Abstract
Background
Hypertrophic cardiomyopathy (HCM) remains the most common cause of sudden cardiac death (SCD) in the young; however, current strategies do not identify all HCM patients at risk. A novel validated algorithm was proposed by the last European Society of Cardiology guidelines to guide implantable cardioverter‐defibrillator (ICD) therapy. Recently, extensive myocardial fibrosis was independently associated with increased risk of SCD events. This study aimed to establish the relation between myocardial fibrosis (late gadolinium enhancement [LGE] extension) and the novel SCD risk‐prediction model in a real population of HCM to evaluate its potential additional value in the different risk groups.
Hypothesis
There is a significant association between LGE extension and the novel SCD risk calculator that may help conflicting ICD decisions.
Methods
Seventy‐seven patients with HCM underwent routine clinical evaluation, echocardiography, and cardiac magnetic resonance study. Their SCD risk at 5 years was calculated using the new model.
Results
Extension of LGE positively correlated with SCD risk prediction (r = 0.7, P < 0.001). Low‐, intermediate‐, and high‐risk groups according to the model showed significantly different extent of LGE (5% ± 6% vs 18% ± 9% vs 17% ± 4%; P < 0.001). Four patients (6%) in the low‐risk group and 5 (62%) in the intermediate‐risk group showed extensive areas of LGE. All patients except 1 (86%) at highest risk (n = 6) showed extensive areas of LGE.
Conclusions
LGE extension is concordant with the novel SCD‐risk model defining low‐ and high‐risk groups; it may provide additional information, allowing better discrimination to support implantable cardioverter‐defibrillator decision. LGE quantification holds promise for SCD stratification in HCM.
Keywords: Hypertrophic Cardiomyopathy, Sudden Cardiac Death, Risk Stratification, Cardiac Magnetic Resonance, Late Gadolinium Enhancement
1. INTRODUCTION
Hypertrophic cardiomyopathy (HCM) remains the most common cause of sudden cardiac death (SCD) in the young, mainly due to fatal arrhythmic events.1, 2, 3, 4 Clinical risk strategies comprise several clinical and imaging features to detect patients at highest risk, candidates for implantable cardioverter‐defibrillator (ICD) therapy. Although primary prevention with ICD has been highly effective for life‐threatening ventricular tachyarrhythmias, conventional risk assessment fails to identify all HCM patients at risk.1, 2, 4, 5, 6, 7 Recently, the detection of myocardial fibrosis as an additional marker of risk is gaining considerable interest, particularly to support the decision in ambiguous patients. Contrast‐enhanced cardiovascular magnetic resonance (CMR) imaging with late gadolinium enhancement (LGE) is capable of noninvasive detection of myocardial fibrosis in ischemic and nonischemic cardiomyopathies.8, 9, 10 Although the presence of LGE in HCM has been associated with ventricular arrhythmias, the association with SCD has been largely questioned.11, 12, 13, 14, 15, 16, 17, 18, 19 Initial research in this scenario considered LGE as a binary variable attributing equal risk to any amount of LGE, from trivial to extensive. In a disease in which the prevalence of LGE oscillates between 60% and 80%, this objective has proved impractical and nonrealistic. Recent evidence has shown a continuous relationship between the amount of LGE and the risk of SCD, independent of conventional risk factors.18
A novel prognostic algorithm was recently developed and validated by UK investigators and has been proposed by current 2014 European Society of Cardiology (ESC) guidelines.1, 20 It was designed with retrospectively collected data, and therefore, new associations with SCD such as the amount of LGE were not explored or included. Thenceforth, the prognostic model has been incorporated into clinical practice to guide ICD therapy based on the estimation of 5‐year risk of SCD.
The aim of this study is to establish the relation between LGE extension and the novel SCD risk‐prediction model in a real population of HCM to evaluate its potential additional value in the different risk groups.
2. METHODS
Seventy‐seven patients with unequivocal diagnosis of HCM and who were referred for clinically indicated CMR from January 2013 to October 2015 were included in this study.10 All subjects underwent clinical evaluation, echocardiography, and a CMR study. SCD risk was assessed by the new validated prediction model.1, 20 Exclusion criteria for all subjects included patient age <16 years, elite athletes, known diagnosis of metabolic/infiltrative diseases and syndromes, and previous procedures of myectomy or alcohol septal ablation. In addition, subjects with generally accepted contraindications to CMR or a history of renal disease with a current estimated glomerular filtration rate <30 mL/min/1.73 m2 were also excluded.
The study protocol was reviewed and approved by the local institutional ethics committees. All procedures were carried out in accordance with the Declaration of Helsinki (2000).
2.1. Echocardiography
All patients underwent clinical transthoracic echocardiography (TTE) according to the recommendations from the European Association of Cardiovascular Imaging.21 Those variables included in the risk‐stratification model were collected. Maximum left ventricular (LV) wall thickness (LVWT) was defined as the greatest thickness using parasternal short‐axis plane in 2D echocardiography. Left atrial (LA) diameter was determined by M‐mode or 2D echocardiography in the parasternal long‐axis plane. The maximum left ventricular outflow tract (LVOT) gradient was determined at rest and with Valsalva provocation using pulsed and continuous‐wave Doppler from the apical 3‐ and 5‐chamber view. Peak LVOT gradients were determined using the modified Bernoulli equation (pressure gradient = 4v2, where v is the peak aortic outflow velocity.
2.2. CMR and image analysis
All patients underwent routine clinical scan protocol for volumes, mass, and tissue characterization by LGE using a 1.5‐Tesla magnetic resonance imaging scanner equipped with an advanced cardiac package and multitransmit technology (Achieva; Philips Healthcare, Best, The Netherlands) following professional recommendation for standardized acquisition.22 All cine images were acquired using a balanced steady‐state free precession sequence in combination with parallel imaging (SENSitivity encoding, factor 2) and retrospective gating during a gentle expiratory breathhold (echo time [TE]/repetition time [TR]/flip‐angle: 1.7 ms/3.4 ms/60°, spatial resolution 1.8 × 1.8 × 8 mm). LGE imaging was performed in a gapless whole heart coverage of short‐axis slices ~15 minutes after administration of 0.2 mmol/kg body‐weight gadobutrol using a mid‐diastolic inversion prepared 2D gradient echo sequence (TE/TR/flip‐angle: 2.0 ms/3.4 ms/25°, interpolated voxel size 0.7 × 0.7 × 8 mm) with a patient‐adapted prepulse delay.
All routine CMR analysis was performed on commercially available software (CMR 42; Circle, Calgary, Canada). Endocardial LV borders were manually traced at end‐diastole and end‐systole. LV end‐diastolic volume (LVEDV) and LV end‐systolic volume (LVESV) were determined using the Simpson rule. Left ventricular ejection fraction (LVEF) was computed as LVEDV − LVESV/LVEDV. LGE images were visually examined for the presence of regional fibrosis showing as bright areas within the myocardium in corresponding longitudinal views and by exclusion of potential artifacts.23 LGE was quantified using the grayscale threshold method of ≥6 SDs. Extensive areas of LGE were defined by the presence of >15% of LGE of the total LV mass.18, 24
Inter‐ and intraobserver reproducibility of LGE quantification were assessed in 10 randomly selected subjects. For agreement measurements, the endocardial and epicardial borders were retraced, and the threshold was redetermined.
2.3. Statistical analysis
Statistical analysis was performed using SPSS software version 21.0 (IBM Corp., Armonk, NY). Normality of distributions was tested with the Kolmogorov‐Smirnov statistic. Categorical data are expressed as percentages; continuous variables are expressed as mean ± SD or median (interquartile range), as appropriate. For comparison of 2 and >2 normally distributed variables, the Student t test, 1‐way analysis of variance (ANOVA, with the Bonferroni post hoc test), and the χ2 test were employed as appropriate. Correlations were assessed using the Pearson correlation coefficient for normally distributed variables and the Spearman correlation coefficient for nonparametric data. Associations were explored by single and multivariate linear regressions. All tests were 2‐tailed, and a P value of <0.05 was considered significant.
3. RESULTS
Clinical and demographic characteristics of the study population are summarized in Table 1. At initial evaluation, 23% of the patients had suffered a previous unexplained syncope, 17% had evidence of nonsustained ventricular tachycardia (NSVT), and 8% had family history of SCD (Table 2). Unexplained syncope was significantly more prevalent in those patients with LGE (38% vs 5%; P = 0.02); however, no differences were found in the prevalence of rest obstruction, presence of NSVT, or family history of SCD between patients with and without LGE (P > 0.05 for all).
Table 1.
Demographic and clinical characteristics of patients with HCM
| Variable | All HCM Patients, N = 77 |
|---|---|
| Age, y | 59 ± 15 |
| Male sex | 52 (67) |
| Body surface area, g/m2 | 1.86 ± 0.3 |
| T2DM | 10 (13) |
| HTN | 42 (55) |
| Hypercholesterolemia | 27 (35) |
| Smoker | 12 (16) |
| eGFR, mL/min/1.73 m2 | 81 ± 20 |
| Hgb, g/dL | 14 ± 1.5 |
| History of CAD | 5 (7) |
| NYHA class | |
| I | 55 (72) |
| II | 17 (22) |
| III/IV | 5 (6) |
| AF | 19 (25) |
| Obstructive HCM | 10 (13) |
Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; HCM, hypertrophic cardiomyopathy; Hgb, hemoglobin; HTN, hypertension; NYHA, New York Heart Association; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Data are presented as n (%) or mean ± SD.
Table 2.
Risk‐stratification variables according to the model
| Variable | All HCM Patients, N = 77 |
|---|---|
| Age, y | 59 ± 15 |
| Family history of SCD n (%) | 6 (8) |
| Unexplained syncope n (%) | 18 (23) |
| LVOT gradient, mm Hg | 20.4 ± 27 |
| Maximum LVWT, mm | 18.7 ± 4.7 |
| LA diameter, mm | 43 ± 7 |
| NSVT n (%) | 13 (17) |
| HCM SCD‐risk score | 2.5 ± 1.6 |
| 5‐Year risk by group n (%) | |
| Low risk, <4% | 62 (81) |
| Intermediate risk, 4%–6% | 8 (10) |
| High risk, 6% | 7 (9) |
Abbreviations: ECG, electrocardiographic; HCM, hypertrophic cardiomyopathy; LA, left atrial; LVOT, left ventricular outflow tract; LVWT, left ventricular wall thickness; NSVT, nonsustained ventricular tachycardia during 24‐ to 48‐hour ambulatory ECG monitoring; SCD, sudden cardiac death; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
LGE was present in 75% of HCM patients (n = 58), and 26% of them (n = 15) showed extensive areas of LGE (>15% of the total LV mass; Figure 1). Extensive LGE was more frequently observed in men, in patients with a previous episode of unexplained syncope, with NSVT, with family history of SCD, with LVWT ≥30 mm, and in patients with New York Heart Association class ≥ II (P < 0.001 for all). HCM patients with extensive areas of LGE showed higher E/e′ ratios, higher LV mass, and maximum LV thickness (P < 0.001 for all). Two patients exhibited extreme hypertrophy (≥30 mm); one of them also showed a wide amount of LGE and was considered at high risk by the model. Apical aneurysm was present in 2 patients, both of them clinically judged to be at low risk. Both exhibited areas of LGE, one representing 8% and the other 18% of the total LV mass. Significant resting LVOT obstruction (gradient >30 mm Hg) was present in 10 patients (13%).
Figure 1.
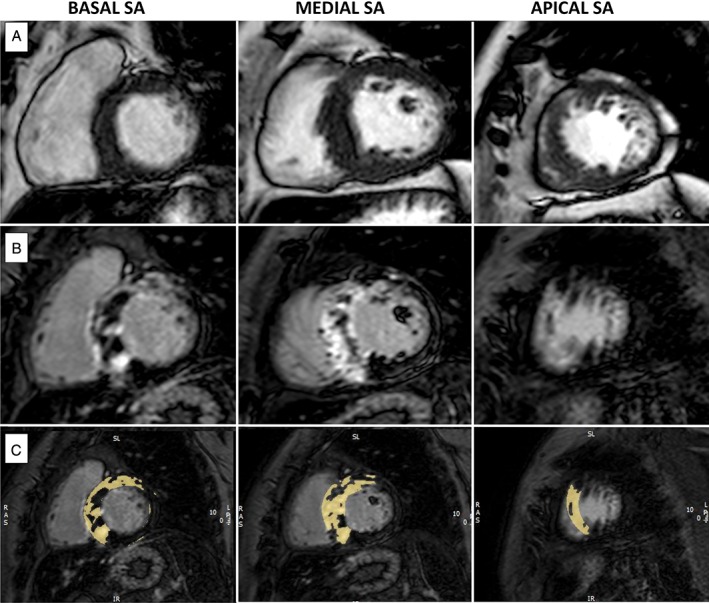
Representative case of a patient with septal HCM, an estimated risk of SCD at 5 years of 5.1% (intermediate risk) and extensive amount of LGE. (A) End‐diastolic cine images from SA views. (B) and (C): Basal, mid, and apical LGE images. Yellow areas delineate LGE extension using the grayscale threshold method of ≥6 SDs above the normal myocardium. Abbreviations: HCM, hypertrophic cardiomyopathy; LGE, late gadolinium enhancement; SA, short‐axis; SCD, sudden cardiac death; SD, standard deviation.
The amount of LGE was significantly higher in patients with unexplained syncope, NSVT, and family history of SCD (12% ± 8% vs 6% ± 8%, 14% ± 7% vs 7% ± 8%, and 22% ± 8% vs 7% ± 7%, respectively; P < 0.01 for all). The median LGE of patients with extreme hypertrophy (n = 2) was 10%, compared with 6% of the other HCM patients (P < 0.05). The extension of LGE was not significantly different in the subgroup of patients with significant LVOT gradient.
According to the proposed 5‐year risk score, 62 patients (81%) were considered as low risk, 8 (10%) intermediate risk, and 7 (9%) high risk. Among imaging findings, maximum LVWT and parameters of diastolic function showed significant differences between risk groups (Table 3). All patients included in intermediate‐risk and high‐risk groups showed areas of LGE, as did 69% of patients in the low‐risk group. Patients with LGE had a significantly higher score compared with patients without LGE (SCD score: HCMLGE: 1.4 ± 0.75 vs 2.9 ± 1.7; P < 0.001). Low‐, intermediate‐, and high‐risk groups showed a significantly different extent of LGE (5 ± 6 vs 18 ± 9 vs 17 ± 4, respectively; P < 0.0001). Only 4 patients (6%) in the low‐risk group showed extensive areas of LGE (see Supporting Information, Table 1, in the online version of this article for detailed characteristics of these patients); however, 5 patients (62%) at intermediate risk and all except 1 at highest risk (n = 6) showed extensive areas of LGE.
Table 3.
Echocardiographic and CMR findings
| Variable | All Patients, N = 77 | Low‐Risk Group, n = 62 | Intermediate‐Risk Group, n = 8 | High‐Risk Group, n = 7 | P Value |
|---|---|---|---|---|---|
| Echocardiographic measures | |||||
| LAD, mm | 43 ± 7 | 42 ± 7 | 46 ± 7 | 44 ± 4 | 0.2 |
| Maximum LVWT, mm | 18.7 ± 4 | 18 ± 4 | 21 ± 5 | 25 ± 5 | 0.001 |
| LVOT gradient, mm Hg | 20 ± 26 | 20 ± 18 | 31 ± 37 | 14 ± 4 | 0.4 |
| Basal LVOT gradient ≥30 mm Hg | 10 (13) | 8 (13) | 2 (25) | 0 (0) | 0.3 |
| E/A ratio | 1.15 ± 0.4 | 1.1 ± 0.4 | 1.6 ± 0.2 | 1.01 ± 0.5 | 0.005 |
| Lateral E/e′ | 9.3 ± 5 | 8.1 ± 4 | 10 ± 5 | 15 ± 5 | 0.007 |
| Septal E/e′ | 12 ± 5 | 11 ± 4 | 13 ± 4 | 18 ± 3 | 0.04 |
| CMR measures | |||||
| LVEDV index, mL/m2 | 43 ± 17 | 43 ± 16 | 44 ± 17 | 33 ± 8 | 0.4 |
| LVEF, % | 62 ± 7 | 66 ± 8 | 62 ± 6 | 69 ± 7 | 0.2 |
| RVEF, % | 69 ± 7 | 68 ± 7 | 67 ± 9 | 69 ± 3 | 0.8 |
| LVMI, mg/m2 | 86 ± 30 | 82 ± 31 | 102 ± 32 | 105 ± 24 | 0.09 |
| Maximal LVWT, mm | 18.8 ± 5 | 18 ± 4 | 21 ± 8 | 24 ± 6 | 0.009 |
| LAVI, mL/m2 | 63 ± 20 | 58 ± 23 | 70 ± 35 | 64 ± 10 | 0.2 |
| LGE | |||||
| LGE present | 58 (75) | 43 (69) | 8 (100) | 7 (100) | <0.05 |
| LGE extent | 7.9 ± 8 | 5 ± 6 | 18 ± 9 | 17 ± 4 | <0.001 |
| LGE extension | <0.001 | ||||
| 1%–5% | 25 (32) | 25 | 0 | 0 | |
| 6%–10% | 12 (16) | 9 | 3 | 0 | |
| 11%–15% | 6 (8) | 5 | 0 | 1 | |
| 16%–20% | 6 (8) | 1 | 1 | 4 | |
| >20% | 9 (12) | 3 | 4 | 2 | |
Abbreviations: CMR, cardiac magnetic resonance imaging; LAD, left atrial diameter; LAVI, left atrial volume index; LGE, late gadolinium enhancement; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; LVOT, left ventricular outflow tract; LVWT, left ventricular wall thickness; RVEF, right ventricular ejection fraction; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
3.1. Analysis of relationships
The SCD‐risk score showed positive moderate correlations with some functional‐imaging parameters included in the proposed SCD score (maximum LVWT and LA size, r = 0.4 and r = 0.5, respectively; P < 0.01 for both). In addition, measurement of diastolic function (medial and lateral E/é, r = 0.4; P < 0.01 for both) and the extension of LGE (r = 0.7; P < 0.0001) were positively correlated with the SCD‐risk model (Figure 2).
Figure 2.
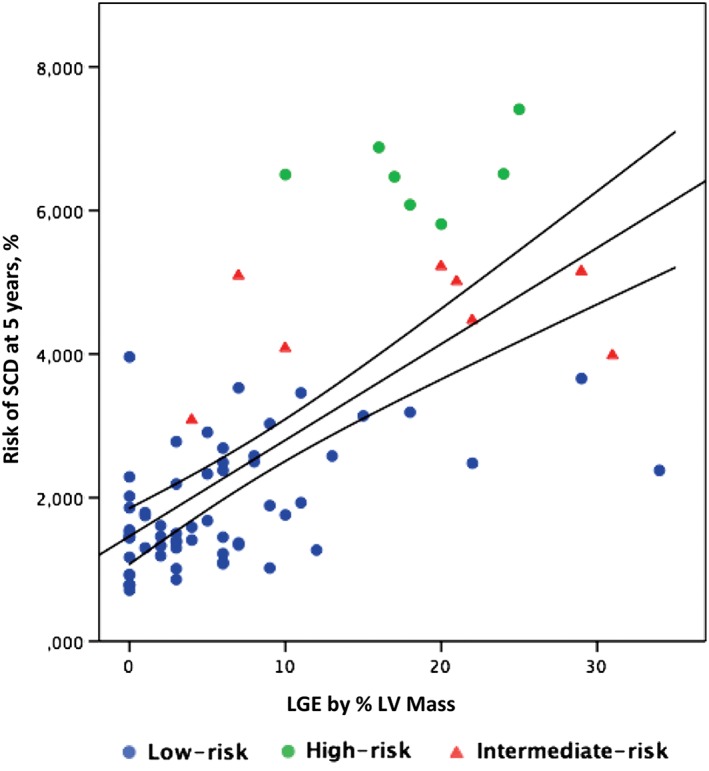
Positive correlation between the amount of LGE and the estimated risk of SCD at 5 years. Blue, low‐risk patients; red, intermediate‐risk patients; green, high‐risk patients. Abbreviations: LGE, late gadolinium enhancement; LV, left ventricular; SCD, sudden cardiac death.
The amount of LGE was predictive between low‐risk and intermediate‐risk + high‐risk groups (<4% and >4% risk) in binary logistic regression analysis independently of LVEF, LV mass, LA volume, and NYHA class, with an area under the curve (AUC) of 0.92 (95% confidence interval: [CI]: 0.86‐0.98; per 1% of LGE, Wald: 8.3, Exp(B): 1.22; P < 0.01). Furthermore, the amount of LGE was an independent predictor between low‐ and high‐risk groups (Wald: 8.99, Exp(B): 1.17; P < 0.001) and between low‐ and intermediate‐risk groups (Wald: 11.26, Exp(B): 1.16; P < 0.001); however, it did not aid prediction between intermediate‐ and high‐risk groups (P < 0.05).
According to conventional SCD risk assessment,1, 3 the amount of LGE was predictive of the presence of ≥1 risk factor (including LVWT ≥30 mm, unexplained syncope, family history of SCD, or evidence of NSVT) with an AUC of 0.84 (95% CI: 0.74‐0.93; per 1% of LGE, Wald: 14.2, Exp(B): 1.24; P < 0.001). Moreover, the amount of LGE was predictive of the presence of ≥2 risk factors with an AUC of 0.88 (95% CI: 0.80‐0.96; per 1% of LGE, Wald: 7.49, Exp(B): 1.12; P < 0.01).
3.2. Reproducibility
Intra‐ and interobserver agreement for the grayscale threshold method (≥6 SD) was high (intraobserver: coefficient of correlation: r = 0.99, P < 0.001, coefficient of variation: 5.2%; interobserver: r = 0.95, P < 0.001, coefficient of variation = 8.1%).
4. DISCUSSION
In a real‐world clinical scenario of patients with HCM, we provide a proof‐of‐concept that LGE extension is concordant with the SCD‐risk model proposed by current ESC guidelines. Although only a minority of patients at low risk exhibited extensive areas of LGE, nearly all of the patients in the high‐risk group showed ≥15% LGE. All patients in the intermediate‐risk and high‐risk groups showed any amount of LGE, suggesting that its absence could identify low‐risk patients, but its presence per se is not enough to define risk. We further show that none or trivial amounts of LGE are predictive of low risk of SCD according to the model. Our findings suggest an additive role of LGE imaging as an arbitrator after clinical risk assessment, particularly when an intermediate risk is estimated.
Identification of HCM patients without a previous history of lethal ventricular arrhythmias, who are at high risk of SCD, remains a challenge with serious clinical implications. Although ICD has proven to be effective in the primary prevention of sudden death in HCM,25, 26, 27 conventional risk stratification is incomplete. First, it does not identify all patients at risk, and second, it overestimates risk, resulting in inappropriate prophylactic ICD implantation,28 which on the whole underlines the importance of more precise identification of those patients at highest risk.
In 2014, a new SCD model was designed and validated with multicenter data, which resulted in higher discrimination compared with the stratification of conventional risk factors used in contemporary clinical practice.20 These results were validated in an external and independent cohort of 2 tertiary European centers.29 The score relies on complex mathematical and statistical modeling to estimate 5‐year risk of SCD. Previously, markers of risk were considered as binary clinical parameters (NSVT, severe hypertrophy, unexplained syncope, family history of SCD, and abnormal blood pressure response to exercise), but most of the parameters included in the new score take into account the continuous association with an increasing risk of SCD (age, LVWT, LA diameter, LVOT gradient). The latest 2014 ESC expert consensus guidelines proposed the new score as the primary method by which patients should be (or should not be) selected for prophylactic ICDs.1 The score stratifies 3 groups of risk: low, intermediate, and high risk, according to the estimated 5‐year risk of SCD (<4%, 4%–6%, and ≥6%, respectively). In the low‐risk group, ICD is “generally not indicated”; in the intermediate‐risk group, it “may be considered”; and in the high‐risk group, ICD “should be considered.” As a result, the new model has been largely incorporated into clinical practice; however definitive decision in intermediate‐risk patients remains challenging. The prognostic model was derived from a retrospective, multicenter longitudinal cohort study and, therefore, novel markers of risk such as amount of LGE have not been included for the moment.
In recent years, there has been an increasing interest in using LGE as a marker of risk. Ventricular arrhythmias represent the most likely mechanism of SCD in HCM and originate from regions of structurally abnormal myocardium. The extension of localized LGE significantly correlates with both the corresponding depolarizing and repolarizing electrical damage causing ventricular arrhythmias in HCM.12 Although prospective outcome studies have demonstrated an association between the presence of LGE and a combined endpoint of adverse HCM‐related events,13, 14, 15, 16, 17, 30 these studies have conflicting results regarding the relation between LGE and SCD. When data from these studies were pooled in a meta‐analysis, LGE showed a nonsignificant trend toward the detection of SCD/aborted SCD.17 As a result, available data did not definitively support its inclusion in current ESC recommendations. HCM is generally a low‐event‐rate disease, and therefore demonstrating novel (and independent to conventional ones) risk markers is a challenging task. Initial CMR studies in HCM were focused largely on the association between the presence of LGE as a binary variable and adverse outcomes, with most of them failing in demonstrating it to be independently predictive of SCD risk. However, any amount of LGE per se cannot be considered a risk marker, because this designation attributes equal predictive weight to a broad spectrum of LGE amounts (from trivial to extensive). Furthermore, HCM exhibits LGE in 60% to 80% of cases, suggesting that its presence represents a more diagnostic than prognostic parameter. Recent evidence demonstrated that SCD event risk increased in a continuous and direct manner with respect to the extent of LGE.18 Chan et al showed that when %LGE was considered together with each of the conventional risk markers, the incremental prognostic value in predicting SCD events was significantly increased. Extensive areas of LGE (≥15% of LV mass) demonstrated a 2‐fold increase in SCD event risk in those patients otherwise considered to be at lower risk by conventional risk stratification, with an estimated likelihood for SCD events of 6% at 5 years. Performance of the SCD event risk model was enhanced by LGE. The additive prognostic relevance of LGE extension has been proven in other scenarios of ischemic and nonischemic cardiomyopathies; however, the lack of clear standardization and definition of %LGE limit its implementation in clinical practice.9, 31, 32, 33, 34
The novel SCD model has been questioned, in part for the lack of novel risk markers such as LGE.7 Our study demonstrates that the amount of LGE is concordant with the new model defining low‐risk and high‐risk groups; the balanced presence of extensive areas of LGE in intermediate‐risk patients defines a subgroup of HCM patients where LGE imaging (its amount) may resolve complex decisions regarding ICDs. Potentially, %LGE may provide additional information (none, minimal, or extensive myocardial fibrosis), tilting the balance in favor of or against an ICD indication.
Although the novel ESC risk‐stratification algorithm seems to outperform previous strategies, the decision to indicate an ICD or not remains challenging, and the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) HCM guidelines still are commonly applied by many HCM clinics in addition to individual clinical judgment. The evidence of patients scored with lowest risk but experiencing SCD7 warrants further attention. In our cohort, 4 patients in this group showed extensive fibrosis, one of them with an apical aneurysm and 3 of them with ≥1 positive risk factors according to traditional risk algorithms.3 These patients would probably benefit from ICD placement, according to latest AHA recommendations.
Furthermore, our results show that the amount of LGE is related to most of the individual risk factors supported by ACCF/AHA HCM guidelines and significantly predicts the presence of 1 or ≥2 risk factors.
Greater insights into the question of myocardial fibrosis and SCD risk will be investigated through the emergence of novel T1‐mapping techniques. Native T1 can accurately detect diffuse myocardial substrate in HCM, which is underestimated by LGE, allowing the discrimination with other cardiomyopathies; native T1 is a strong and independent predictor of adverse cardiovascular outcomes in nonischemic cardiomyopathies.35, 36, 37 Additional limitations of the model, such as the use of LA anteroposterior diameter instead of LA volume index or even LA function, also may be resolved in futures studies.
4.1. Study limitations
This represents an observational and descriptive study with a relatively small number of patients. Our present initiative does not attempt to associate LGE with SCD or validate the new ESC method of risk stratification; rather, it is intended to describe the LGE findings after applying the new stratification score in a real‐world scenario of HCM. Given the concordance with low‐ and high‐risk groups, our results suggest that LGE may play the role of arbitrator in subgroups of HCM patients with ambiguous individual risk. Given the lack of prognostic data and the small number of patients in this intermediate‐risk group, our results should be confirmed in future studies. The proportionally low percentage of patients in the intermediate‐ and high‐risk groups represents the current clinical scenario and may explain the lack of significant differences in the extent of LGE on regression analysis.
There are different techniques for LGE quantification. The high grayscale threshold method used in this study has shown high reproducibility and has been validated by histopathology, providing the best representation of total fibrosis burden.24, 38 Most important, Chan et al, whose results have been used as the background to define the LGE extension according to the SCD risk, applied this grayscale threshold method in their study.18
5. CONCLUSION
LGE extension is concordant with the SCD risk model defining low‐ and high‐risk groups. An extensive area of LGE identifies higher‐risk patients, whereas minimal LGE or the absence of LGE predicts low individual risk. In intermediate‐risk patients, it may provide additional information, allowing better discrimination to support an ICD decision. With the growing penetration of CMR into clinical cardiovascular practice, LGE quantification holds promise for SCD stratification in HCM.39
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Supplementary material.
Table S1. Detail characteristics of low‐risk patients according to ESC model but extensive amount of LGE. LA: left atrial. Max LVWT: maximum left ventricular wall thickness. LVOT: Left ventricular outflow tract. FH of SCD: Family history of sudden cardiac death. NSVT: non‐sustained ventricular tachycardia during 24–48 hour ambulatory ECG monitoring. NS: not significant
Hinojar R, Zamorano JL, Gonzalez Gómez A, et al. ESC sudden‐death risk model in hypertrophic cardiomyopathy: Incremental value of quantitative contrast‐enhanced CMR in intermediate‐risk patients. Clin Cardiol. 2017;40:853–860. 10.1002/clc.22735
REFERENCES
- 1. Elliott PM, Anastasakis A, Borger MA, et al. ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy [published correction appears in Circulation. 2010;122:e7]. Circulation. 2010;121:445–456. [DOI] [PubMed] [Google Scholar]
- 3. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA Guidelines for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:e212–e260. [DOI] [PubMed] [Google Scholar]
- 4. Elliott PM, Poloniecki J, Dickie S, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. [DOI] [PubMed] [Google Scholar]
- 5. Maron BJ, Maron MS, Lesser JR, et al. Sudden cardiac arrest in hypertrophic cardiomyopathy in the absence of conventional criteria for high risk status. Am J Cardiol. 2008;101:544–547. [DOI] [PubMed] [Google Scholar]
- 6. Bongioanni S, Spirito P, Masi AS, et al. Extensive myocardial fibrosis in a patient with hypertrophic cardiomyopathy and ventricular tachycardia without traditional high‐risk features. Circ Cardiovasc Imaging. 2009;2:349–350. [DOI] [PubMed] [Google Scholar]
- 7. Maron BJ, Casey SA, Chan RH, et al. Independent assessment of the European Society of Cardiology sudden death risk model for hypertrophic cardiomyopathy. Am J Cardiol. 2015;116:757–764. [DOI] [PubMed] [Google Scholar]
- 8. Mewton N, Liu CY, Croisille P, et al. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy [published correction appears in JAMA. 2013;310:99]. JAMA. 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 10. Cardim N, Galderisi M, Edvardsen T, et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging. 2015;16:280. [DOI] [PubMed] [Google Scholar]
- 11. Adabag AS, Maron BJ, Appelbaum E, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–1374. [DOI] [PubMed] [Google Scholar]
- 12. Sakamoto N, Kawamura Y, Sato N, et al. Late gadolinium enhancement on cardiac magnetic resonance represents the depolarizing and repolarizing electrically damaged foci causing malignant ventricular arrhythmia in hypertrophic cardiomyopathy. Heart Rhythm. 2015;12:1276–1284. [DOI] [PubMed] [Google Scholar]
- 13. Maron MS, Appelbaum E, Harrigan CJ, et al. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail. 2008;1:184–191. [DOI] [PubMed] [Google Scholar]
- 14. O'Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. [DOI] [PubMed] [Google Scholar]
- 15. Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875–887. [DOI] [PubMed] [Google Scholar]
- 16. Rubinshtein R, Glockner JF, Ommen SR, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast‐enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51–58. [DOI] [PubMed] [Google Scholar]
- 17. Green JJ, Berger JS, Kramer CM, et al. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370–377. [DOI] [PubMed] [Google Scholar]
- 18. Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast‐enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–495. [DOI] [PubMed] [Google Scholar]
- 19. Ismail TF, Jabbour A, Gulati A, et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart. 2014;100:1851–1858. [DOI] [PubMed] [Google Scholar]
- 20. O'Mahony C, Jichi F, Pavlou M, et al; Hypertrophic Cardiomyopathy Outcomes Investigators . A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk‐SCD). Eur Heart J . 2014;35:2010–2020. [DOI] [PubMed] [Google Scholar]
- 21. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 22. Kramer CM, Barkhausen J, Flamm SD, et al; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols . Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson . 2013;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schulz‐Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson. 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrigan CJ, Peters DC, Gibson CM, et al. Hypertrophic cardiomyopathy: quantification of late gadolinium enhancement with contrast‐enhanced cardiovascular MR imaging. Radiology. 2011;258:128–133. [DOI] [PubMed] [Google Scholar]
- 25. Maron BJ, Spirito P, Shen WK, et al. Implantable cardioverter‐defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy [published correction appears in JAMA. 2007;298:1516]. JAMA. 2007;298:405–412. [DOI] [PubMed] [Google Scholar]
- 26. Maron BJ, Spirito P, Ackerman MJ, et al. Prevention of sudden cardiac death with implantable cardioverter‐defibrillators in children and adolescents with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;61:1527–1535. [DOI] [PubMed] [Google Scholar]
- 27. Maron BJ, Shen WK, Link MS, et al. Efficacy of implantable cardioverter‐defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med. 2000;342:365–373. [DOI] [PubMed] [Google Scholar]
- 28. O'Mahony C, Tome‐Esteban M, Lambiase PD, et al. A validation study of the 2003 American College of Cardiology/European Society of Cardiology and 2011 American College of Cardiology Foundation/American Heart Association risk stratification and treatment algorithms for sudden cardiac death in patients with hypertrophic cardiomyopathy. Heart. 2013;99:534–541. [DOI] [PubMed] [Google Scholar]
- 29. Vriesendorp PA, Schinkel AF, Liebregts M, et al. Validation of the 2014 European Society of Cardiology guidelines risk prediction model for the primary prevention of sudden cardiac death in hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol. 2015;8:829–835. [DOI] [PubMed] [Google Scholar]
- 30. Hinojar R, Botnar R, Kaski JC, et al. Individualized cardiovascular risk assessment by cardiovascular magnetic resonance. Future Cardiol. 2014;10:273–289. [DOI] [PubMed] [Google Scholar]
- 31. Kuruvilla S, Adenaw N, Katwal AB, et al. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta‐analysis. Circ Cardiovasc Imaging. 2014;7:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. [DOI] [PubMed] [Google Scholar]
- 33. Kancharla K, Weissman G, Elagha AA, et al. Scar quantification by cardiovascular magnetic resonance as an independent predictor of long‐term survival in patients with ischemic heart failure treated by coronary artery bypass graft surgery. J Cardiovasc Magn Reson. 2016;18:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neilan TG, Coelho‐Filho OR, Danik SB, et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:944–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Puntmann VO, Voigt T, Chen Z, et al. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:475–484. [DOI] [PubMed] [Google Scholar]
- 36. Hinojar R, Varma N, Child N, et al. T1 mapping in discrimination of hypertrophic phenotypes: hypertensive heart disease and hypertrophic cardiomyopathy: findings from the International T1 Multicentre Cardiovascular Magnetic Resonance Study. Circ Cardiovasc Imaging . 2015;8:pii:e003285. [DOI] [PubMed] [Google Scholar]
- 37. Puntmann VO, Carr‐White G, Jabbour A, et al; International T1 Multicentre CMR Outcome Study . T1‐mapping and outcome in nonischemic cardiomyopathy: all‐cause mortality and heart failure. JACC Cardiovasc Imaging . 2016;9:40–50. [DOI] [PubMed] [Google Scholar]
- 38. Moravsky G, Ofek E, Rakowski H, et al. Myocardial fibrosis in hypertrophic cardiomyopathy: accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging. 2013;6:587–596. [DOI] [PubMed] [Google Scholar]
- 39. von Knobelsdorff‐Brenkenhoff F, Schulz‐Menger J. Role of cardiovascular magnetic resonance in the guidelines of the European Society of Cardiology. J Cardiovasc Magn Reson. 2016;18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Table S1. Detail characteristics of low‐risk patients according to ESC model but extensive amount of LGE. LA: left atrial. Max LVWT: maximum left ventricular wall thickness. LVOT: Left ventricular outflow tract. FH of SCD: Family history of sudden cardiac death. NSVT: non‐sustained ventricular tachycardia during 24–48 hour ambulatory ECG monitoring. NS: not significant


