Abstract
Background
Acute coronary syndrome (ACS) patients are increasingly older. Conventional prognostic scales include chronological age but do not consider vulnerability. In elderly patients, a frail phenotype represents a better reflection of biological age.
Hypothesis:
This study aims to determine the prevalence of frailty and its influence on patients age ≥75 years with ACS.
Methods
Patients age ≥75 years admitted due to type 1 myocardial infarction were included in 2 tertiary hospitals, and clinical data were collected prospectively. Frailty was defined at admission using the previously validated Survey of Health Ageing and Retirement in Europe Frailty Index (SHARE‐FI) tool. The primary endpoint was the combination of death or nonfatal myocardial reinfarction during a follow‐up of 6 months. Major bleeding (hemoglobin decrease ≥3 g/dL or transfusion needed) and readmission rates were also explored.
Results
A total of 234 consecutive patients were included. Frail patients (40.2%) had a higher‐risk profile, based on higher age and comorbidities. On multivariate analysis, frailty was an independent predictor of the combination of death or nonfatal myocardial reinfarction (adjusted hazard ratio [aHR]: 2.54, 95% confidence interval [CI]: 1.12‐5.79), an independent predictor of the combination of death, nonfatal myocardial reinfarction, or major bleeding (aHR: 2.14, 95% CI: 1.13‐4.04), and an independent predictor of readmission (aHR: 1.80, 95% CI: 1.00‐3.22).
Conclusions
Frailty phenotype at admission is common among elderly patients with ACS and is an independent predictor for severe adverse events. It should be considered in future risk‐stratification models.
Keywords: Acute Coronary Syndrome, Frailty, Acute Myocardial Infarction, Prognosis, Aging
1. INTRODUCTION
A recent analysis based on previous registries and official population statistics estimates a significant increase in the incidence of acute coronary syndrome (ACS) over the next 35 to 40 years, parallel to population aging. Thus, from 2016 to 2049, ACS incidence is expected to increase between 69% and 116% in the elderly population.1
It is known that age itself is a prognostic factor for adverse events in ACS patients, but it also predicts an inappropriate treatment, not based on available evidence. Furthermore, the patient group age ≥75 years constitutes a minor role in published ACS studies.2, 3, 4
Aging confers increased morbidity, hospital admissions, falls, institutionalization, and dependence due to a lower resistance to stressors and less functional reserve. This decrease in resilience is known as frailty.5 Frailty is also associated with loss of muscle mass, functional decline, neuroendocrine system dysregulation, or immune suppression. This embodies a different environment, one in which medical acts should be individualized.
The aim of this study is to clarify the role of frailty in the ACS process. Our main goals were (1) to determine the prevalence of frailty at admission and (2) to evaluate the impact of frailty on mortality and reinfarction in patients age ≥75 years with type 1 myocardial infarction (MI).
2. METHODS
This prospective, observational study was conducted in 2 tertiary hospitals in Spain. Both institutional review boards approved the study protocol. Recruitment began in October 2013 and concluded in November 2015.
Patients included were those age ≥75 years admitted to the cardiology department due to an acute MI of type 1 (according to the Universal Definition of Myocardial Infarction6) and who agreed to participate through informed content. Type 1 MI is defined as a spontaneous MI related to ischemia due to a primary coronary event, such as plaque erosion and/or rupture, fissuring, or dissection.6 In our study, patients with type 1 MI were identified by clinical presentation and by discarding other causes that could be responsible for myocardial damage, such those with an increasing in oxygen demand (eg, atrial fibrillation).
Patients who presented in cardiogenic shock, with severe cognitive impairment, with severe dependence measured as an E in the Katz Index of Independence in Activities of Daily Living (ADL), or had a life expectancy <1 year due to oncological disease were excluded. All patients were followed until hospital discharge and 6 months after the event. The attending physician was unaware of the results of frailty assessment throughout the admission phase and follow‐up, so therapeutic strategy was not influenced by our study.
Frailty was assessed in the first 48 hours of admission by an experienced professional using the Survey of Health Ageing and Retirement in Europe Frailty Index (SHARE‐FI) questionnaire, which is validated for the European population.7
The SHARE‐FI index is based on Fried criteria,5 collected in a standardized questionnaire addressing exhaustion, appetite, ambulation, resistance, physical activity, and handgrip strength measurement. Some of these symptoms may reflect angina or heart failure (HF) due to the coronary disease, so questions were literally defined and inquire about physical state over the last month, or over the last 3 months in the case of walking difficulties. Individual ALD parameters were defined as follows:
Exhaustion: “In the last month, have you had too little energy to do the things you wanted to do?” (yes/no)
Diminution of appetite: “Have you been eating more or less than usual?” (yes/no)
Ambulation and resistance: “Because of a health problem, have you had difficulty walking 100 m or climbing 1 flight of stairs without resting in the last 3 months?” (yes/no)
Physical activity: “How often do you engage in activities that require a moderate level of energy, such as cleaning the car or taking a walk?” (an ordinal variable)
Handgrip strength measurement: the Smedley Spring Hand Dynamometer (Saehan Corporation, Seoul, South Korea)
The SHARE‐FI score results in a continuous variable that allows later classification as frail, pre‐frail, and nonfrail. For the purpose of this study, 2 groups were distinguished; frail patients and nonfrail patients (composed by SHARE‐FI pre‐frail and nonfrail). Frailty was analyzed as a dichotomous variable.
Baseline characteristics were collected at admission, including demographic, anthropometric, and clinical information, as well as prognostic indexes: Global Registry of Acute Coronary Events (GRACE) 6‐month mortality, Thrombolysis in Myocardial Infarction (TIMI), and Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Event Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines CRUSADE bleeding scores also were collected at admission. Dependency level was assessed by the Katz ADL index and burden of comorbidity by the Charlson Comorbidity Index. All patients were followed during admission and through 6 months through previously scheduled appointments, urgent admissions or telephone contact, collecting the therapeutic strategy and medical treatment employed in each case.
2.1. Study endpoints
The primary endpoint was to study the relation between frailty at admission and major adverse cardiovascular events at 6 months, expressed by a composite of death or reinfarction. Reinfarction was defined as type 1 according to the Universal Definition of Myocardial Infarction.6
Secondary endpoints were to establish the relation between frailty at admission and 6‐month all‐cause mortality; reinfarction; major bleeding (defined as loss of ≥3 g/dL hemoglobin and/or requiring transfusion); a combination of reinfarction, all‐cause mortality, and major bleeding; and readmission for any cause.
Major bleeding was defined as equivalent to Bleeding Academic Research Consortium (BARC) category ≥3a. Intracranial, intradural, or intraocular bleeding were also registered.
2.2. Statistical analysis
Assuming a prevalence of 50% of frail phenotype among our ACS population age ≥75 years (40%–62%, according to previous studies),8, 9 a major adverse event odds ratio (OR) of 2.2 for the frail population10 and an adverse events rate of 15% for nonfrail population, a total of 217 patients would be required (α = 0.05; β = 0.2; 2‐sided contrast).
Statistical differences between groups were assessed using the χ2 test and Fisher exact test when appropriate for categorical variables, The Student t test was used for continuous variables of normal distribution, and the Mann‐Whitney U test for non‐normal distribution.
Statistical analysis was conducted in 2 phases. First, a univariate analysis was performed to stratify patients according to frailty. Subsequently, a multivariate analysis was done using multiple logistic regression, including within the models potential confounders identified in the univariate analysis. A 2‐sided P < 0.05 was considered significant.
Continuous variables were described as mean ± SD, and median (interquartile range) for cases with skewed distribution. Categorical variables were exposed as frequencies and percentages. The data were analyzed using SPSS version 22.0 (IBM Corp., Armonk, NY).
3. RESULTS
3.1. Baseline characteristics
A total of 234 consecutive patients were included; of those, 94 (40.2%) were identified as frail, 66 (28.2%) as pre‐frail, and 74 (31.6%) as nonfrail patients according to the SHARE‐FI index. For the purpose of this study, 2 groups were identified: frail (n = 94; 40.2%) and nonfrail (n = 140; 59.8%).
Table 1 shows baseline data of the included patients. There were important baseline differences among the studied groups. The frail population was older and more often female, with higher rates of diabetes mellitus (DM), especially insulin‐dependent DM, and with more frequent comorbidities and dependency status. In addition, frail patients had higher rates of previous MI and prior HF. Table 2 shows risk estimation at admission, in‐hospital clinical data, and the therapeutic strategy taken. Overall, frail patients, despite having a higher risk profile, were less frequently managed with an invasive strategy and more frequently discharged without complete revascularization. In‐hospital stay was longer in the frail group.
Table 1.
Baseline characteristics
| Nonfrail, n = 140 | Frail, n = 94 | P Value | |
|---|---|---|---|
| Age, y | 81.6 ± 4.2 | 84.4 ± 5.8 | 0.0001 |
| Female sex | 45 (32.1) | 50 (53.2) | 0.001 |
| BMI, kg/m2 | 26.8 ± 3.6 | 27.3 ± 4.9 | 0.590 |
| HTN | 110 (78.6) | 81 (86.2) | 0.141 |
| DM | 52 (37.1) | 47 (50.0) | 0.051 |
| Insulin dependent | 12 (8.6) | 18 (19.1) | 0.018 |
| Non‐insulin dependent | 40 (28.6) | 29 (30.9) | 0.708 |
| Dyslipidemia | 68 (48.6) | 53 (56.4) | 0.241 |
| Tobacco history | 54 (38.6) | 20 (21.3) | 0.005 |
| ADL dependencea | 1 (0.7) | 45 (48.9) | 0.0001 |
| Previous stroke | 13 (9.3) | 12 (12.8) | 0.398 |
| Previous peptide ulcer | 6 (4.3) | 3 (3.2) | 0.670 |
| Previous MI | 22 (15.7) | 26 (27.7) | 0.027 |
| Previous CABG | 5 (3.6) | 5 (5.3) | 0.517 |
| Previous angioplasty | 10 (7.1) | 16 (17.0) | 0.018 |
| PAD | 16 (11.4) | 17 (18.1) | 0.151 |
| Valvular prosthesis | 1 (0.7) | 2 (2.1) | 0.346 |
| AF | 32 (22.9) | 23 (24.5) | 0.776 |
| Previous HF | 8 (5.7) | 16 (17.0) | 0.005 |
| CCI score | 6.38 ± 1.58 | 8.04 ± 2.11 | 0.0001 |
| Previous medical treatment | |||
| ASA | 49 (35.3) | 53 (56.4) | 0.001 |
| Clopidogrel | 13 (9.4) | 15 (16.0) | 0.128 |
| Ticagrelor | 1 (0.7) | 0 (0.0) | 0.410 |
| VKA/NOAC | 31 (22.5) | 16 (17.0) | 0.311 |
| ACEI/ARB | 82 (59.4) | 61 (64.9) | 0.400 |
| β‐Blocker | 41 (29.5) | 36 (38.3) | 0.161 |
| Loop diuretic | 25 (18) | 35 (37.2) | 0.001 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ADL, activities of daily living; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid (aspirin); BMI, body mass index; CABG, coronary artery bypass grafting; CCI, Charlson Comorbidity Index; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; MI, myocardial infarction; NOAC, novel oral anticoagulant; PAD, peripheral arterial disease; SD, standard deviation; VKA, vitamin K antagonist.
Dependence ≥ B in Katz Index of Independence in ADL.
Data are presented as n (%) or mean ± SD.
Table 2.
Clinical data at admission and therapeutic strategy
| Nonfrail, n = 140 | Frail, n = 94 | P Value | |
|---|---|---|---|
| STEMI | 57 (40.7) | 30 (31.9) | 0.172 |
| SBP, mm Hg | 138 ± 24.1 | 140 ± 31.4 | 0.994 |
| Heart rate, bpm | 75 ± 16.0 | 84 ± 19.0 | 0.0001 |
| Killip‐Kimball class | 0.001 | ||
| I | 100 (71.4) | 43 (45.7) | |
| II | 32 (22.9) | 40 (42.6) | |
| III | 7 (5.0) | 11 (11.7) | |
| Admission LVEF, % | 53 ± 10.65 | 52 ± 12.75 | 0.601 |
| Blood tests | |||
| Peak TnI, ng/mL | 43.7 ± 73.7 | 29.71 ± 56.0 | 0.403 |
| Maximum Cr, mg/dL | 1.13 ± 0.49 | 1.49 ± 0.94 | 0.0001 |
| Admission Hgb, g/dL | 13.78 ± 1.81 | 12.42 ± 1.69 | 0.0001 |
| Maximum BNP, pg/mL | 570 ± 758.77 | 808.49 ± 732.91 | 0.002 |
| Admission CRP, mg/L | 32.0 ± 61.7 | 53.0 ± 75.5 | 0.012 |
| 1,25 vitamin D, ng/mL | 52.9 ± 36.4 | 40.1 ± 34.0 | 0.013 |
| Risk estimation | |||
| GRACE score | 142.4 ± 21.7 | 155.3 ± 24.9 | 0.0001 |
| TIMI score | 3.4 ± 1.2 | 4.2 ± 1.3 | 0.0001 |
| CRUSADE bleeding index | 33.1 ± 12.8 | 48.4 ± 13.4 | 0.0001 |
| In‐hospital therapeutic strategy | |||
| Coronary angiography | 132 (94.3) | 65 (69.1) | 0.0001 |
| Culprit‐lesion angioplasty | 107 (81.7) | 43 (59.7) | 0.001 |
| Complete revascularization at discharge | 71 (53.8) | 25 (32.9) | 0.004 |
| CABG | 3 (2.2) | 1 (1.2) | 0.813 |
| In‐hospital stay, d | 5.93 ± 5.18 | 9.41 ± 12.46 | 0.004 |
| Treatment at discharge | |||
| ASA | 128 (92.8) | 78 (88.6) | 0.288 |
| Clopidogrel | 78 (56.5) | 39 (44.3) | 0.073 |
| Ticagrelor | 43 (31.2) | 20 (22.7) | 0.168 |
| DAPT | 117 (84.8) | 57 (64.8) | 0.0001 |
| OAC | 32 (23.2) | 21 (23.9) | 0.907 |
| DAPT + OAC | 19 (13.8) | 6 (6.8) | 0.104 |
| Aldosterone antagonist | 14 (10.1) | 14 (15.9) | 0.200 |
| ACEI/ARB | 103 (73.6) | 64 (68.1) | 0.735 |
| β‐Blocker | 106 (76.8) | 64 (72.7) | 0.488 |
| Loop diuretic | 43 (31.2) | 52 (59.1) | 0.0001 |
| Statin | 129 (93.5) | 73 (83) | 0.012 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid (aspirin); BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; Cr, creatinine; CRP, C‐reactive protein; DAPT, dual antiplatelet therapy; Hgb, hemoglobin; LVEF, left ventricular ejection fraction; OAC, oral anticoagulant therapy; SBP, systolic blood pressure; SD, standard deviation; STEMI, ST‐segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction; TnI, troponin I.
Data are presented as n (%) or mean ± SD.
Table 2 also shows the treatment strategy at discharge. Frail patients less frequently received dual antiplatelet therapy or statins and were more frequently treated with loop diuretics than were nonfrail patients.
3.2. Impact of frail phenotype on follow‐up
Frail patients had a higher rate of adverse outcomes during the follow‐up. In univariate analysis, the primary endpoint (combined mortality or reinfarction) was significantly higher in the frail group (23 [28.0%] vs 12 [8.8%]; P = 0.0001). Secondary endpoints, except major bleeding, showed a significant association with frailty in univariate analysis (Figure 1).
Figure 1.
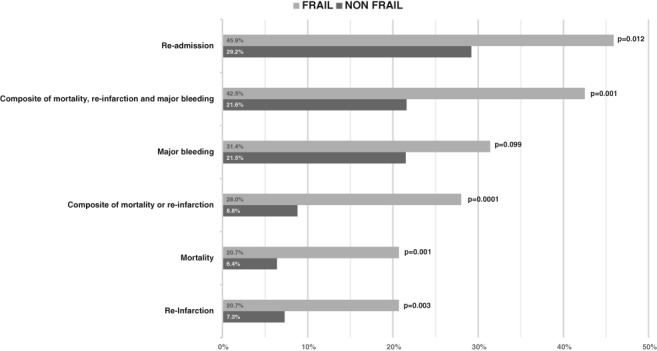
Adverse outcomes at 6 months.
Multivariate analysis of the endpoints is shown in Table 3. After adjustment for age, DM, previous MI, and GRACE score as a continuous variable at admission, frailty was an independent prognostic marker for the composite of death or reinfarction (adjusted hazard ratio: 2.54, 95% confidence interval: 1.12‐5.79). The impact of frailty on the combination of mortality, reinfarction, or major bleeding was also adjusted. Frailty was also independent from age, GRACE score, previous HF, and the presence of peripheral arterial disease for the composite of death, reinfarction, or major bleeding. Frailty was also an independent marker of readmission in 6 months.
Table 3.
Multivariate endpoint analysis (final models)
| Adjusted HR (95% CI) | P Value | |
|---|---|---|
| Factors related to the composite of death or reinfarction. Variables included age, DM, previous infarction, GRACE score, and frailty. | ||
| DM | 2.29 (1.02‐5.17) | 0.046 |
| Previous infarction | 2.32 (0.97‐5.55) | 0.059 |
| GRACE score | 1.03 (1.01‐1.05) | 0.002 |
| Frailty | 2.54 (1.12‐5.79) | 0.026 |
| Factors related to the composite of death, reinfarction, or major bleeding. Variables included age, DM, previous HF, PAD, GRACE score, and frailty. | ||
| PAD | 2.38 (1.04‐5.41) | 0.039 |
| GRACE score | 1.02 (1.01‐1.03) | 0.008 |
| Frailty | 2.14 (1.13‐4.04) | 0.019 |
| Factors related to the possibility of readmission. Variables included age, PAD, previous HF, GRACE score, and frailty. | ||
| PAD | 2.22 (1.00‐4.91) | 0.049 |
| Previous HF | 2.68 (1.03‐6.97) | 0.043 |
| Frailty | 1.80 (1.00‐3.22) | 0.049 |
Abbreviations: CI, confidence interval; DM, diabetes mellitus; HF, heart failure; HR, hazard ratio; PAD, peripheral arterial disease.
Figure 2 shows Kaplan‐Meier curves for the composite of mortality or reinfarction and readmission, respectively, stratified by frailty status.
Figure 2.
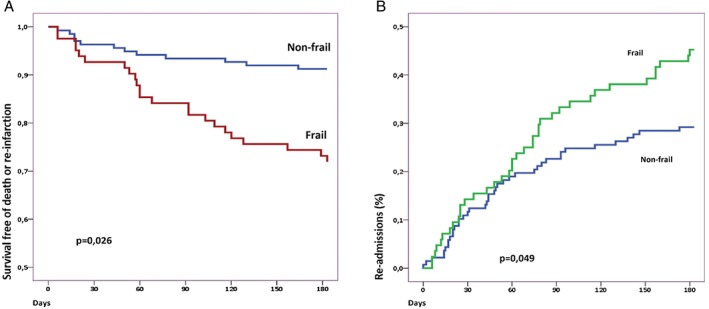
(A) Kaplan‐Meier curve for the composite of reinfarction or mortality stratified according to frailty. (B) Kaplan‐Meier curve for readmission stratified according to frailty.
4. DISCUSSION
In the present study, we found that frailty is an independent and important predictor of higher mortality or reinfarction after type 1 MI. This illustrates the importance of assessing biological age in elderly patients, which predicts worse outcomes regardless of chronological age.10 An important aspect of this study is that it provides support for use of SHARE‐FI in the ACS setting. The SHARE‐FI questionnaire, together with handgrip evaluation, is a simple and effective method for a baseline objective assessment of frailty phenotype at bedside, without need for blood tests or calculating gait speed.
Although the relationship between frailty and prognosis had been extensively studied in association with HF or heart surgery, the impact of prospective evaluation of frailty status in ACS has not been fully explored. Furthermore, methods used for assessment of frailty varied, ranging from retrospective review of patient files9, 11 to adaptation of Fried criteria in a substudy of the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes (TRILOGY ACS) trial investigators12 or subjective, less extensively validated scales.13, 14 The body of information from these studies also suggests a strong relationship between frailty status and poor adverse outcomes in elderly ACS patients.
In another interesting study, Sanchis et al prospectively evaluated a cohort of patients age >65 years who survived an ACS using several geriatric measurements, including frailty (using Fried and Green scores), assessment of physical disability with the Barthel Index, instrumental disability, cognitive impairment, and comorbidity.15 The Green score was the only geriatric independent predictor of mortality. The main limitation of this study is that the evaluation of frailty was performed at discharge, precluding the impact of frailty on acute‐phase adverse outcomes.
Another interesting finding, previously suggested,16, 17 is that elderly, frail patients were less frequently treated with evidence‐based effective strategies, such as cardiac catheterization and revascularization, dual antiplatelet therapy, or statins. Especially important was the selection of a more conservative strategy, considering the clearly higher‐risk profile at admission present in this subgroup. Recent data have shown that an invasive strategy can dramatically improve outcomes in patients age ≥80 years with non–ST‐segment elevation ACS or unstable angina18; however, the role of invasive strategy in the frail population remains unclear.
4.1. Study limitations
This study has several limitations that should be considered. This is a relatively small observational study with prospective data obtained from only 2 centers. Moreover, the number of events was relatively small, so this may weaken the strength of the conclusions.
Frailty assessment, especially handgrip strength, could be influenced by the acute process, and we did not search for possible changes in frailty and dependency status after discharge that could have provided interesting additional information. Bleeding evaluation was limited to severe bleeding (equivalent to BARC type 3 bleeding). We did not collect information on moderate or mild bleeding, such as BARC type 1 or 2, that also could have had implications on physicians’ selection of treatment strategies or patients’ compliance with antiplatelet agents.
Importantly, our risk analysis included variables that could be assessed at the admission level, before decisions regarding treatment selection were made, including coronary invasive studies or revascularization. Therefore, our risk‐model analyses do not consider the impact of an invasive strategy or revascularization on patient outcomes. Finally, our study was not powered to evaluate the impact of different therapeutic strategies on clinical outcomes.
5. CONCLUSION
Frailty is an independent prognostic marker of mortality, reinfarction, and readmission at 6 months in patients age ≥75 years admitted due to acute type 1 MI. Despite having a higher‐risk profile, frail patients less frequently receive evidence‐based treatment, including revascularization strategies.
Author contributions
Drs. G.L. Alonso Salinas, M. Sanmartín‐Fernández, J. Luis Zamorano, and M. Pascual Izco planned the study and analyzed the data. M. Sanmartín‐Fernández and G.L. Alonso Salinas wrote the first draft of the manuscript. All authors reviewed the article, made contributions, and take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflicts of interest
The authors declare no conflicts to disclose.
Supporting information
AppendixS1. Survey of Health Ageing and Retirement in Europe Frailty Index (SHARE–FI)
Alonso Salinas GL, Sanmartin M, Pascual Izco M, et al. Frailty is an independent prognostic marker in elderly patients with myocardial infarction. Clin Cardiol. 2017;40:925–931. 10.1002/clc.22749
Funding information Funding for this study was received from Ministerio de Economía y Competitividad, Fondo de Investigación en Salud–Instituto de Salud Carlos III program ref.: PI13/01973.
REFERENCES
- 1. Dégano IR, Elosua R, Marrugat J. Epidemiology of acute coronary syndromes in Spain: estimation of the number of cases and trends from 2005 to 2049. Rev Esp Cardiol (Engl Ed). 2013;66:472–481. [DOI] [PubMed] [Google Scholar]
- 2. Alexander KP, Newby LK, Cannon CP, et al; American Heart Association Council on Clinical Cardiology; Society of Geriatric Cardiology. Acute coronary care in the elderly, part I: non–ST‐segment elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology, in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–2569. [DOI] [PubMed] [Google Scholar]
- 3. Alexander KP, Newby LK, Armstrong PW, et al; American Heart Association Council on Clinical Cardiology; Society of Geriatric Cardiology. Acute coronary care in the elderly, part II: ST‐segment elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology, in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2570–2589. [DOI] [PubMed] [Google Scholar]
- 4. Alegre O, Ariza‐Solé A, Vidán MT, et al. Impact of frailty and other geriatric syndromes on clinical management and outcomes in elderly patients with non–ST‐segment elevation acute coronary syndromes: rationale and design of the LONGEVO‐SCA Registry. Clin Cardiol. 2016;39:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M146–M156. [DOI] [PubMed] [Google Scholar]
- 6. Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 7. Romero‐Ortuno R, Walsh CD, Lawlor BA, et al. A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC Geriatr. 2010;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Afilalo J, Karunananthan S, Eisenberg MJ, et al. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. [DOI] [PubMed] [Google Scholar]
- 9. Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with 1‐year mortality for elderly patients with non–ST‐segment elevation myocardial infarction. Eur J Prev Cardiol. 2014;21:1216–1224. [DOI] [PubMed] [Google Scholar]
- 10. Alonso Salinas GL, Sanmartín‐Fernández M, Pascual Izco M, et al. Frailty is a short‐term prognostic marker in acute coronary syndrome of elderly patients. Eur Heart J Acute Cardiovasc Care. 2016;5:434–440. [DOI] [PubMed] [Google Scholar]
- 11. Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short‐term outcomes for elderly patients with non–ST‐segment elevation myocardial infarction. Circulation. 2011;124:2397–2404. [DOI] [PubMed] [Google Scholar]
- 12. White HD, Westerhout CM, Alexander KP, et al; TRILOGY ACS investigators . Frailty is associated with worse outcomes in non–ST‐segment elevation acute coronary syndromes: Insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes (TRILOGY ACS) trial. Eur Heart J Acute Cardiovasc Care. 2016;5:231–242. [DOI] [PubMed] [Google Scholar]
- 13. Sujino Y, Tanno J, Nakano S, et al. Impact of hypoalbuminemia, frailty, and body mass index on early prognosis in older patients (≥85 years) with ST‐elevation myocardial infarction. J Cardiol. 2015;66:263–268. [DOI] [PubMed] [Google Scholar]
- 14. Graham MM, Galbraith PD, O'Neill D, et al. Frailty and outcome in elderly patients with acute coronary syndrome. Can J Cardiol. 2013;29:1610–1615. [DOI] [PubMed] [Google Scholar]
- 15. Sanchis J, Bonanad C, Ruiz V, et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J. 2014;168:784–791. [DOI] [PubMed] [Google Scholar]
- 16. Kang L, Zhang SY, Zhu WL, et al. Is frailty associated with short‐term outcomes for elderly patients with acute coronary syndrome? J Geriatr Cardiol. 2015;12:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alonso Salinas GL, Sanmartín Fernández M, Pascual Izco M, et al. Frailty predicts major bleeding within 30 days in elderly patients with acute coronary syndrome. Int J Cardiol. 2016;222:590–593. [DOI] [PubMed] [Google Scholar]
- 18. Tegn N, Abdelnoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non–ST‐elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open‐label randomised controlled trial. Lancet. 2016;387:1057–1065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS1. Survey of Health Ageing and Retirement in Europe Frailty Index (SHARE–FI)


