Abstract
Background
Inflammation is closely related to atrial fibrillation (AF) pathogenesis, and interleukin‐37 (IL‐37) represents a new member of the anti‐inflammatory cytokines.
Hypothesis
IL‐37 might play an important role in AF development and act as a potential risk factor for AF diagnosis.
Methods
The mRNA level of IL‐37 in peripheral blood mononuclear cells (PBMCs) and serum IL‐37 levels in AF patients and healthy controls were measured by real‐time polymerase chain reaction (RT‐PCR) and enzyme‐linked immunosorbent assay (ELISA). PBMCs from AF patients were stimulated with recombinant IL‐37. Levels of pro‐inflammatory cytokines IL‐6 and C‐reactive protein were determined by RT‐PCR and ELISA.
Results
IL‐37 mRNAs and serum protein levels were higher in patients with AF or lone AF compared with healthy controls. Patients with paroxysmal AF or persistent AF showed higher IL‐37 mRNAs and serum protein levels compared with those with permanent AF as well as healthy controls. In vitro, IL‐37 inhibited the production of IL‐6 and C‐reactive protein in PBMCs of patients with AF.
Conclusions
IL‐37 is elevated in AF patients and its expression is closely associated with AF subgroups. Thus, IL‐37 may provide a novel research target for the pathogenesis and therapy of AF. This study is the first to document elevated IL‐37 in AF patients.
Keywords: Interleukin‐37, Inflammation, Atrial Fibrillation, IL‐37, AF
1. INTRODUCTION
Atrial fibrillation (AF) is the most common abnormal heart rhythm in clinical practice with high incidence, prevalence, and related mortality.1, 2, 3, 4 AF causes irregular blood flow in the heart chamber and formation of blood clots, which lead to stroke or heart failure (HF).5 In patients with existing HF, the development of AF doubles mortality; and in patients with existing AF, the development of HF triples mortality.6 When AF and HF occur in combination, clinical outcomes are particularly poor.7, 8, 9, 10 This makes identifying and treating AF extremely important.
Advanced age, HF, diabetes mellitus, obesity, and cigarette smoking are established risk factors for AF.11, 12, 13 Inflammation is activated in all these conditions. Furthermore, increasing pathological evidence suggests a direct link between inflammation and atrial remodeling and, consequently, maintenance of AF.14 Previous data have shown that inflammation is closely related to AF pathogenesis.15, 16 AF occurring after cardiac surgery is closely associated with the activation of the complement system and release of pro‐inflammatory cytokines.17, 18 Inflammatory infiltrates, myocyte necrosis, and fibrosis have been demonstrated in the atrial biopsies of patients with lone AF refractory to antiarrhythmic therapy.14
However, most studies only focused on the pro‐inflammatory factors, such as C‐reactive protein (CRP) and interleukin‐6 (IL‐6).17, 18, 19, 20, 21, 22, 23 Actually, both pro‐inflammatory and anti‐inflammatory factors work together to maintain immune‐system homeostasis, and the imbalance of these 2 groups of factors would cause detrimental consequences to the tissues.24 Interleukin‐37 (IL‐37) represents a new member of the anti‐inflammatory cytokines and exerts an anti‐inflammatory effect in inflammatory diseases.24, 25 Recent studies found that IL‐37 is elevated significantly in peripheral blood of patients with acute coronary syndrome26 and acute myocardial infarction,27 and it had a cardioprotective effect in a mouse model of myocardial infarction.28
However, the level of IL‐37 in AF patients and how the expression of IL‐37 relates to AF have not been explored. In this study, we compared the level of IL‐37 mRNA in peripheral blood mononuclear cells (PBMCs) and serum IL‐37 protein levels in AF patients with the levels in healthy controls. Moreover, we investigated the effects of IL‐37 on the expression of pro‐inflammatory cytokine IL‐6 and CRP in the PBMCs of AF patients.
2. METHODS
2.1. Patients
Using a case–control study design, IL‐37 levels in a group of patients with AF were compared with those of a control group of patients in sinus rhythm who were undergoing routine physical examination. We prospectively enrolled 125 consecutive patients with AF into this study. An age‐ and sex‐matched group of 42 healthy people was considered the control group. Detailed medical history, physical examination, and routine biochemical testing were performed, in addition to a 12‐lead electrocardiogram. Using transthoracic echocardiography, valve function, left ventricular size and function (left ventricular ejection fraction), and left atrial diameter were evaluated as previously described. The diameter of the left atrium was measured in the parasternal long‐axis view. AF duration was determined by the patient description of a well‐defined and abrupt‐onset palpitation with subsequent electrocardiographic evidence of AF at the time of presentation. Exclusion criteria were as follows: known coronary artery disease or acute coronary syndromes; surgery within 60 days; a history of infection; chronic inflammatory, hepatic, or malignant disease; chronic renal failure; use of anti‐inflammatory drugs; and LVEF <40%.The protocol of this study was approved by the hospital's ethics committee, and each patient gave written consent.
2.2. AF classification
Lone AF was defined as AF occurring in the absence of structural heart disease and could include patients with hypertension but without structural heart disease. AF lasting ≤7 days was defined as paroxysmal AF.29, 30, 31, 32, 33 AF duration of >7 days was further divided into 2 subgroups: persistent and permanent. Persistent AF was successfully converted to sinus rhythm (electrical cardioversion), whereas AF was considered permanent when cardioversion was a failure or it was not considered due to excessive dilated left atrium or low success rate.29, 30, 31, 32
2.3. Enzyme‐linked immunosorbent assay
Blood samples were collected from peripheral veins. PBMCs were isolated by Ficoll‐Paque Plus (Stem Cell Tech Inc., Vancouver, Canada) density gradient centrifugation and stored at −80°C until RNA extraction. Serums were frozen at −80°C until cytokines were detected. CRP was assayed by immunoturbidimetric method using an Abbott Aeroset automated analyzer (Abbott Laboratories, Abbott Park, Illinois) in serum samples according to the manufacturer's protocol. CRP concentrations were determined with a detection limit of ≈ 0.005 mg/dL. IL‐6 was measured by immunoenzymetric method using an IL‐6 enzyme‐amplified sensitivity immunoassay (EASIA) kit (BioSource Europe S.A., Nivelles, Belgium) in plasma samples, as described previously.
Serum and cell culture supernatant IL‐37 levels were determined by ELISA following the manufacturer's instructions. IL‐37 was quantified using ELISA reagent kits purchased from Adipogen Corp. (San Diego, California).
2.4. Recombinant human IL‐37 protein cloning
IL‐37 gene (Homo sapiens [human]) was amplified from cDNA of PBMCs using the primer pair 5′‐CGGGATCCATGGTTCACACAAGTCCA‐3′ and 5′‐CCCAAGCTTCTAATCGCTGACCTCACT‐3′. The PCR fragments were double digested with restriction endonucleases and ligated into the prokaryotic expression vector. The fusion protein was expressed in a stable prokaryotic expression system. The plasmids of positive clones were then sequenced by the Sanger method with 100% identification with the published sequence (GenBank: AF167368). The induced and uninduced cultures were analyzed by sodium‐dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) to identify the expression of recombinant protein. The harvested cells were resuspended in NaCl‐Tris–HCl buffer, sonicated in an ice bath, centrifuged at 12 000 rpm for 30 minutes, and then the supernatants were collected. The supernatants were added to a 1‐mL HisTrap HP column (GE Healthcare, Piscataway, New Jersey) that had been equilibrated with NaCl‐Tris–HCl buffer. Different concentrations of imidazole buffer were used to elute the recombinant protein. Collected target protein peaks were examined by SDS‐PAGE electrophoresis and immunoblot analysis using antihuman IL‐37 antibody (Abcam, Cambridge, United Kingdom). The eluted recombinant protein was dialyzed in phosphate‐buffered saline at 4°C overnight. The concentration was detected by the Bradford method, and the recombinant protein was stored at −20°C.
2.5. Cell culture condition
The culture medium consisted of RPMI 1640 (Hyclone; Thermo Fisher Scientific, Logan, Utah) supplemented with 10% fetal calf serum (Hyclone), 100 IU/mL penicillin, and 100 µg/mL streptomycin (Hyclone). Whole PBMCs were cultured in 24‐well, flat‐bottomed plates (5 × 105 cells/mL/well) for 24 hours. PBMCs were stimulated with or without human recombinant IL‐37 at 100 ng/mL for 24 hours and then incubated further with LPS (1 µg/mL) for 6 hours. Total RNAs were extracted, and cytokine transcriptions were analyzed by RT‐PCR. Culture supernatants were harvested and frozen at −80°C for later cytokine analysis by ELISA.
2.6. RNA extraction and RT‐PCR
Total RNA was extracted from PBMCs with Trizol (Invitrogen, Carlsbad, California) according to the manufacturer's instructions. Then the quantity and purity of RNA was determined by absorbance on a FilterMax F5 multi‐mode microplate reader (Molecular Devices, Sunnyvale, California) at 260 nm and 280 nm. Samples with ratios from 1.8 to 2.0 were accepted for next reverse transcription reaction. cDNA was prepared by using the iScript cDNA Synthesis kit (Bio‐Rad Laboratories, Hercules, California). Polymerase chain reaction primers (Generay Biotechnology, Shanghai, China) used for RT‐PCR were as follows: for IL‐37, sense: 5′‐AGTGCTGCTTAGAAGAC CCGG‐3′ and antisense: 5′‐AGAGTCCAGGACCAGTACTTTGTGA‐3′; for IL‐6, sense: 5′‐AGCCACTCACCTCTTCAGAAC‐3′ and antisense: 5′‐ACATGTCTCCTTTCTCAGGGC‐3′; for CRP, sense: 5′‐AGCCTCTCTCATGCTTTTGG‐3′ and antisense: 5′‐TGTCTCTTGGTGGCATACGA‐3′; and for β‐actin, sense: 5′‐CCTGACTGACTACCTCATGAAG‐3′ and antisense: 5′‐GACGTAGCACAGCTTCTCCTTA‐3′. RT‐PCR amplification reaction was prepared with the SYBR Green PCR kit (Bio‐Rad) and performed using the 7500 Fast RT‐PCR system (Applied Biosystems, Carlsbad, California). PCR products were verified by melting curve analysis. Relative mRNA levels of target genes were calculated by the 2‐ΔΔCt method.
2.7. Statistical analysis
Data were expressed as mean ± SE and analyzed by GraphPad Prism version 5.00 (GraphPad Software, San Diego, California). Comparisons between groups were made using the nonparametric Mann–Whitney U test. P values <0.05 were considered statistically significant.
3. RESULTS
3.1. Patient population
IL‐37, IL‐6, and CRP were assayed in 125 patients with atrial fibrillation (AF) and 42 control patients. Of all 125 AF patients, 30 had paroxysmal AF, 41 had permanent AF, and 54 had persistent AF. Lone AF was present in 38 patients (18 paroxysmal, 4 permanent, and 16 persistent). AF patients had higher prevalence of hypertension, valvular heart disease, and cardiomyopathy (see Supporting Information, Table 1, in the online version of this article). Persistent and permanent AF subgroups had similar characteristics except for left atrial diameter and duration of AF (see Supporting Information, Table 2, in the online version of this article).
3.2. Increased serum levels of IL‐37 and pro‐inflammatory cytokines in AF patients
To compare the protein level of IL‐37 between the patients with AF and healthy controls (HCs), ELISA was used to measure serum IL‐37 level. The results showed that the serum IL‐37 levels in AF patients and lone AF patients were significantly higher than those from HCs (P = 0.0006 and P < 0.0001, respectively; Figure 1A). To investigate the protein level of major pro‐inflammatory cytokines involved in AF pathogenesis, the serum levels of IL‐6 and CRP were measured by ELISA in AF patients and controls, respectively; pro‐inflammatory cytokine IL‐6 (P < 0.0001; Figure 1B) and CRP (P < 0.0001; Figure 1C) serum levels in AF patients and lone AF patients were significantly higher than those from HCs. These results showed that IL‐37 protein levels were elevated in AF patients, and also pro‐inflammatory factor IL‐6 and CRP, indicating that IL‐37 is probably associated with the pathogenesis of AF.
Figure 1.
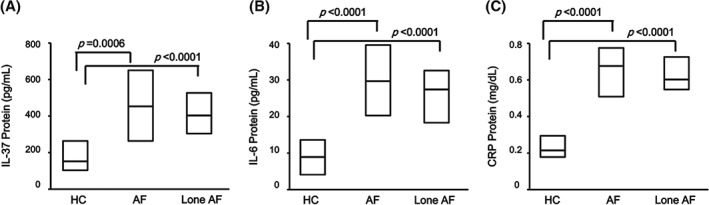
Comparison of (A) serum IL‐37 protein levels, (B) serum IL‐6 concentrations, and (C) serum CRP concentrations in overall AF patients (N = 125), lone AF patients (n = 38), and HCs (n = 42), as determined by ELISA. Results are depicted as box plots; middle line indicates median, bottom of box indicates 25th percentile, and top of box indicates 75th percentile. Mann–Whitney U test and associated P values are indicated. Abbreviations: AF, atrial fibrillation; CRP, C‐reactive protein; ELISA, enzyme‐linked immunoassay; HC, healthy controls; IL, interleukin.
3.3. Up‐regulated expression of IL‐37 mRNA and pro‐inflammatory cytokines in PBMCs of AF patients
The mRNA level of IL‐37 and pro‐inflammatory cytokines in PBMCs were investigated in AF patients and HCs. Compared with controls, the IL‐37 mRNA level was significantly higher in PBMCs of patients with AF (P < 0.0001) and lone AF (P < 0.0001; Figure 2A). Consistently, the expression of pro‐inflammatory cytokine IL‐6 (P < 0.0001; Figure 2B) and CRP (P < 0.0001; Figure 2C) were also notably higher in AF and lone AF patients than in HCs. These data indicated that the expression of IL‐37 in PBMCs was elevated in AF patients, the same as pro‐inflammatory cytokine IL‐6 and CRP.
Figure 2.
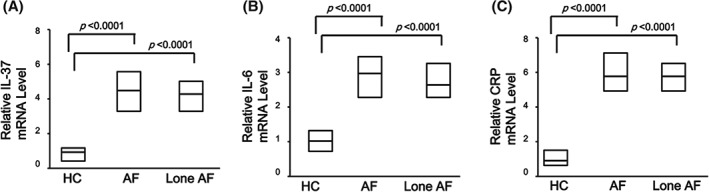
Comparison of (A) level of IL‐37 mRNA in PBMCs, (B) level of IL‐6 mRNA in PBMCs, and (C) level of CRP mRNA in PBMCs in overall AF patients (N = 125), lone AF patients (n = 38), and HCs (n = 42), as determined by RT‐PCR. Results are depicted as box plots; middle line indicates median, bottom of box indicates 25th percentile, and top of box indicates 75th percentile. Mann–Whitney U test and associated P values are indicated. Abbreviations: AF, atrial fibrillation; CRP, C‐reactive protein; HC, healthy controls; IL, interleukin; PBMC, peripheral blood mononuclear cell; RT‐PCR, real‐time polymerase chain reaction.
3.4. IL‐37 mRNA and serum protein levels were higher in paroxysmal and persistent AF compared with permanent AF
We further investigated whether the expression of IL‐37 was related to AF subgroups. Serum IL‐37 level was higher in paroxysmal AF patients compared with HCs, persistent AF patients, and permanent AF patients (P < 0.0001; Figure 3A). Serum IL‐37 level was higher in persistent AF patients compared with HCs and permanent AF patients (P < 0.0001; Figure 3A), whereas there was no significant difference between HCs and permanent AF patients (P = 0.09; Figure 3A). IL‐37 mRNA level analysis in PBMCs showed the same pattern (Figure 3B). These results showed that the expression of IL‐37 corresponded to the AF subgroups.
Figure 3.
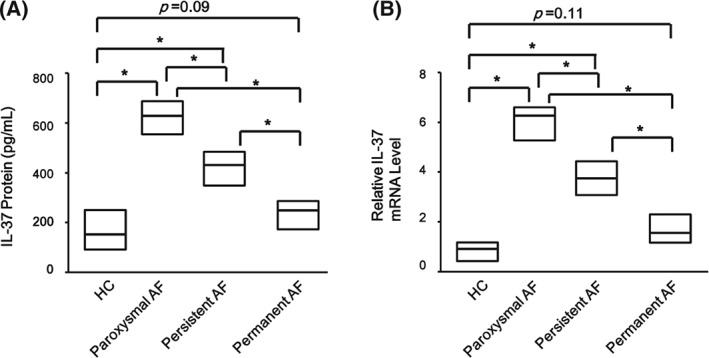
Comparison of IL‐37 protein and mRNA levels among paroxysmal AF, persistent AF, and permanent AF patients with HCs. (A) Serum IL‐37 protein level in AF subgroups compared by ELISA, and (B) IL‐37 mRNA level in PBMCs in AF subgroups compared by RT‐PCR with those from HCs (paroxysmal AF, n = 30; persistent AF, n = 54; permanent AF, n = 41; HCs, n = 42). Results are depicted as box plots; middle line indicates median, bottom of box indicates 25th percentile, and top of box indicates 75th percentile. Mann–Whitney U test and associated P values are indicated. Abbreviations: AF, atrial fibrillation; ELISA, enzyme‐linked immunosorbent assay; HC, healthy controls; IL, interleukin; PBMC, peripheral blood mononuclear cells; RT‐PCR, real‐time polymerase chain reaction. * Indicates P < 0.0001.
3.5. IL‐37 suppressed production of pro‐inflammatory cytokines in PBMCs of AF patients
It has been demonstrated that IL‐37 inhibited the excessive inflammatory response in autoimmune diseases. To investigate whether the elevated IL‐37 was responsible for the down‐regulation of pro‐inflammatory cytokines in PBMCs in AF patients, PBMCs from all AF patients were cultured and either treated or untreated with purified recombinant human IL‐37 protein at concentrations of 100 ng/mL for 24 hours. The cells and cultural supernatants were harvested for RT‐PCR and ELISA analysis, separately. The recombinant IL‐37 markedly reduced the secretion of pro‐inflammatory cytokines IL‐6 (P = 0.0178; Figure 4A) and CRP (P = 0.0112; Figure 4B) in PBMCs of AF patients. Moreover, treatment with recombinant IL‐37 also significantly suppressed the mRNA level of IL‐6 (P = 0.0013; Figure 5A) and CRP (P = 0.0092; Figure 5B) in PBMCs of AF patients. These results suggested that IL‐37 suppressed the excessive inflammation by inhibiting the expression of pro‐inflammatory cytokines.
Figure 4.
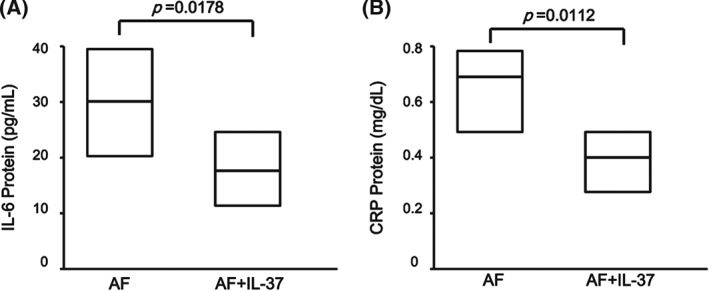
Interleukin‐37 inhibits the secretion of pro‐inflammatory cytokines in PBMCs of patients with AF. PBMCs from AF patients (N = 125) and HCs (n = 42) were stimulated with IL‐37 (100 ng/mL) for 24 hours, and the supernatants were examined for (A) IL‐6 and (B) CRP levels by ELISA. Results are depicted as box plots; middle line indicates median, bottom of box indicates 25th percentile, and top of box indicates 75th percentile. Mann–Whitney U test and associated P values are indicated. Abbreviations: AF, atrial fibrillation; CRP, C‐reactive protein; ELISA, enzyme‐linked immunosorbent assay; HC, healthy controls; IL, interleukin; PBMC, peripheral blood mononuclear cells.
Figure 5.
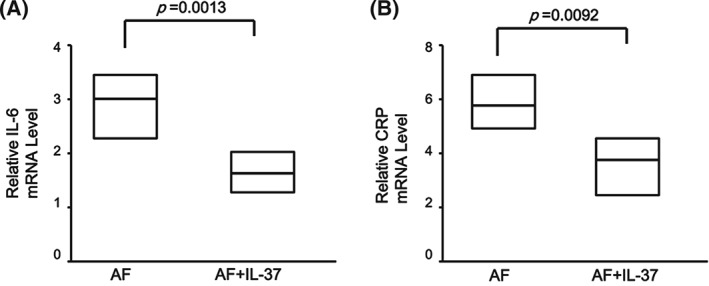
Interleukin‐37 inhibits the expression of pro‐inflammatory cytokines in PBMCs of patients with AF. PBMCs from AF patients (N = 125) and HCs (n = 42) were stimulated with IL‐37 (100 ng/mL) for 24 hours, and the total RNAs were extracted and analyzed for (A) IL‐6 and (B) CRP mRNA levels by RT‐PCR. Results are depicted as box plots; middle line indicates median, bottom of box indicates 25th percentile, and top of box indicates 75th percentile. Mann–Whitney U test and associated P values are indicated. Abbreviations: AF, atrial fibrillation; CRP, C‐reactive protein; ELISA, enzyme‐linked immunosorbent assay; HC, healthy controls; IL, interleukin; PBMC, peripheral blood mononuclear cell; RT‐PCR, real‐time polymerase chain reaction.
4. DISCUSSION
It is well known that there is a significant relationship between atherosclerosis and inflammation.34, 35 Similarly, increasing attention has been paid to the relationship between AF and inflammation. It is considered that this arrhythmia may result from fibrosis or degeneration of atrial muscles, to which underlying heart disease or aging can lead.36
Several studies showed that inflammation process is involved in the pathogenesis of AF.14, 15, 21, 23 It is suggested that inflammation contributes to both occurrence and persistence of AF. Inflammation may impair intracellular calcium current, leading to electrical remodeling in atrial tissue.37 It was shown that higher inflammation status during sinus rhythm potentially increases the risk of occurrence of AF in a follow‐up period.38 These data support that inflammation contributes to occurrence of AF by means of gradually stimulating electrical remodeling.
In the present study, we found the novel association of AF with IL‐37, a marker of anti‐inflammation. IL‐37 was higher in AF patients and lone AF patients when compared with control patients with no history of atrial arrhythmia. In subgroup analyses, higher IL‐37 levels were observed in patients with paroxysmal AF and persistent AF compared with permanent AF, as well as in control patients in sinus rhythm. These results suggest that elevated IL‐37 may be related to the “burden” or type of AF. The association of IL‐37 elevation with AF provides more evidence of the inflammation mechanism in AF development. Treating the PBMCs with IL‐37 significantly suppressed the expression of pro‐inflammatory factor IL‐6 and CRP. It may be considered that inflammation with low anti‐inflammation cytokines may be responsible for resistance to cardioversion in AF patients.
4.1. Study limitations
Our hypothesis requires further studies with more patients and more analysis of pro‐inflammatory and anti‐inflammatory factors. Furthermore, the exclusion criteria are very broad in the current study, and it needs further investigations in detail.
5. CONCLUSION
Our study provides additional support for the role of inflammation in the occurrence of AF, demonstrating that the expression level of IL‐37 is correlated with the clinical manifestations of AF. It is unclear at present whether promotion of IL‐37 level would have a beneficial effect on the clinical incidence or persistence of AF. It is conceivable that the prevention of AF in patients with elevated inflammation might be improved by the use of anti‐inflammatory agents. Nevertheless, the inflammation pathways may provide a potential target for pharmacological interruption or reversal of atrial structural remodeling and AF. Currently available pharmacological treatments for AF have limited efficacy and potentially toxic side effects. Inflammatory mechanisms may form a basis for new, better‐tolerated pharmacological approaches for treating AF. The anti‐inflammatory factor IL‐37 is closely related to AF pathogenesis and subgroups, and it may be useful for preventing AF development.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Supporting Table 1. Patient Characteristics
Supporting Table 2. Patient Characteristics: persistent versus permanent atrial fibrillation
Li W, Li S, Li X, Jiang S and Han B. Interleukin‐37 elevation in patients with atrial fibrillation. Clin Cardiol, 2017;40(2):66–72.
REFERENCES
- 1. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence [published correction appears in Circulation. 2006;114:e498]. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 3. Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrade J, Khairy P, Dobrev D, et al. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 5. Ling LH, Kistler PM, Kalman JM, et al. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol. 2016;13:131–147. [DOI] [PubMed] [Google Scholar]
- 6. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 7. Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new‐onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–1056. [DOI] [PubMed] [Google Scholar]
- 8. Dries DL, Exner DV, Gersh BJ, et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703. [DOI] [PubMed] [Google Scholar]
- 9. Olsson LG, Swedberg K, Ducharme A, et al; CHARM Investigators. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart Failure–Assessment of Reduction in Mortality and Morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997–2004. [DOI] [PubMed] [Google Scholar]
- 10. Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure: a study of 390 patients. Circulation. 1991;84:40–48. [DOI] [PubMed] [Google Scholar]
- 11. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population‐based cohort: the Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 12. Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 13. Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 14. Frustaci A, Chimenti C, Bellocci F, et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. [DOI] [PubMed] [Google Scholar]
- 15. Aviles RJ, Martin DO, Apperson‐Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. [DOI] [PubMed] [Google Scholar]
- 16. Anumonwo JM, Kalifa J. Risk factors and genetics of atrial fibrillation. Heart Fail Clin. 2016;12:157–166. [DOI] [PubMed] [Google Scholar]
- 17. Gaudino M, Andreotti F, Zamparelli R, et al. The ‐174G/C interleukin‐6 polymorphism influences postoperative interleukin‐6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108(suppl 1):II195–II199. [DOI] [PubMed] [Google Scholar]
- 18. Bruins P, te Velthuis H, Yazdanbakhsh AP, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C‐reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–3548. [DOI] [PubMed] [Google Scholar]
- 19. Hijazi Z, Aulin J, Andersson U, et al; ARISTOTLE Investigators. Biomarkers of inflammation and risk of cardiovascular events in anticoagulated patients with atrial fibrillation. Heart. 2016;102:508–517. [DOI] [PubMed] [Google Scholar]
- 20. Masson S, Aleksova A, Favero C, et al; GISSI‐AF Investigators. Predicting atrial fibrillation recurrence with circulating inflammatory markers in patients in sinus rhythm at high risk for atrial fibrillation: data from the GISSI atrial fibrillation trial. Heart. 2010;96:1909–1914. [DOI] [PubMed] [Google Scholar]
- 21. Gedikli O, Dogan A, Altuntas I, et al. Inflammatory markers according to types of atrial fibrillation. Int J Cardiol. 2007;120:193–197. [DOI] [PubMed] [Google Scholar]
- 22. Conway DS, Buggins P, Hughes E, et al. Prognostic significance of raised plasma levels of interleukin‐6 and C‐reactive protein in atrial fibrillation. Am Heart J. 2004;148:462–466. [DOI] [PubMed] [Google Scholar]
- 23. Chung MK, Martin DO, Sprecher D, et al. C‐reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. [DOI] [PubMed] [Google Scholar]
- 24. Banchereau J, Pascual V, O'Garra A. From IL‐2 to IL‐37: the expanding spectrum of anti‐inflammatory cytokines. Nat Immunol. 2012;13:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen HM, Fujita M. IL‐37: a new player in immune tolerance. Cytokine. 2015;72:113–114. [DOI] [PubMed] [Google Scholar]
- 26. Ji Q, Zeng Q, Huang Y, et al. Elevated plasma IL‐37, IL‐18, and IL‐18BP concentrations in patients with acute coronary syndrome. Mediators Inflamm. 2014;2014:165742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Cai X, Chen L, et al. The evaluation of plasma and leukocytic IL‐37 expression in early inflammation in patients with acute ST‐segment elevation myocardial infarction after PCI. Mediators Inflamm. 2015;2015:626934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu D, Wang A, Jiang F, et al. Effects of interleukin‐37 on cardiac function after myocardial infarction in mice. Int J Clin Exp Pathol. 2015;8:5247–5251. [PMC free article] [PubMed] [Google Scholar]
- 29. Fuster V, Rydén LE, Cannom DS, et al; American College of Cardiology Foundation/American Heart Association Task Force. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e269–e367. [DOI] [PubMed] [Google Scholar]
- 30. Fuster V, Rydén LE, Cannom DS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines, European Society of Cardiology Committee for Practice Guidelines, European Heart Rhythm Association, Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society [published correction appears in Circulation 2007;116:e138]. Circulation. 2006;114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 31. Fuster V, Rydén LE, Asinger RW, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines, European Society of Cardiology Committee for Practice Guidelines and Policy Conferences, North American Society of Pacing and Electrophysiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. Circulation. 2001;104:2118–2150. [PubMed] [Google Scholar]
- 32. Camm AJ, Kirchhof P, Lip GY, et al; for the European Heart Rhythm Association, European Association for Cardio‐Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) [published correction appears in Eur Heart J. 2011;32:1172]. Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 33. January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society [published correction appears in Circulation. 2014;130:e272–e274]. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C‐reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. [DOI] [PubMed] [Google Scholar]
- 35. Ridker PM. High‐sensitivity C‐reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. [DOI] [PubMed] [Google Scholar]
- 36. Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J. 1972;34:520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Korantzopoulos P, Kolettis T, Siogas K, et al. Atrial fibrillation and electrical remodeling: The potential role of inflammation and oxidative stress. Med Sci Monit. 2003;9:RA225–RA229. [PubMed] [Google Scholar]
- 38. Watanabe T, Takeishi Y, Hirono O, et al. C‐reactive protein elevation predicts the occurrence of atrial structural remodeling in patients with paroxysmal atrial fibrillation. Heart Vessels. 2005;20:45–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table 1. Patient Characteristics
Supporting Table 2. Patient Characteristics: persistent versus permanent atrial fibrillation


