Abstract
Background
Inflammation has a key role in the process of atherosclerosis. Production of leukotrienes by 5‐lipoxygenase has been linked to atherosclerotic plaques and cardiovascular events.
Hypothesis
In this study, a selective 5‐LO inhibitor will slow plaque progression using serial cardiac computed tomographic angiography (CCTA).
Methods
Patients with recent acute coronary syndrome (ACS) were prospectively assigned to one of 3 VIA‐2291 doses (25 mg, 50 mg, 100 mg) or placebo by oral administration. All groups underwent CCTA at baseline and at 6 months’ follow‐up. Plaque types such as low‐attenuation plaque (LAP), fibro‐fatty tissue (FF), fibro‐calcified plaque (FC), and dense calcium plaque (DC) were measured based upon predefined density threshold, and changes from baseline CCTA were analyzed.
Results
The final analysis included 54 patients (age, 56 ± 9 years; 85.1% male) with CCTA at baseline and 24 weeks. Evaluating on treatment VIA‐2291 (all 3 doses, n = 37) demonstrated significant reductions in plaque progression compared with placebo (n = 17). VIA‐2291 significantly reduced LAP (5.9 ± 20.7 mm3 vs −9.7 ± 33.3 mm3), FF (11.1 mm3 ± 13.3 mm3 vs −0.9 ± 2.7 mm3), and FC (−0.1 ± 6.22 mm3 vs −14.3 ± 6.2 mm3; all P < 0.05) and retarded the progression of DC (3.9 ± 3.2 mm3 vs 0.2 ± 0.4 mm3) compared with placebo.
Conclusions
VIA‐2291 resulted in slowed plaque progression compared with placebo across different plaque subtypes in patients with recent ACS (http://ClinicalTrials.gov NCT00358826).
Keywords: Imaging, computed tomography, Clinical trials, Ischemic heart disease, acute coronary syndromes
1. INTRODUCTION
Traditional cardiovascular therapy, such as aspirin and statins, improves outcomes in patients with atherosclerotic cardiovascular disease (ASCVD). Patients with recent acute coronary syndrome (ACS), however, still have residual cardiovascular risk even with these treatments.1
In the process of atherosclerosis, inflammation has a key role. Atherosclerotic inflammation is thought to be an outcome of various biochemical processes, including one that involves leukotrienes (LTs), which is a class of eicosanoids, and its enzymatic regulator, 5‐lipoxgenase (5‐LO).2, 3, 4 A previous study demonstrated that a 5‐LO inhibitor (atreleuton, VIA‐2291) can significantly reduce levels of leukotriene B4 and leukotriene E4 in humans (VIA‐ACS study).5 In their study, the effect of VIA‐2291 on coronary artery plaque volume using cardiac computed tomography angiography (CCTA) was also evaluated. However, only patients with low‐attenuation plaque (LAP; density <60 Hounsfield units [HU]) and good CT image quality were evaluated in their study, leading to a sample of only 34 patients out of an initial 60. Given the recent improvements in imaging techniques and analysis, advanced identification of plaque composition, including fibro‐fatty plaque (FF), fibro‐calcified plaque (FC), and dense calcified plaque (DC), can be identified by CCTA, along with LAP.6
The objective of this study is to evaluate the effect of VIA‐2291 on various coronary plaque types using current CCTA methodology and new algorithms to quantitatively measure plaque.
2. METHODS
2.1. Study population
The current study design, including major inclusion and exclusion criteria, has been described previously.5 In brief, men and women age 30 to 80 years with an acute myocardial infarction or unstable angina 21 (±3) days before study enrollment were randomized. Patients were randomly assigned to receive a once‐daily dose of placebo or VIA‐2291 (atreleuton) at 25 mg, 50 mg, or 100 mg for 12 weeks. Patients who consented to undergo CCTA were extended to 24 weeks and underwent CCTA at baseline and follow‐up. Patients currently enrolled in another trial within 3 months of enrollment were excluded. The institutional review board at each center approved this study protocol, and all patients signed written informed consent.
2.2. CT acquisition
A 64‐slice multidetector CT scanner (GE LightSpeed VCT; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) was used to acquire images, as previously reported.5 Prior to the scan, β‐blockers were administered to achieve a resting heart rate <65 bpm. Sublingual nitroglycerine 0.4 mg was also administered before each scan. Contrast‐enhanced CT1 examination was performed using a triphasic bolus of 60 mL of iodinated contrast material (iodixanol 320), followed by 50 mL of mixed contrast (50%) and saline (50%), and finally followed by 25 mL of saline injected into antecubital vein at 5 mL/s. The following imaging and reconstruction parameters were applied: collimation, 64 × 0.625 mm; tube voltage, 120 kV; tube current, 550 to 800 mA (depending on patient weight). Retrospective electrocardiographic gating studies were performed from 0% to 90% of the R‐R interval at 10% increments. Automatic reconstructions of the 75% phase were also obtained.
2.3. Coronary plaque assessment
All CCTA images were evaluated at an independent core laboratory (Los Angeles Biomedical Research Institute at Harbor–UCLA, Los Angeles, California). The data were transferred to the semiautomated vessel extraction software, Vitrea SUREPlaque, version 4.0 (Vital Images, Minnetonka, Minnesota). Studies were blinded for VIA‐2291 and for placebo use, allowing expert readers to assess the coronary arteries objectively.
Automated software was used to identify plaque within the coronary system. The software traced the contours of the outer vessel wall and inner lumen. In each case we manually modified each trace to exactly fit the inner lumen and vessel wall. Any vessels with a diameter of ≥2.0 mm were evaluated and assessed based on the 17‐segment American Heart Association coronary tree model.7 We excluded those segments that could not be evaluated because of severe artifacts on either serial scans. Any segments with stents were also omitted. We identified plaque locations and used an automated plaque‐detection tool to measure plaque volume. Coronary plaque volumes, LAP, FF, FC, and DC were calculated by predefined HU8 threshold. The fixed HU cutoff used for classification were −30 to 30 for LAP, 31 to 130 for FF, 131 to 350 for FC, and >350 for DC. The overall change for each plaque type at follow‐up from baseline was calculated for each patient. The intraobserver and interobserver variability for plaque quantification using this software already have been published.9
2.4. Statistical analysis
Continuous variables were expressed as mean ± SD. The plaque volume changes from baseline CCTA after 6 months were evaluated for each study group. Additionally, placebo was compared against each of the 3 VIA‐2291 groups individually and also against all VIA‐2291 groups together using the Student t test. Categorical variables were expressed as numbers and percentages; the χ2 test was used for comparisons between placebo and each VIA‐2291 group. A P value of <0.05 was considered statistically significant. All statistical analyses ware performed using SAS software, version 9.3 (SAS Institute, Inc., Cary, North Carolina).
3. RESULTS
3.1. Baseline characteristics
Of the 191 patients enrolled in this VIA‐ACS study, 60 patients underwent CCTA both at baseline and 24 weeks. We excluded 6 patients from the analysis because of imaging artifacts due to excessive calcium or motion. Ultimately, this gave us a total of 54 patients to analyze for this study (Figure). The baseline patient characteristics are presented in Table 1. Among the VIA‐2291 and placebo groups, there were no significant differences in baseline characteristics.
Table 1.
Baseline characteristics of the study population
| Placebo, n = 17 | VIA‐2291 25 mg, n = 12 | VIA‐2291 50 mg, n = 12 | VIA‐2291 100 mg, n = 13 | All VIA‐2291 Groups, n = 37 | |
|---|---|---|---|---|---|
| Age, y | 55.1 ± 8.3 | 57.6 ± 8.9 | 55.4 ± 9.7 | 54.5 ± 8.2 | 55.8 ± 8.8 |
| Male sex | 13 (76.5) | 10 (83.3) | 11 (91.7) | 12 (92.3) | 33 (89.2) |
| Risk factors | |||||
| HTN | 12 (70.6) | 5 (40.7) | 10 (83.3) | 11 (84.6) | 26 (70.3) |
| Smoking | 9 (60.0) | 5 (71.4) | 10 (83.3) | 8 (72.7) | 23 (76.7) |
| Hyperlipidemia | 4 (26.7) | 0 (0) | 1 (8.33) | 2 (18.2) | 3 (10) |
| DM | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Medications | |||||
| ASA | 17 (100) | 11 (91.7) | 12 (100) | 13 (100) | 36 (97.3) |
| Clopidogrel | 17 (100) | 11 (91.7) | 12 (100) | 13 (100) | 36 (97.3) |
| β‐Blocker | 17 (100) | 10 (83.3) | 10 (83.3) | 13 (100) | 33 (89.2) |
| ACEIs | 9 (52.9) | 4 (33.3) | 9 (75.0) | 10 (76.9) | 23 (62.3) |
| ARBs | 3 (17.7) | 1 (8.3) | 1 (8.3) | 2 (15.4) | 4 (10.8) |
| Statins | 17 (100) | 12 (100) | 12 (100) | 13 (100) | 37 (100) |
| Blood pressure, mm Hg | |||||
| SBP | 111.6 ± 3.2 | 111.5 ± 8.6 | 119.9 ± 13.7 | 111.0 ± 4.0 | 114.0 ± 2.2 |
| DBP | 67.0 ± 9.5 | 66.3 ± 2.5 | 73.3 ± 6.8 | 68.3 ± 8.7 | 69.3 ± 8.0 |
| Lipid values, mg/dL | |||||
| Total cholesterol | 137.01 ± 38.1 | 123.6 ± 22.8 | 111.3 ± 34.9 | 133.9 ± 8.3 | 123.55 ± 30.3 |
| LDL‐C | 85 ± 40.2 | 63.2 ± 16.5 | 63 ± 24.3 | 78.2 ± 20.0 | 68.5 ± 21.2 |
| HDL‐C | 36.8 ± 8.7 | 37.1 ± 14.4 | 34.7 ± 10.4 | 32.0 ± 5.1 | 34.5 ± 10.4 |
| TG | 147.8 ± 69.3 | 192.1 ± 50.0 | 152.4 ± 82.8 | 169.2 ± 83.0 | 171.2 ± 117.6 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid (aspirin); DBP, diastolic blood pressure; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation; TG, triglycerides; VIA‐2291, atreleuton.
Data are presented as n (%) or mean ± SD.
Figure 1.
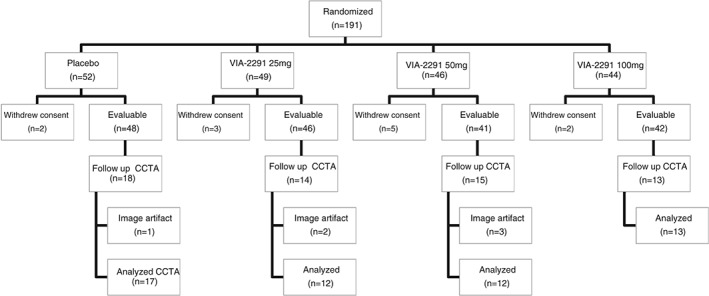
Flow diagram of patient enrollment in the study. Abbreviations: CCTA, cardiac computed tomography angiography; VIA‐2291, atreleuton.
3.2. Plaque characteristics at baseline and follow‐up, and the change among placebo and VIA‐2291 groups
The baseline plaque data are presented in Table 2. FF plaque was significantly higher in the VIA‐2291 (50 mg) group compared with the placebo group (P = 0.023) at baseline. However, there was no significant difference between the placebo and VIA‐2291 groups for other baseline plaque types. The changes in each plaque type over 6 months in the placebo and VIA‐2291 groups are demonstrated in Table 2. Evaluating on treatment VIA‐2291 (all 3 doses, n = 37) demonstrated significant reductions in plaque progression compared with placebo (n = 17). VIA‐2291 significantly reduced LAP (5.9 ± 20.7 mm3 vs −9.7 ± 33.3 mm3), FF (11.1 mm3 ± 13.3 mm3 vs −0.9 ± 2.7 mm3), and FC (−0.1 ± 6.22 mm3 vs −14.3 ± 6.2 mm3; all P < 0.05) and retarded the progression of DC (3.9 ± 3.2 mm3 vs 0.2 ± 0.4 mm3), when compared with placebo. No significant difference was seen in plaque volume changes between placebo and the 3 individual doses of VIA‐2291 due to the small sample size.
Table 2.
Change in each plaque characteristic after 24 weeks of placebo vs VIA‐2291 in patients with recent ACS
| Placebo, n = 17 | VIA‐2291 25 mg, n = 12 | VIA‐2291 50 mg, n = 12 | VIA‐2291 100 mg, n = 13 | All VIA‐2291 groups, n = 37 | |
|---|---|---|---|---|---|
| LAP, mm3 | |||||
| Baseline | 47.2 ± 74.7 | 54 ± 60.4 | 75.9 ± 71.7 | 36.0 ± 27.2 | 54.8 ± 10.3 |
| Follow‐up | 53.9 ± 93.4 | 36.3 ± 31.1 | 67.4 ± 62.6 | 35.3 ± 29.3 | 46.4 ± 44.8 |
| Change from baseline | 5.9 ± 20.7 | −17.7 ± 52.6 | −8.1 ± 16.9 | −0.7 ± 9.4 | −9.7 ± 33.3 |
| P value vs placebo | 0.10 | 0.06 | 0.27 | <0.05 | |
| FF, mm3 | |||||
| Baseline | 39.2 ± 32.1 | 33.1 ± 22.0 | 79.3 ± 57.2a | 39.6 ± 32.5 | 50.3 ± 43.8 |
| Follow‐up | 50.3 ± 69.7 | 27.4 ± 16.7 | 80.6 ± 52.1 | 42.8 ± 35.8 | 50.1 ± 42.9 |
| Change from baseline | 11.1 ± 13.3 | −5.7 ± 15.8 | 1.4 ± 14.5 | 3.3 ± 19.0 | −0.9 ± 2.7 |
| P value vs placebo | 0.25 | 0.55 | 0.62 | <0.05 | |
| FC, mm3 | |||||
| Baseline | 75.5 ± 87.9 | 83.3 ± 78.7 | 88.3 ± 44.2 | 115.1 ± 88.5 | 96.3 ± 13.1 |
| Follow‐up | 75.3 ± 86.8 | 81.0 ± 80.0 | 64.8 ± 79.8 | 93.8 ± 56.8 | 80.2 ± 71.6 |
| Change from baseline | −0.1 ± 6.22 | −2.3 ± 26.8 | −16.1 ± 46.5 | −21.3 ± 42.3 | −14.3 ± 6.2 |
| P value vs placebo | 0.82 | 0.24 | 0.10 | <0.05 | |
| DC, mm3 | |||||
| Baseline | 0.8 ± 1.4 | 2.1 ± 3.2 | 1.0 ± 1.9 | 5.2 ± 9.0 | 2.8 ± 5.9 |
| Follow‐up | 4.7 ± 13.3 | 2.0 ± 3.3 | 0.8 ± 1.4 | 5.0 ± 9.0 | 2.7 ± 5.8 |
| Change from baseline | 3.9 ± 3.2 | −0.2 ± 2.3 | −0.2 ± 1.0 | −0.2 ± 2.4 | 0.2 ± 0.4 |
| P value vs placebo | 0.30 | 0.30 | 0.29 | <0.05 |
Abbreviations: ACS, acute coronary syndrome; DC, dense calcified plaque; FC, fibro‐calcified plaque; FF, fibro‐fatty plaque; LAP, low‐attenuation plaque; SD, standard deviation.
Data are presented as mean ± SD.
Different from placebo (P < 0.05).
4. DISCUSSION
This is one of the first studies to evaluate changes in atherosclerosis progression under the influence of an anti‐inflammatory agent. The progression of atherosclerosis occurs from the accumulation of lipid within the intima, which is followed by inflammation. Factors such as 5‐LOs have one of the most important roles in the inflammatory process and in the formation of vulnerable plaque because of their function in producing and regulating levels of LTs.2, 3, 4 Therefore, it is reasonable to identify 5‐LO as a predictor of atherosclerosis.
Despite the numerous established therapies, CAD still remains the leading cause of morbidity and mortality worldwide. Therefore, novel prediction strategies that also target various aspects of atherosclerosis, including the inflammation process, are thought to be needed. Furthermore, because VIA‐2291 has shown promise in reducing the inflammatory nature of other disorders,10 it may serve as a useful to tool in reducing the inflammatory process associated with atherosclerotic progression.
This current study demonstrated that VIA‐2291 significantly reduced each component of noncalcified plaque, including LAP, FC, and FF, and retarded the progression of DC, compared with placebo at 6‐month follow‐up. This is consistent with the main VIA‐ACS study, which showed significantly reduced levels of leukotriene E4 and leukotriene B4 after VIA‐2291 therapy. These LT markers are thought to be good predictors of atherosclerosis.5
Necrotic core, which is a component of plaque, is formed by the necrosis of foam cells phagocytosing pools of lipids within the intima.11 Pathological studies using intravascular ultrasound (IVUS) have shown that the culprit lesion of ACS likely has a lipid‐rich necrotic core, which is covered by a thin fibrous cap.1 Furthermore, previous studies comparing CCTA and virtual histology IVUS showed that necrotic core is observed as a LAP using CCTA,6 which is well known as a feature of vulnerable plaque.12 This current study demonstrated that combined VIA‐2291 significantly reduced the LAP; therefore, it is expected that VIA‐2291 may stabilize the plaque by reducing the LAP. On the contrary, we could not show the dose‐dependent effect of VIA‐2291 on LAP. The lowest dose of VIA‐2291 demonstrated the maximum effect on the LAP. Several effects might be included, such as baseline characteristics of the patient, medication use, and baseline plaque volume. Further prospective study with a larger sample size is needed.
Although the effect of FF, FC, and DC on the cardiovascular system is still unclear, these plaque types are considered to be vulnerable especially when they are presented in large quantities within in the coronaries. Previous IVUS studies have indicated that the progression of plaque volume can increase a patient's risk of an adverse cardiovascular event.8 The current study found that patients on VIA‐2291 showed a decrease in plaque progression for each plaque type, which may have a favorable effect on prevention of ASCVD.
Regarding the change of DC, despite the favorable effect of the VIA‐2291, almost no change was seen in each VIA‐2291 group. Our group has previously investigated the effect of statin treatment on coronary plaque, and DC also was not changed significantly in the statin group, even after 1 year of follow‐up.13 Thus, dense calcification may be stabilizing and not associated with cardiovascular risk.
Given the improvement in workstations for analyzing plaque volume using CCTA, we were able to evaluate more plaque types in this study and in larger numbers of participants than the prior VIA‐ACS study. Although IVUS is traditionally used for evaluating the efficacy of medication or other interventions, CCTA also can be employed to noninvasively evaluate almost all segments of the coronary arteries, with the exception of small distal vessels. CCTA has been used in various other studies to understand the natural history of coronary artery plaque14 and also the effect of statins on plaque progression.13, 15 This current study further supports the existing literature on the utility of CCTA in understanding the nature of plaque and its usefulness in evaluating the outcome of such interventions on atherosclerosis.
4.1. Study limitations
The present analysis has several limitations. First, this study did not have enough power to show the dose‐dependent effect of VIA‐2291 due to its small sample size and relatively short time until follow‐up. Second, all participants began this study after initial ACS diagnosis and subsequent treatment. Because many treatments exist for ACS, our study participants were on various medications before and after study enrollment. A recent study investigated the effect of VIA‐2291 on vascular inflammation using fluorodeoxyglucose–positron emission tomography and did not show a significant anti‐inflammatory effect of VIA‐2291.16 This may be due to severe arterial inflammation after ACS, but the authors could not exclude the potential effect of anti‐inflammatory changes of statin therapy. Thus, although there was no significant difference between placebo and the VIA‐2291 group in baseline characteristics, we cannot exclude the effect of such medications on plaque progression. Third, there were nonsignificant differences in groups regarding baseline characteristics (fewer men, fewer smokers, more hyperlipidemia, higher low‐density lipoprotein cholesterol, and lower triglycerides in the placebo group). There also was a relatively wide range regarding baseline plaque volume. We did not perform a multivariable analysis considering these differences because of small sample size; thus, we cannot exclude the effect of such differences. Finally, we used a novel method to measure plaque, using semiautomated plaque quantification software, and highly reproducible results were reported with this software.17, 18 Moreover, advancements in image acquisition, image processing, and software improvements on newer CT scanners can potentially improve the ability of CCTA to track atherosclerosis. Therefore, larger sample‐size outcome studies are needed to conclusively demonstrate the efficacy of VIA‐2291 on ASCVD.
5. CONCLUSION
This is the first anti‐inflammatory demonstrating a positive effect on plaque progression in a double‐blinded prospective study. VIA‐2291 resulted in lower plaque progression compared with placebo across different plaque subtypes on serial CCTA over 6 months. Further studies are needed to evaluate whether VIA‐2291 has the ability to stabilize vulnerable plaque and to decrease ASCVD.
Conflicts of interest
Dr. Budoff and Dr. Tardif receive funding from Tallikut Pharmaceuticals. Drs. Matsumoto, Ibrahim, Grégoire, L'Allier, and Pressacco report no conflicts of interest.
Matsumoto S, Ibrahim R, Grégoire JC, L'Allier PL, Pressacco J, Tardif J‐C and Budoff MJ. Effect of treatment with 5‐lipoxygenase inhibitor VIA‐2291 (atreleuton) on coronary plaque progression: a serial CT angiography study, Clin Cardiol, 2016. doi: 10.1002/clc.22646
REFERENCES
- 1. Stone GW, Maehara A, Lansky AJ, et al; PROSPECT Investigators . A prospective natural‐history study of coronary atherosclerosis [published correction appears in N Engl J Med. 2011;365:2040]. N Engl J Med. 2011;364:226–235. [DOI] [PubMed] [Google Scholar]
- 2. Spanbroek R, Grabner R, Lotzer K, et al. Expanding expression of the 5‐lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci U S A. 2003;100:1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dwyer JH, Allayee H, Dwyer KM, et al. Arachidonate 5‐lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. [DOI] [PubMed] [Google Scholar]
- 4. Bäck M. Inflammatory signaling through leukotriene receptors in atherosclerosis. Curr Atheroscler Rep. 2008;10:244–251. [DOI] [PubMed] [Google Scholar]
- 5. Tardif JC, L'Allier PL, Ibrahim R, et al. Treatment with 5‐lipoxygenase inhibitor VIA‐2291 (Atreleuton) in patients with recent acute coronary syndrome. Circ Cardiovasc Imaging. 2010;3:298–307. [DOI] [PubMed] [Google Scholar]
- 6. Motoyama S, Kondo T, Anno H, et al. Atherosclerotic plaque characterization by 0.5‐mm‐slice multislice computed tomographic imaging. Circ J. 2007;71:363–366. [DOI] [PubMed] [Google Scholar]
- 7. Raff GL, Abidov A, Achenbach S, et al; Society of Cardiovascular Computed Tomography. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr . 2009;3:122–136. [DOI] [PubMed] [Google Scholar]
- 8. Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound–derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol. 2010;55:2399–2407. [DOI] [PubMed] [Google Scholar]
- 9. Hamirani YS, Zeb I, Pagali SR, et al. Normalization of automatic plaque quantification in cardiac computed tomography (CCT). Int J Cardiol. 2011;146:282–290. [DOI] [PubMed] [Google Scholar]
- 10. Lehnigk B, Rabe KF, Dent G, et al. Effects of a 5‐lipoxygenase inhibitor, ABT‐761, on exercise‐induced bronchoconstriction and urinary LTE4 in asthmatic patients. Eur Respir J. 1998;11:617–623. [PubMed] [Google Scholar]
- 11. Falk E, Nakano M, Bentzon JF, et al. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34:719–728. [DOI] [PubMed] [Google Scholar]
- 12. Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. [DOI] [PubMed] [Google Scholar]
- 13. Zeb I, Li D, Nasir K, et al. Effect of statin treatment on coronary plaque progression—a serial coronary CT angiography study. Atherosclerosis. 2013;231:198–204. [DOI] [PubMed] [Google Scholar]
- 14. Papadopoulou SL, Neefjes LA, Garcia‐Garcia HM, et al. Natural history of coronary atherosclerosis by multislice computed tomography. JACC Cardiovasc Imaging. 2012;(3 suppl):S28–S37. [DOI] [PubMed] [Google Scholar]
- 15. Inoue K, Motoyama S, Sarai M, et al. Serial coronary CT angiography–verified changes in plaque characteristics as an endpoint: evaluation of effect of statin intervention. JACC Cardiovasc Imaging. 2010;3:691–698. [DOI] [PubMed] [Google Scholar]
- 16. Gaztanaga J, Farkouh M, Rudd JH, et al. A phase 2 randomized, double‐blind, placebo‐controlled study of the effect of VIA‐2291, a 5‐lipoxygenase inhibitor, on vascular inflammation in patients after an acute coronary syndrome. Atherosclerosis. 2015;240:53–60. [DOI] [PubMed] [Google Scholar]
- 17. Matsumoto S, Nakanishi R, Li D, et al. Aged garlic extract reduces low attenuation plaque in coronary arteries of patients with metabolic syndrome in a prospective randomized double‐blind study. J Nutr. 2016;146:427S–432S. [DOI] [PubMed] [Google Scholar]
- 18. Papadopoulou SL, Garcia‐Garcia HM, Rossi A, et al. Reproducibility of computed tomography angiography data analysis using semiautomated plaque quantification software: implications for the design of longitudinal studies. Int J Cardiovasc Imaging. 2013;29:1095–1104. [DOI] [PubMed] [Google Scholar]


