Abstract
Frequent premature atrial complexes (PACs) are universal in the general population; however, their clinical significance is unclear. We hypothesize that frequent PACs are associated with increased risk of stroke and death. The PubMed (from 1966 to April 2017) and Embase (from 1974 to April 2017) databases were searched for longitudinal studies that reported the relation of PACs with incidence of stroke and death with various etiologies. Study quality was evaluated, and the relative risks (RR) of unfavorable outcomes in subjects with frequent PACs vs those without were calculated. Eleven studies with overall high quality were eligible according to inclusion criteria. The meta‐analysis demonstrated that frequent PACs were associated with an increased risk of stroke (unadjusted RR: 2.20, 95% confidence interval [CI]: 1.79‐2.70; adjusted RR: 1.41, 95% CI: 1.25‐1.60) and death from all causes (unadjusted RR: 2.17, 95% CI: 1.80‐2.63; adjusted RR: 1.26, 95% CI: 1.13‐1.41), cardiovascular diseases (unadjusted RR: 2.89, 95% CI: 2.20‐3.79; adjusted RR: 1.38, 95% CI: 1.24‐1.54), and coronary artery disease (unadjusted RR: 2.74, 95% CI: 1.64‐4.58; adjusted RR: 1.74, 95% CI: 1.27‐2.37). No significant publication bias was detected. The association was robust in sensitivity analysis, subgroup analysis, and pooled analysis of estimates adjusting for confounding factors. Frequent PACs are not benign phenomena; they are associated with higher risk of unfavorable outcomes. Further research on the optimal management of subjects with frequent PACs is urgently required.
Keywords: Death, Meta‐analysis, Premature Atrial Complex, Stroke
1. INTRODUCTION
Premature atrial complexes (PACs) are not rare in the general population, and their prevalence increase with age.1 Just because of this, PACs are usually considered benign phenomena and there is an absence of evidence regarding how to manage patients with PACs effectively. However, it is more likely that PACs are indicators of underlying pathological changes of left atrial myocardium.2 Accumulating evidence shows that left atrial enlargement and P‐wave terminal force in lead V1 in the electrocardiogram (ECG), which reflected left atrial pressure, were related to future unfavorable prognosis in subjects without apparent cardiovascular (CV) disease.3, 4 Moreover, a close relationship was observed between PACs and atrial fibrillation (AF),5 a proven risk factor of stroke, myocardial infarction, and mortality. Therefore, it is necessary to reexamine the predictive value of PACs in generally healthy subjects, especially considering that a large number of subjects suffer from PACs and the detection of those abnormalities is effortless.
2. METHODS
2.1. Study inclusion
We searched the PubMed (from 1966 to April 2017) and Embase (from 1974 to April 2017) databases for longitudinal studies reporting the relation of PACs with stroke or all‐cause or cause‐specific death. The following keywords were used to construct the search strategy: (premature atrial contraction OR atrial premature beat OR atrial premature contraction OR atrial premature complex OR atrial extrasystole OR atrial ectopic beat OR supraventricular premature contraction OR superventricular premature beat OR supraventricular extrasystole) AND (stroke OR death OR mortality OR survival) AND (cohort OR case–control OR longitudinal OR prospective OR retrospective OR follow‐up). All potential studies were initially screened without language restriction. Additionally, the references lists were manually searched for other eligible studies.
Two authors independently screened and judged the eligibility of identified articles, and all the controversies were resolved by consensus. The included studies had to meet the following criteria: (1) enrollment of subjects age ≥ 18 years; (2) follow‐up period ≥1 year; and (3) the events number or effect estimates (relative risk [RR] or hazard ratio) of the outcomes of interest were reported in a PACs group compared with a no‐PACs group. We excluded studies that included patients with specific diseases (eg, acute stroke, chronic kidney disease) and those with a cross‐sectional design, from duplicate cohorts, without a control group, or in an abstract format.
The following data were extracted by 2 independent authors: cohort name, location, cohort design, sample size, follow‐up period, numbers of interested outcomes, study population, exclusion criteria for participants, age, sex, PAC prevalence, definition of frequent PACs, PAC measurement method, outcome ascertainment, effect estimates (RR or hazard ratio) and 95% confidence interval (CI), and adjusted variables. We used the Newcastle‐Ottawa Scale to evaluate study quality based on the selection of the cohort, comparability of cohorts, and assessment of outcomes. Two authors assessed the study quality independently and discrepancies were resolved by consensus.
2.2. Statistical analysis
The primary outcomes were nonfatal and fatal stroke, including ischemic and hemorrhagic. The secondary outcomes were all‐cause death and cause‐specific death, including CV death and death from coronary artery disease (CAD). Data of each study were pooled to estimate the relation of PACs with outcomes using fixed‐effects models or random‐effects models, depending on the heterogeneity across studies, which was evaluated with I 2 statistics (>50% was considered significant). We pooled the unadjusted and adjusted effect estimates separately, for the purpose of investigating the real‐world association and independent relation between PACs and clinical outcomes. The pooled‐effect estimates were represented as RRs and 95% CIs. The Egger test was used and funnel plot was constructed to evaluate publication bias. All the statistical analyses were performed with Stata version 12.0 software (StataCorp LP, College Station, TX).
3. RESULTS
The database search identified 325 potentially eligible studies. After screening titles/abstracts and retrieving full‐text articles, a total of 11 studies including 129 514 participants fulfilled the inclusion criteria,6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 2 of which were from the same study: Atherosclerosis Risk in Communities (ARIC; see Supporting Information, Figure 1, in the online version of this article).7, 14
Table 1 and Table 2 demonstrate the characteristics of the included studies. Three studies were conducted in Europe,6, 9, 11 3 in the United States,7, 14, 15, 16 and 4 in Asia.8, 10, 12, 13 All but 2 of the studies6, 12 were of a prospective design. All of the studies enrolled middle‐aged and elderly participants. Population‐based subjects were included in 7 studies,7, 9, 10, 11, 13, 14, 15, 16 whereas subjects referring to Holter monitoring for symptoms were enrolled in 3 studies.6, 8, 12 The percentage of male participants ranged from 32% to 100%. Baseline frequent PACs prevalence varied from 0.8% to 38.6%. Eligible studies used different measurement methods to determine PACs: routine screening ECG was used in 3 studies,10, 15, 16 15‐s ECG in 1 study,13 2‐min ECG in 1 study,7, 14 and Holter monitoring in 5 studies (4 studies monitored for 24 h and 1 for 48 h).6, 8, 9, 11, 12 Any presence of PAC was defined as exposure in all studies using routine ECG, whereas the definition of frequent PACs in studies using Holter monitoring ranged from 76 beats of PACs recorded per day to >30% of the recording time per day. It is noteworthy that the studies that enrolled subjects with higher CV risk, such as symptomatic subjects and those of older ages, had lower thresholds for frequent PACs. Study duration of follow‐up was >10 years in all but 2 studies.6, 8 Endpoint of fatal or nonfatal stroke was reported in 8 studies,8, 9, 10, 11, 12, 13, 14, 15 all‐cause death in 6 studies,8, 9, 10, 12, 13, 16 CV death in 4 in studies,10, 12, 13, 16 and death from CAD in 5 studies.6, 7, 10, 12, 16 (See Supporting Information, Table 1, in the online version of this article for details of the assessment of quality of included studies.) All studies had scores >7 for all items evaluated, indicative of relatively high quality.
Table 1.
Characteristics of studies included in meta‐analysis
| First Author | Year | Cohort Name | Location | Cohort Design | No. of Participants | Mean Age, y | Male Sex, % | PACs Prevalence, % | Average Follow‐up, y | No. of Stroke | No. of ACD | No. of CV Death | Stroke Death | CAD Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Algra6 | 1993 | NA | Netherlands | RC | 6693 | ≥60: 50.3% | 58 | 7.1 | 2 | NA | NA | NA | NA | 245 |
| Engström9 | 2000 | MB | Sweden | PC | 402 | 68 | 100 | 19.2 | 10.6 | 58 | 181 | 89 | NA | NA |
| Cheriyath7 | 2011 | ARIC | United States | PC | 14 574 | 54 | 43 | 5.0 | 14 | NA | NA | NA | NA | 288 |
| Chong8 | 2012 | NA | Hong Kong | PC | 428 | 66 | 44 | 25 | 6.1 | 41 | 60 | NA | NA | NA |
| Ofoma14 | 2012 | ARIC | United States | PC | 14 493 | 54 | 43 | 4.9 | 13 | 509 | NA | NA | NA | NA |
| Inohara10 | 2013 | NIPPON DATA 90 | Japan | PC | 7692 | 53 | 42 | 0.8 | 14 | NA | 1211 | 338 | 138 | 68 |
| Qureshi16 | 2014 | NHANES III | United States | PC | 7504 | 60 | 53 | 1.2 | 13 | NA | 2386 | 963 | NA | 511 |
| Larsen11 | 2015 | CHS | Denmark | PC | 678 | 65 | 59 | 10.3 | Up to 15 | NA | NA | NA | NA | NA |
| Lin12 | 2015 | TPVGH | Taiwan | RC | 5371 | 62 | 60 | 38.6 | 10 | NA | 1209 | 291 | 18 | 66 |
| Murakoshi13 | 2015 | IPHS | Japan | PC | 63 197 | 59 | 32 | 6.1 | 14 | NA | 8712 | 2527 | 1208 | NA |
| O'Neal15 | 2016 | REGARDS | United States | PC | 22 975 | 64 | 44 | 7.3 | Up to 11 | 549 | NA | NA | NA | NA |
Abbreviations: ACD, all‐cause death; ARIC, Atherosclerosis Risk in Communities; CAD, coronary artery disease; CHS, Copenhagen Holter Study; CV, cardiovascular; ECG, electrocardiographic; IPHS, Ibaraki Prefectural Health Study; MB, Men Born in 1914; NA, not available; NHANES III, The Third National Health and Nutrition Examination Survey; NIPPON DATA 90, National Integrated Project for Prospective Observation of Non‐communicable Disease and Its Trends in the Aged, 1990–2005; PACs, premature atrial complexes; PC, prospective cohort; RC, retrospective cohort; REGARDS, Reasons for Geographic and Racial Differences in Stroke; TPVGH, Registry of 24‐h ECG monitoring at Taipei Veterans General Hospital database.
Table 2.
Characteristics of study subjects, definition of exposure, assessment of outcomes in meta‐analysis
| First Author, Year | Study Population | Major Exclusion Criteria | Exposure Definition | Exposure Ascertainment | Outcome Definition | Outcome Ascertainment | Variables Controlled |
|---|---|---|---|---|---|---|---|
| Algra, 19936 | Patients referring for 24‐h ECG for various indications | NA | PAC ≥30% of the recording time | 24‐h ECG | SCD | Records of general practitioners and hospitals | NA |
| Engström, 20009 | Random sample of 68‐year‐old men living in Malmö, Sweden | History of MI and stroke | ≥218 PACs per 24 h | 24‐h ambulatory ECG | Mortality, stroke (IS, SH, and IH) | Mortality Register of the Swedish National Bureau of Statistics, autopsy findings; Stroke Registry of Malmö; National Cause of Death Registry; Swedish Hospital Discharge Register; clinical presentation at CT, lumbar puncture, or necropsy | SBP, DM, history of angina pectoris, and smoking |
| Cheriyath, 20117 | ARIC, population‐based sample of subjects | Participants with known history of CHD or stroke | Presence of PAC | 2‐min 12‐lead ECG | SCD, incident CHD, and fatal CHD | Hospital records and death certificates, physician questionnaires, and interviews with next of kin | Age, race, sex, education, smoking, BMI, LDL/HDL ratio, DM, HTN, serum potassium, magnesium, heart rate, and use of heart rhythm medications |
| Chong, 20128 | Patients with palpitations, dizziness, or syncope | AF, high‐grade AVB, pacemaker or ICD, chronic RHD, history of CHF, or IS | >100 PACs/d | 24‐h ECG monitoring | Composite of IS, CHF, or death | Medical records and discharge summaries | NA |
| Ofoma, 201214 | ARIC, population‐based study | Individuals with history of stroke or CHD, and those developing SH or IH during follow‐up | Presence of PAC | 2‐min ECG | IS | Medical records, death certificates, interview of involved healthcare providers and next of kin | Age, race, sex, BMI, TC, DM, HTN, smoking, and PVC |
| Inohara, 201310 | Otherwise‐healthy participants | History of a known vascular condition, such as MI or stroke; presence of AF or AFL | ≥1 beat of APC | Screening 12‐lead ECG | Death from different underlying causes | National Vital Statistics Database of Japan | Age, sex, BMI, smoking habit, drinking habit, hypercholesterolemia, DM, SBP, serum Cr, and other ECG findings |
| Qureshi, 201416 | Nationally‐representative, community‐dwelling individuals | Individuals with known CVD, ECG evidence of MI, paced rhythms or AF | Presence of any APC | Standard 12‐lead ECG | ACD, CVD‐related mortality, and IHD‐related mortality | National Death Index, death certificates | Age, sex, race/ethnicity, smoking status, SBP, BMI, BP medications, TC, DM, cancer and pulmonary disease, LVH, and QTc |
| Larsen, 201511 | Middle‐age and elderly subjects with or without CV risk factors | Subjects with AF, manifest CVD, stroke, cancer, or other life‐threatening conditions | ≥30 PACs/h | 48‐h Holter monitoring | IS, combined endpoint of ACD or first event of stroke | National central patient registry, discharge letters, patient files | Age, sex, smoking, TC, DM, BMI, and SBP |
| Lin, 201512 | Patients referring for Holter monitoring for various indications and clinical follow‐up according to physician discretion | Participants with AF or AFL, a PPM, or a history of ablation | >76 beats/d | 24‐h ECG monitoring | Death, all‐cause hospitalization, CV hospitalization, occurrence of new‐onset AF, and PPM implantation | Bureau of National Health Insurance of Taiwan | Age, sex, HTN, CHD, previous MI, CHF, and use of anti‐HTN medication |
| Murakoshi, 201513 | Community‐based health checkups | Subjects receiving medical treatment for heart disease and subjects with AF at baseline | Presence of any APC | 15‐s ECG | Death from total stroke (including IS and HS), any CV cause, or all‐cause | Death certificates and resident registration | Age, BMI, SBP, anti‐HTN therapy, past history of stroke, DM, TC, HDL‐C, TG, eGFR, current smoking, drinking habit, and other abnormal ECG findings |
| O'Neal, 201615 | Oversampling of blacks and residents of the Stroke Belt | Individuals with stroke/TIA, AVB and evidence of non–sinus rhythm on the baseline ECG, and those with frequent PVCs | Presence of PAC | Routine screening ECG | IS | Medical record review, death certificates and/or proxy interviews | Age, sex, race, age*race, education, income, region of residence, SBP, smoking, DM, LDL‐C, LVH, anti‐HTN medications, lipid‐lowering therapies, ASA, and CHD |
Abbreviations: ACD, all‐cause death; AF, atrial fibrillation; AFL, atrial flutter; APC, atrial premature complex; ARIC, Atherosclerosis Risk in Communities; ASA, aspirin; AVB, atrioventricular block; BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; CHF, congestive heart failure; Cr, creatinine; CT, computed tomography; CV, cardiovascular; CVD, cardiovascular disease; DM, diabetes mellitus; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; HS, hemorrhagic stroke; HTN, hypertension; ICD, implantable cardioverter‐defibrillator; IH, intracerebral hemorrhage; IS, ischemic stroke; LDL‐C, low‐density lipoprotein cholesterol; LVH, left ventricular hypertrophy; MI, myocardial infarction; NA, not available; PAC, premature atrial complex; PPM, permanent pacemaker; PVC, premature ventricular complex; QTc, corrected QT interval; RHD, rheumatic heart disease; SBP, systolic blood pressure; SCD, sudden cardiac death; SH, subarachnoid hemorrhage; TC, total cholesterol; TG, triglycerides; TIA, transient ischemic attack.
Eight studies reported the unadjusted estimates of risk of nonfatal and fatal stroke comparing frequent PACs with no frequent PACs.8, 9, 10, 11, 12, 13, 14, 15 The random‐effects pooled RR was 2.20 (95% CI: 1.79‐2.70; I 2 = 57.4%; P = 0.016), indicating a 120% increased risk of stroke associated with PACs (Figure 1). To explore the reason for the high heterogeneity, each study was removed one at a time. The results showed that only Murakoshi's study fully accounted for the heterogeneity; after excluding this study, the RR was 1.86 (95% CI: 1.57‐2.20; I 2 = 0%; P = 0.445). We then conducted subgroup analyses (definition of endpoint, study subjects, PACs measurement method, frequent PACs prevalence, threshold of frequent PACs, age, sex, study location, sample size) to further explore the sources of heterogeneity (see Supporting Information, Table 2, in the online version of this article). Both of definition of endpoint and study location were responsible for the high heterogeneity. Moreover, the sensitivity analyses, as well as subgroup analyses, consistently validated the stability of the results, which showed increased risk of stroke in patients with frequent PACs. The magnitude of risk was attenuated to 41% when the meta‐analysis was rerun with 5 studies adjusting for potential confounding factors9, 11, 13, 14, 15; nevertheless, the higher risk was still statistically significant (RR: 1.41, 95% CI: 1.25‐1.60; I 2 = 0%; P = 0.506; Figure 2).
Figure 1.
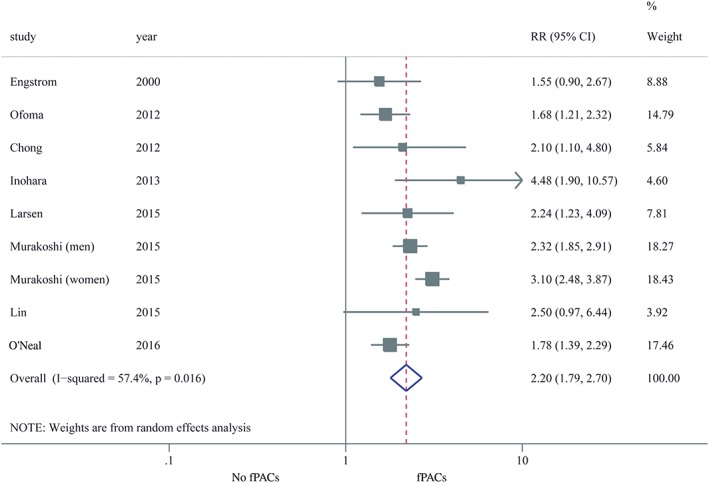
Forest plot of the relation of fPACs with stroke (unadjusted RR). Abbreviations: CI, confidence interval; fPACs, frequent premature atrial complexes; RR, relative risk
Figure 2.
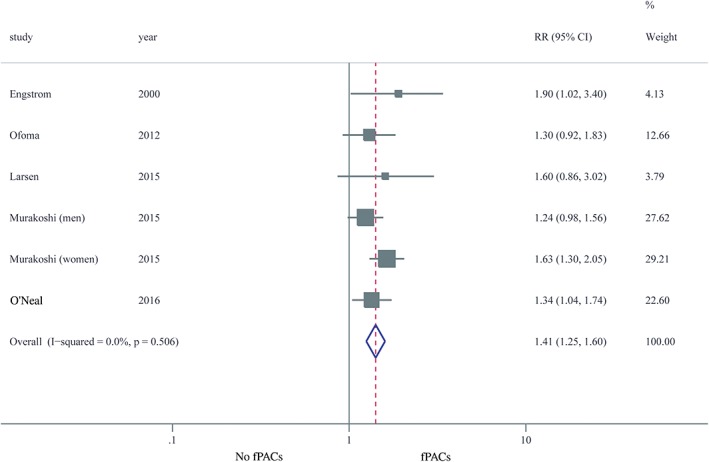
Forest plot of the relation of fPACs with stroke (adjusted RR). Abbreviations: CI, confidence interval; fPACs, frequent premature atrial complexes; RR, relative risk
Six studies reported the outcome of all‐cause death.8, 9, 10, 12, 13, 16 In the pooled analysis of the 6 studies presenting unadjusted estimates, the RR for all‐cause death comparing frequent PACs with no frequent PACs was 2.17 (95% CI: 1.80‐2.63; I 2 = 89%; P < 0.001; see Supporting Information, Figure 2, in the online version of this article). The increased risk was decreased to 26% but still significant when the meta‐analysis was rerun with 5 studies adjusting for confounders (RR: 1.26, 95% CI: 1.13‐1.41; I 2 = 65.9%; P = 0.012).9, 10, 12, 13, 16 (See Supporting Information, Figure 3, in the online version of this article.)
Four studies reported the outcome of CV death.10, 12, 13, 16 Both the results of pooled analysis of 4 studies reporting unadjusted estimates (RR: 2.89, 95% CI: 2.20‐3.79; I 2 = 85.0%; P < 0.001) and 3 studies presenting data adjusted for confounders (RR: 1.38, 95% CI: 1.24‐1.54; I 2 = 43.8%; P = 0.148) showed significant higher risk of CV death comparing frequent PACs with no frequent PACs (see Supporting Information, Figures 4 and 5, in the online version of this article).
Five studies evaluated death from CAD as outcome.6, 7, 10, 12, 16 The RR was 2.74 (95% CI: 1.64‐4.58; I 2 = 78.6%; P = 0.001) when unadjusted estimates were pooled and 1.74 (95% CI: 1.27‐2.37; I 2 = 7.9%; P = 0.297) when adjusted estimates were pooled comparing frequent PACs with no frequent PACs (see Supporting Information, Figures 6 and 7, in the online version of this article).
Risk of publication bias was assessed by inspection of funnel plots and Egger's tests. Both methods demonstrated lack of publication bias regarding the outcomes of stroke and all‐cause death, respectively (see Supporting Information, Figures 8 and 9, in the online version of this article).
4. DISCUSSION
In the present study, we found that frequent PACs were associated with increased risks of fatal or nonfatal stroke and death from all causes, CV etiology, and CAD. Other studies that did not meet the inclusion criteria also supported the relation of PACs with adverse CV events. It has been reported that more than half of patients with cryptogenic stroke have frequent PACs (≥200 per 24 h).17 In a prospective cohort study, Vinther et al. observed that patients with ischemic stroke and excessive PACs carried similar risk of recurrent strokes or death compared with those with AF.18 Additionally, Pinho et al. investigated a dose‐effect relationship between PACs and recurrence of stroke or transient ischemic attack.19
The concept of “atrial cardiomyopathy” emphasizes the importance of the role of the atrium in cardiac physiological and pathological conditions,2 which may be further consolidated by our study. Several mechanisms could be used to explain our findings. First, PACs, stroke, and other CV events share some common risk factors (eg, older age, prevalent CV disease, obstructive sleep apnea, indices reflecting increased left ventricular filling pressure, and unhealthy lifestyles).1, 20 Second, AF might serve as an intermediary between PACs and CV outcomes. PACs independently predicted incidence of AF5, 21, 22, 23 and significantly improved the predictive performance of Framingham AF risk model.24, 25 On the other hand, PACs might be a surrogate of undetected occult paroxysmal AF in patients with stroke.26, 27, 28 The relation of PACs and AF can be further reinforced by the fact that increased risk of late recurrence of AF observed in patients with PACs at 6 months after pulmonary vein isolation.29 Third, PACs and AF may be different phenotypes of atrial cardiomyopathy, which is a surrogate of subclinical cardiac damage. In turn, PACs could accelerate the process of atrial remodeling and contribute to atrial dysfunction.30 Fourth, unique hemodynamic changes occurred in patients with PACs. Irregular atrial rhythm and the result of variant coupling interval cause blood‐flow stasis and promote thrombus development. In addition, the effect of premature ventricular contractions on ventricular dilation and function decline could be exacerbated by frequent PACs.31
Based on available evidence, some issues are still not addressed: What is the threshold value of PACs in predicting adverse outcomes? Which CV risk factors have interactions with PACs; in other words, which types of patients with frequent PACs are most prone to poor outcomes? Would treatment of PACs per se, such as radiofrequency ablation and/or anticoagulation therapy in the absence of apparent AF, add benefit for hard endpoints? Are there alternative and effective upstream therapies for the management of PACs? What could we do when subjects with frequent PACs are identified, even though we know they carry a higher risk of long‐term unfavorable prognosis?
The past several years have witnessed rapid development of wearable devices, including those equipped with ECG recording function. More asymptomatic subjects with frequent PACs will be easily detected with early monitoring for future clinical outcomes. Prospective studies are needed to clarify the risk factors, gene susceptibility, natural progression or disease trajectory, and clinical approach of management of frequent PACs, especially in apparently healthy subjects. The additional discrimination capability of frequent PACs beyond traditional CV risk models needs evaluation. Besides, whether early intervention of apparently healthy patients with frequent PACs translates to improvement of prognosis deserves further investigation.
4.1. Study limitations
Our findings should be interpreted with caution. First, statistical heterogeneity was evident. We conducted several methods to explore the source of heterogeneity and found that both the definition of endpoint and the study location were primarily responsible for it. Second, as all included studies were observational in nature, the influence of residual confounders on the association could not be fully excluded, even among apparently healthy participants. PACs could be the signs of noncardiac diseases or indicators of subclinical CV diseases, which might dampen the independent association between PACs and adverse events. Furthermore, lack of information on left ventricular function, serum electrolytes, and detailed drug therapies and doses in most of the included studies was a non‐negligible limitation. In addition, although subjects with baseline AF were excluded in the majority of studies, only 1 study considered the potential influence of new‐onset AF during the period of follow‐up. Third, not all of the studies enrolled generally healthy subjects, which could restrict the generalization of the conclusions. Nevertheless, subgroup analysis demonstrated that whether in population‐based studies or hospital‐based studies, frequent PACs were associated with increased risk of stroke. Fourth, stroke subtypes were variable across the included studies. Although most studies investigated incident ischemic stroke as an endpoint, others did not differentiate subtypes of stroke. Fifth, until now there have been no randomized controlled trials addressing treatment of frequent PACs; thus, whether therapies directed to this condition would change the prognosis remains to be investigated.
5. CONCLUSION
Our study suggests that presence of frequent PACs is associated with a higher risk of unfavorable prognosis. As PACs are universal in the general population, our results provide evidence of PACs as an indicator of risk stratification and have substantial social significance. Our findings call for further research on the approaches in the early management of subjects with frequent PACs.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Appendix S1 SUPPLEMENTAL MATERIAL
sTable 1. Quality evaluation of included studies.
sTable 2. Subgroup analyses of the association between PACs and stroke (unadjusted).
sFigure 1. Flow diagram of study selection.
sFigure 2. Forest plot of the relation of fPACs with all‐cause death (unadjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 3. Forest plot of the relation of fPACs with all‐cause death (adjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 4. Forest plot of the relation of fPACs with cardiovascular death (unadjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 5. Forest plot of the relation of fPACs with cardiovascular death (adjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 6. Forest plot of the relation of fPACs with death from coronary artery disease (unadjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 7. Forest plot of the relation of fPACs with death from coronary artery disease (adjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 8. Funnel plot for publication bias in studies for stroke (unadjusted).
sFigure 9. Funnel plot for publication bias in studies for all‐cause death (unadjusted).
Huang B., Huang F., Peng Y. et al. Relation of premature atrial complexes with stroke and death: Systematic review and meta‐analysis. Clin Cardiol. 2017;40:962–969. 10.1002/clc.22780
Funding Information
This work was supported by the National Natural Science Foundation of China (grant nos. 81370219 and 81400267, Beijing, China), the Supporting Project of Sichuan Provincial Department of Science and Technology (grant no. 2014SZ0004, Sichuan, China), and the Sichuan province Science and Technology Innovation Team (grant no. 2017TD0004, Sichuan, China). The funding sources had no involvement in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
REFERENCES
- 1. Conen D, Adam M, Roche F, et al. Premature atrial contractions in the general population: frequency and risk factors. Circulation. 2012;126:2302–2308. [DOI] [PubMed] [Google Scholar]
- 2. Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18:1455–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahabadi AA, Geisel MH, Lehmann N, et al. Association of computed tomography–derived left atrial size with major cardiovascular events in the general population: the Heinz Nixdorf Recall Study. Int J Cardiol. 2014;174:318–323. [DOI] [PubMed] [Google Scholar]
- 4. Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120:472–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Acharya T, Tringali S, Bhullar M, et al. Frequent atrial premature complexes and their association with risk of atrial fibrillation. Am J Cardiol. 2015;116:1852–1857. [DOI] [PubMed] [Google Scholar]
- 6. Algra A, Tijssen JG, Roelandt JR, et al. Contribution of the 24 hour electrocardiogram to the prediction of sudden coronary death. Br Heart J. 1993;70:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheriyath P, He F, Peters I, et al. Relation of atrial and/or ventricular premature complexes on a two‐minute rhythm strip to the risk of sudden cardiac death (the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol. 2011;107:151–155. [DOI] [PubMed] [Google Scholar]
- 8. Chong BH, Pong V, Lam KF, et al. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace. 2012;14:942–947. [DOI] [PubMed] [Google Scholar]
- 9. Engström G, Hedblad B, Juul‐Möller S, et al. Cardiac arrhythmias and stroke: increased risk in men with high frequency of atrial ectopic beats. Stroke. 2000;31:2925–2929. [DOI] [PubMed] [Google Scholar]
- 10. Inohara T, Kohsaka S, Okamura T, et al; NIPPON DATA 80/90 Research Group . Long‐term outcome of healthy participants with atrial premature complex: a 15‐year follow‐up of the NIPPON DATA 90 cohort. PLoS One. 2013;8:e80853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larsen BS, Kumarathurai P, Falkenberg J, et al. Excessive atrial ectopy and short atrial runs increase the risk of stroke beyond incident atrial fibrillation. J Am Coll Cardiol. 2015;66:232–241. [DOI] [PubMed] [Google Scholar]
- 12. Lin CY, Lin YJ, Chen YY, et al. Prognostic significance of premature atrial complexes burden in prediction of long‐term outcome. J Am Heart Assoc. 2015;4:e002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murakoshi N, Xu D, Sairenchi T, et al. Prognostic impact of supraventricular premature complexes in community‐based health checkups: the Ibaraki Prefectural Health Study. Eur Heart J. 2015;36:170–178. [DOI] [PubMed] [Google Scholar]
- 14. Ofoma U, He F, Shaffer ML, et al. Premature cardiac contractions and risk of incident ischemic stroke. J Am Heart Assoc. 2012;1:e002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Neal WT, Kamel H, Kleindorfer D, et al. Premature atrial contractions on the screening electrocardiogram and risk of ischemic stroke: the Reasons for Geographic and Racial Differences in Stroke Study. Neuroepidemiology. 2016;47:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qureshi W, Shah AJ, Salahuddin T, et al. Long‐term mortality risk in individuals with atrial or ventricular premature complexes (Results from the Third National Health and Nutrition Examination Survey). Am J Cardiol. 2014;114:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Todo K, Moriwaki H, Saito K, et al. Frequent premature atrial contractions in stroke of undetermined etiology. Eur Neurol. 2009;61:285–288. [DOI] [PubMed] [Google Scholar]
- 18. Vinther KH, Tveskov C, Möller S, et al. Excessive premature atrial complexes and the risk of recurrent stroke or death in an ischemic stroke population. J Stroke Cerebrovasc Dis. 2017;26:1163–1170. [DOI] [PubMed] [Google Scholar]
- 19. Pinho J, Braga CG, Rocha S, et al. Atrial ectopic activity in cryptogenic ischemic stroke and TIA: a risk factor for recurrence. J Stroke Cerebrovasc Dis. 2015;24:507–510. [DOI] [PubMed] [Google Scholar]
- 20. Kawano Y, Tamura A, Ono K, et al. Association between obstructive sleep apnea and premature supraventricular contractions. J Cardiol. 2014;63:69–72. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen KT, Vittinghoff E, Dewland TA, et al. Electrocardiographic predictors of incident atrial fibrillation. Am J Cardiol. 2016;118:714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cabrera S, Vallès E, Benito B, et al. Simple predictors for new‐onset atrial fibrillation. Int J Cardiol. 2016;221:515–520. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki S, Sagara K, Otsuka T, et al. Usefulness of frequent supraventricular extrasystoles and a high CHADS2 score to predict first‐time appearance of atrial fibrillation. Am J Cardiol. 2013;111:1602–1607. [DOI] [PubMed] [Google Scholar]
- 24. Dewland TA, Vittinghoff E, Mandyam MC, et al. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumarathurai P, Mouridsen MR, Mattsson N, et al. Atrial ectopy and N‐terminal pro‐B‐type natriuretic peptide as predictors of atrial fibrillation: a population‐based cohort study. Europace. 2017;19:364–370. [DOI] [PubMed] [Google Scholar]
- 26. Gladstone DJ, Dorian P, Spring M, et al; EMBRACE Steering Committee and Investigators . Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke. 2015;46:936–941. [DOI] [PubMed] [Google Scholar]
- 27. Kochhäuser S, Dechering DG, Dittrich R, et al. Supraventricular premature beats and short atrial runs predict atrial fibrillation in continuously monitored patients with cryptogenic stroke. Stroke. 2014;45:884–886. [DOI] [PubMed] [Google Scholar]
- 28. Wallmann D, Tüller D, Kucher N, et al. Frequent atrial premature contractions as a surrogate marker for paroxysmal atrial fibrillation in patients with acute ischaemic stroke. Heart. 2003;89:1247–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gang UJ, Nalliah CJ, Lim TW, et al. Atrial ectopy predicts late recurrence of atrial fibrillation after pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2015;8:569–574. [DOI] [PubMed] [Google Scholar]
- 30. Hasdemir C, Simsek E, Yuksel A. Premature atrial contraction–induced cardiomyopathy. Europace. 2013;15:1790. [DOI] [PubMed] [Google Scholar]
- 31. Pacchia CF, Akoum NW, Wasmund S, et al. Atrial bigeminy results in decreased left ventricular function: an insight into the mechanism of PVC‐induced cardiomyopathy. Pacing Clin Electrophysiol. 2012;35:1232–1235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 SUPPLEMENTAL MATERIAL
sTable 1. Quality evaluation of included studies.
sTable 2. Subgroup analyses of the association between PACs and stroke (unadjusted).
sFigure 1. Flow diagram of study selection.
sFigure 2. Forest plot of the relation of fPACs with all‐cause death (unadjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 3. Forest plot of the relation of fPACs with all‐cause death (adjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 4. Forest plot of the relation of fPACs with cardiovascular death (unadjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 5. Forest plot of the relation of fPACs with cardiovascular death (adjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 6. Forest plot of the relation of fPACs with death from coronary artery disease (unadjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 7. Forest plot of the relation of fPACs with death from coronary artery disease (adjusted RR). fPACs, frequent premature atrial complexes; RR, relative risk; CI, confidence interval.
sFigure 8. Funnel plot for publication bias in studies for stroke (unadjusted).
sFigure 9. Funnel plot for publication bias in studies for all‐cause death (unadjusted).


