table 2.
Multivariate analysis: Risk of new PM implantation by TAVI year
| OR | 95% CI | P Value | |
|---|---|---|---|
| TAVI calendar year as a continuous measure | |||
| Per 1‐year increment | 0.81 | 0.70‐0.94 | 0.004 |
| TAVI period categorized by quintiles | |||
| Q1: 2008–2010 | Ref | ||
| Q2: 2011 | 0.54 | 0.28‐1.02 | 0.057 |
| Q3: 2012 | 0.69 | 0.37‐1.26 | 0.221 |
| Q4: 2013 | 0.23 | 0.10‐0.50 | <0.001 |
| Q5: 2014 | 0.50 | 0.26‐0.97 | 0.039 |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; OR, odds ratio; PCI, percutaneous coronary intervention; PM, pacemaker; Q, quintile; Ref, reference; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Adjusted for age, sex, LVEF, STS score, DM, AF, COPD, baseline mean transaortic gradient, and history of PCI and mitral valve replacement.
3.4. Clinical endpoints
There was a low event rate with no apparent temporal trend in periprocedural myocardial infarction (MI), stroke, and tamponade, as well as in the rate of major vascular complications, which was 8% in 2008–2010, decreased to 4.5% in 2011, and remained constant thereafter (4.1%–5.4%; P = 0.345). On the other hand, moving from the initial to the last time cohort, there was a witnessed shift regarding several other outcome measurements. To begin with, the new PPM insertion rate decreased from 25% in 2008–2010 to 12% in 2014 (P < 0.001), and multivariable analyses (Table 2) demonstrated that each upturn in calendar year was associated with 20% reduction in need for new PPM (odds ratio [OR]: 0.81, 95% confidence interval [CI]: 0.67‐0.94, P = 0.004) and 15% reduction in need for new PPM or LBBB (OR: 0.85, 95% CI: 0.75‐0.96, P = 0.008). Other factors that were associated with increased need for new PPM include history of right bundle branch block and utilization of self‐expandable or large device. Likewise, there was a consistent reduction in the rate of in‐hospital infections (14.5% [2008–2010] to 5% [2014]; P = 0.006), and multivariable analyses demonstrated each calendar year to be associated with 12% fewer infections (OR: 0.88, 95% CI: 0.76‐1.02, P = 0.082). Correspondingly, in‐hospital admission days were halved from 12.5 (±38) days in the 2008–2010 cohort to 6.6 (± 12) days in the 2014 cohort (P < 0.001). Finally, Kaplan‐Meier curves showed an inverse relationship between procedure year and cumulative mortality risk (Figure 3). By multivariate analysis (see Supporting Information, Table 3, in the online version of this article), there was no significant mortality decrease (hazard ratio per calendar‐year increment: 0.96, 95% CI: 0.83‐1.10, P = 0.576).
Figure 3.
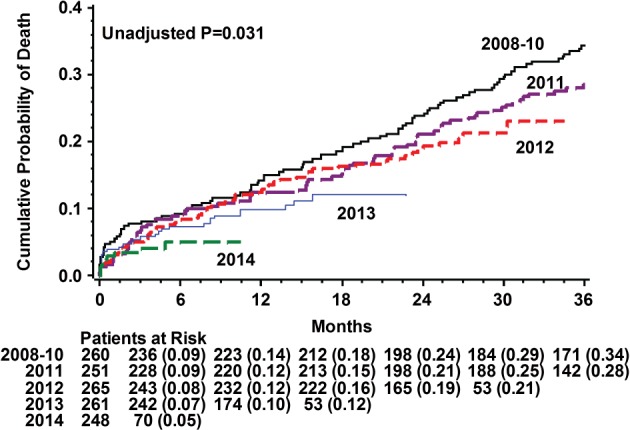
Kaplan‐Meier survival curves of TAVI patients for each of the 5 time quintiles. Abbreviations: TAVI, transcatheter aortic valve implantation.
4. DISCUSSION
In this multicenter Israeli TAVI registry, we report on the temporal trends accompanying the vigorous era of TAVI evolution from preliminary technique to conventional therapy. Our findings show several temporal trends that deserve emphasis: (1) a shift to a lower‐risk patient population; (2) adoption of less‐invasive operative techniques (ie, more practice of conscious sedation, less employment of balloon predilatation, and further restriction to transfemoral access); (3) a decrease in adverse events, such as need for new PPM, LBBB, and in‐hospital infections; (4) a shorter hospital stay; and (5) an increase in overall survival rate.
4.1. Patient characteristics
We found that TAVI is being applied in lower‐risk patients. This shifting paradigm is broadly reported both in clinical practice and in clinical trials in Europe5, 6, 7, 8, 9 and in the United States.10, 11, 12, 13, 14, 15, 16 Although the mean STS score of patients in the early cohort (2008–2010) was already within the customary intermediate STS risk range, all patients were deemed as inoperable/high surgical risk based on other comorbidities and/or technical issues (ie, porcelain aorta, hostile chest) not captured by the STS score. A prominent element was the considerably higher proportion of patients in the early 2008–2010 cohort that were judged to be frail, in comparison to the more recent (2011–2014) cohorts: 28% vs 15% (P < 0.001). Contemporary data demonstrate that compared with surgery, lower‐risk patients do very well with TAVI,15, 16, 17, 18, 19 with the recent Placement of Aortic Transcatheter Valves (PARTNER) IIA and PARTNER IIS3i studies providing (along with the soon‐to‐be‐reported Safety and Efficacy Study of the Medtronic CoreValve System in the Treatment of Severe, Symptomatic Aortic Stenosis in Intermediate‐Risk Subjects Who Need Aortic Valve Replacement [SURTAVI] trial) more robust answers to this issue. Refinement in technology, patient selection, and operator techniques, together with long‐term durability appreciation, are expected to further expand the percutaneous approach to lower‐risk patients.
4.2. Procedural characteristics
The extent of valve‐in‐valve procedures increased significantly to constitute 17% of all TAVI volume at the end of the study. This emerging alternative for patients with a failed bioprosthetic valve at high risk for revision surgery has been explored as safe and very effective procedure,20 and its frequency will most probably increase even further in the near future. One of the medical centers participating in the current study performs the highest number of valve‐in‐valve interventions in our country, and thus explains the high degree of valve‐in‐valve TAVI exhibited. We encountered an accelerated practice of using conscious sedation over the years. Despite this, there was a temporal decrease in the need to urgently convert from conscious sedation to general anesthesia during the procedures (from 2% to 0.5%; P < 0.001)—a finding that should strengthen the comfort operators feel with light anesthesia. There was a decrease in the nonfemoral approach and in use of balloon predilatation, which in sum indicates a drift on the road to more restrained, minimal procedures. Thanks to the recent advances in technology and smaller catheter platform available, procedural simplification is becoming consolidated and most certainly has some effect on outcomes, which is the next subject to be discussed.
4.3. Clinical outcomes
Post‐TAVI conduction abnormalities result primarily from mechanical compression by the device on the specialized adjacent conduction system. The key risk factors for this complication are CoreValve device utilization and baseline right bundle branch block, but it may also depend on other procedural factors. We found that each calendar‐year increment was associated with an adjusted 20% reduction in the need for a new PPM (P < 0.001), along with an equal balloon‐expandable to self‐expandable valve utilization ratio during those periods after adjusting for baseline conduction abnormalities. Consistently, each year increment was associated with an adjusted 15% reduction in requirement for a new PPM or LBBB. Holmes et al assessed the temporal trends among 26 414 patients who underwent TAVI from 2012 through 2014 in the US Transcatheter Valve Therapy (TVT) registry.14 The investigators found that the most common periprocedural cardiac complication was a need for a new PPM, which actually increased from 7.5% in 2012 to 2013 to 10.5% in 2014 (P for trend <0.001). This contrasting trend can be explained by the fact that the CoreValve prosthesis has been included in the US registry only since 2013. The device, which has been associated with a higher incidence of conduction‐system abnormalities,21 most likely confounded the calendar‐year influence. Indeed, we also found CoreValve implantation to be an independent risk for new PPM; but, on the contrary, the CoreValve 2008 to 2014 utilization rate remained fairly steady in the practice of the 3 participating medical centers; hence, the mechanism behind the decrease seen in conduction abnormalities most probably relates to some modifications in procedural technique, such as the decrease in balloon predilatation (see Supporting Information, Table 1, in the online version of this article) and perhaps more accurate valve sizing and positioning associated with more frequent use of preprocedural computed tomography, operator learning curve, and the appreciation that higher implantation may be optimal.22, 23, 24 In line with our findings, a recent report on the temporal trends from the UK TAVI registry25 did not show differences in the total need for PPM or a new LBBB, but it did find a reduction from ~30% (2007–2008) to ~15% (2012) in PPM requirement with CoreValve implantation (P < 0.001). Although the impact of PM implantation on mortality has not been demonstrated, lowering this risk would be preferable, especially in younger patients,3 as it will decrease PM‐associated complications and may affect the TAVI cost‐effectiveness profile, as PPM implantation accounts for the largest attributable cost for TAVI in the United States.26
The event rates for periprocedural stroke, MI, tamponade, major vascular complications, and in‐hospital mortality were all small, and no temporal trends were identified. Similar periprocedural stroke rate and temporal consistent were reported in other studies,14, 27, 28 although the TVT did demonstrate a statistically significant (but numerically/clinically very small) reduction in periprocedural MI.14 Although other studies did find a temporal decrease in the rate of vascular complications,14, 22, 27 we did not. An important consideration on the subject of vascular complications is that smaller catheter sheaths allow the pursuit after higher degree of transfemoral access, so that peripheral vasculatures that were inaccessible 5 years ago are now being accessed, instead of referring patients to alternative approaches associated with inferior results.29 We sampled a subgroup of 375 transfemoral‐access TAVI patients and calculated the patients with index femoral artery minimal diameter <6.5 mm (which is associated with increased vascular complications).29, 30 We found such patients to account for 4.5% during 2008 to 2010 and 20% during 2014. This may imply that operators choose for themselves and their patients undergo some risk for a vascular complication “penalty” rate in favor of higher adherence to transfemoral access.
We found no temporal trend with regard to post‐TAVI paravalvular leak (> moderate) risk, with incidence of 8.2% in 2008 to 2010 and 6.3% in 2014 (P = 0.941). Paravalvular leak was somewhat lower over time in the TVT registry, but without clinically substantial change (from 5.5% to 4.8%). This may feel disappointing, as paravalvular leak is associated with poor outcome15, 21 and represents to some the Achilles heel of TAVI. Nevertheless, keeping in mind that 99% of the valves in this registry were first‐generation transcatheter valves, the degree of regurgitation may change over time with the current shift toward second‐generation devices, as was already demonstrated in many recent reports.15, 16, 31
An additional observation was the temporal reduction in in‐hospital infections and length of stay. The mean hospital stay went down from 12.5 (± 38) to 6.6 (± 12) days during the study (P < 0.001). Shorter hospital stay has important implications on patient comfort and rehabilitation, as well as on cost‐effectiveness. Moreover, it may serve as a good short‐term surrogate indicator for the combined temporal trends in TAVI: lower‐risk patients undergoing more restrained procedures with lower complication rates.
Finally, we found a significant temporal trend in survival, with improved long‐term survival as the procedure calendar year advanced. There was no significant difference between the cohorts in short‐term mortality rate (see Supporting Information, Table 2, in the online version of this article), and the long‐term survival variance lost its significance once we adjusted for age, multiple comorbidities, and STS score (see Supporting Information, Table 3, in the online version of this article). The same findings were demonstrated in the UK TAVI registry and by others,25, 27 and they most likely reflect the fact that the shift in mortality is mainly driven from patient selection rather than from technical/procedural matters, as these would be expected to be projected during/early after the intervention and to be consistent after correcting for the former covariates. Nevertheless, it is possible that our study was underpowered to expose such procedural‐driven mortality trends because the short‐term mortality event rate was very low, so larger studies are needed to examine this issue.
4.4. Study limitations
This is a retrospective study, which carries the concern of unmeasured confounding variables and/or possible missing reported outcomes. Study results can be influenced by differences in disease assessment and documentation patterns at participating institutions. The event rates of some outcome measures (eg, stroke, MI, tamponade, in‐hospital mortality) were small, and our study probably was underpowered to detect any temporal changes in these. Importantly, our aim was to define temporal trends and associations, and no cause‐and‐effect suppositions can be drawn.
5. CONCLUSION
Temporal trends accompanying the evolution of TAVI include its increasing utilization in a lower‐risk population, increased practice of conscious sedation, and adherence to the transfemoral access. Over time, TAVI patients require a shorter hospital stay and are experiencing lower rates of NPP, LBBB, and in‐hospital infections. Post‐TAVI survival is increasing.
Supporting information
Figure S1 Society of Thoracic Surgeons (STS) score predicted risk of mortality in TAVI patients over time. Classified as low STS risk (STS score < 4%, in orange), intermediate STS risk (4% < STS score < 8%, in light blue) and high STS risk (8% < STS score, in purple).
Figure S2 Device type (balloon vs. self‐expandable valve) utilization ratio during the study years
Table S1 Procedural characteristics stratified according to transcatheter aortic valve implantation year
Table S2 Procedural and in‐hospital outcomes stratified according to transcatheter aortic valve implantation year
Table S3 Multivariate analysis: risk of death by transcatheter aortic valve implantation year
Landes U, Barsheshet A, Finkelstein A, Guetta V, Assali A, Halkin A, Vaknin‐Assa H, Segev A, Bental T, Ben‐Shoshan J, Barbash IM and Kornowski R. Temporal trends in transcatheter aortic valve implantation, 2008–2014: patient characteristics, procedural issues, and clinical outcome, Clin Cardiol, 2017;40(2):82–88.
REFERENCES
- 1. Nishimura R, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014;63:2489]. J Am Coll Cardiol. 2014;63:57–185. [Google Scholar]
- 2. Vahanian A, Alfieri O, Andreotti F, et al; Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio‐Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 3. Cribier A, Durand E. Patient selection for TAVI in 2014: is it justified to treat low‐ or intermediate‐risk patients? The cardiologist's view. EuroIntervention. 2014;10(suppl U):U16–U21. [DOI] [PubMed] [Google Scholar]
- 4. Kappetein A, Head S, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 5. Gilard M, Eltchaninoff H, Iung B, et al; FRANCE 2 Investigators. Registry of transcatheter aortic‐valve implantation in high‐risk patients. N Engl J Med. 2012;366:1705–1715. [DOI] [PubMed] [Google Scholar]
- 6. Eltchaninoff H, Prat A, Gilard M, et al; FRANCE Registry Investigators. Transcatheter aortic valve implantation: early results of the FRANCE (French Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32:191–197. [DOI] [PubMed] [Google Scholar]
- 7. Thomas M, Schymik G, Walther T, et al. Thirty‐day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122:62–69. [DOI] [PubMed] [Google Scholar]
- 8. Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. [DOI] [PubMed] [Google Scholar]
- 9. Barbash IM, Finkelstein A, Barsheshet A, et al. Outcomes of patients at estimated low, intermediate, and high risk undergoing transcatheter aortic valve implantation for aortic stenosis. Am J Cardiol. 2015;116:1916–1922. [DOI] [PubMed] [Google Scholar]
- 10. Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 11. Mack MJ, Brennan JM, Brindis R, et al; STS/ACC TVT Registry. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069–2077. [DOI] [PubMed] [Google Scholar]
- 12. Adams DH, Popma JJ, Reardon MJ, et al; US CoreValve Clinical Investigators. Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med. 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 13. Abdel‐Wahab M, Mehilli J, Frerker C, et al; CHOICE Investigators. Comparison of balloon‐expandable vs self‐expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311:1503–1514. [DOI] [PubMed] [Google Scholar]
- 14. Holmes DR Jr, Nishimura RA, Grover FL, et al; for the STS/ACC TVT Registry . Annual outcomes with transcatheter valve therapy: from the STS/ACC TVT Registry. J Am Coll Cardiol. 2015;66:2813–2823. [DOI] [PubMed] [Google Scholar]
- 15. Leon MB, Smith CR, Mack MJ, et al; for the PARTNER‐2 Investigators . Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 16. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate‐risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. [DOI] [PubMed] [Google Scholar]
- 17. Piazza N, Kalesan B, Van Mieghem N, et al. A 3‐center comparison of 1‐year mortality outcomes between transcatheter aortic valve implantation and surgical aortic valve replacement on the basis of propensity score matching among intermediate‐risk surgical patients. JACC Cardiovasc Interv. 2013;6:443–451. [DOI] [PubMed] [Google Scholar]
- 18. D'Errigo P, Barbanti M, Ranucci M, et al; OBSERVANT Research Group . Transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis: results from an intermediate risk propensity‐matched population of the Italian OBSERVANT study. Int J Cardiol. 2013;167:1945–1952. [DOI] [PubMed] [Google Scholar]
- 19. Thyregod H, Steinbrüchel D, Ihlemann N, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1‐year results from the All‐Comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015;65:2184–2194. [DOI] [PubMed] [Google Scholar]
- 20. Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the Global Valve‐in‐Valve Registry. Circulation. 2012;126:2335–2344. [DOI] [PubMed] [Google Scholar]
- 21. Siontis G, Jüni P, Pilgrim T, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta‐analysis. J Am Coll Cardiol. 2014;64:129–140. [DOI] [PubMed] [Google Scholar]
- 22. Willson AB, Webb JG, Freeman M, et al. Computed tomography–based sizing recommendations for transcatheter aortic valve replacement with balloon‐expandable valves: comparison with transesophageal echocardiography and rationale for implementation in a prospective trial. J Cardiovasc Comput Tomogr. 2012;6:406–414. [DOI] [PubMed] [Google Scholar]
- 23. Jilaihawi H, Kashif M, Fontana G, et al. Cross‐sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol. 2012;59:1275–1286. [DOI] [PubMed] [Google Scholar]
- 24. Dvir D, Webb JG, Piazza N, et al. Multicenter evaluation of transcatheter aortic valve replacement using either SAPIEN XT or CoreValve: degree of device oversizing by computed tomography and clinical outcomes. Catheter Cardiovasc Interv. 2015;86:508–515. [DOI] [PubMed] [Google Scholar]
- 25. Ludman PF, Moat N, de Belder MA, et al; UK TAVI Steering Committee and the National Institute for Cardiovascular Outcomes Research. Transcatheter aortic valve implantation in the United Kingdom: temporal trends, predictors of outcome, and 6‐year follow‐up: a report from the UK Transcatheter Aortic Valve Implantation (TAVI) Registry, 2007 to 2012. Circulation. 2015;131:1181–1190. [DOI] [PubMed] [Google Scholar]
- 26. Baron SJ, Arnold SV, Reynolds MR, et al. Costs of periprocedural complications among patients treated with a self‐expanding transcatheter aortic valve prosthesis: results from the CoreValve US Pivotal Extreme Risk Study. J Am Coll Cardiol. doi: 10.1016/j.jacc.2014.07.757. [DOI] [Google Scholar]
- 27. Van Mieghem NM, Chieffo A, Dumonteil N, et al. Trends in outcome after transfemoral transcatheter aortic valve implantation. Am Heart J. 2013;165:183–192. [DOI] [PubMed] [Google Scholar]
- 28. Mastoris L, Schoos MM, Dangas GD, et al. Stroke after transcatheter aortic valve replacement: incidence, risk factors, prognosis, and preventive strategies. Clin Cardiol. 2014;37:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Généreux P, Webb JG, Svensson LG, et al. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of Aortic Transcatheter Valve) Trial. J Am Coll Cardiol. 2012;60:1043–1052. [DOI] [PubMed] [Google Scholar]
- 30. Hayashida K, Lefèvre T, Chevalier B, et al. Transfemoral aortic valve implantation: new criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4:851–858. [DOI] [PubMed] [Google Scholar]
- 31. Manoharan G, Walton AS, Brecker SJ, et al. Treatment of symptomatic severe aortic stenosis with a novel resheathable supra‐annular self‐expanding transcatheter aortic valve system. JACC Cardiovasc Interv. 2015;8:1359–1367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Society of Thoracic Surgeons (STS) score predicted risk of mortality in TAVI patients over time. Classified as low STS risk (STS score < 4%, in orange), intermediate STS risk (4% < STS score < 8%, in light blue) and high STS risk (8% < STS score, in purple).
Figure S2 Device type (balloon vs. self‐expandable valve) utilization ratio during the study years
Table S1 Procedural characteristics stratified according to transcatheter aortic valve implantation year
Table S2 Procedural and in‐hospital outcomes stratified according to transcatheter aortic valve implantation year
Table S3 Multivariate analysis: risk of death by transcatheter aortic valve implantation year


