Abstract
Background
With multiple cholesterol guidelines, we evaluated the accuracy of recommended statin therapy on identifying coronary artery calcium (CAC) and cardiovascular disease (CVD) events by 2004 NCEP/ ATP III, 2016 ESC/EAS, and 2013 ACC/AHA guidelines.
Hypothesis
ACC/AHA guidelines are more accurate in identifying persons at risk for CVD.
Methods
5002/6814 participants age <75 years and free of CVD were included. CAC categories (>0, ≥100, and ≥300) and 10 years of CVD outcomes were considered. Sensitivity (SN), specificity (SP), positive and negative predictive value (PPV and NPV), and likelihood ratios (LR) were calculated. Mean age was 59 years; 47% of subjects were males.
Results
1297 (26%), 1381 (28%), and 2538 (51%) had class I indications for statin/LLT by the NCEP ATP III, ESC/EAS, and AHA/ACC guidelines, respectively. SN, SP, NPV, and PPV for CAC ≥300 were: NCEP ATP III (41.1%, 75.5%, 93.3% and 13.4%), ESC/EAS (54.1%, 74.8%, 94.6% and16.6%), and ACC/AHA (87.2%, 52.6%, 97.8% and 14.5%). SN, SP, PPV, and NPV for corresponding CVD outcomes were: NCEP ATP III (45.8%, 75.1%, 96.3%, and 8.9%), ESC/EAS (50.5%, 72.9%, 98.7%, and 3.6%), and AHA/ACC (79.6%, 50.7%, 98%, and 7.7%). ESC/EAS had significantly higher positive LR 2.15 (95% CI, 1.95 – 2.38) and ACC/AHA had significantly lower negative LR [0.24, (95% CI 0.19 – 0.31)] for corresponding CVD.
Conclusions
Despite the increased in SN of statin eligibility by the ACC/AHA, it has similar NPV and PPV for CAC/future CVD events. The ACC/AHA class I indications for statin may be a superior screening tool for subclinical and clinical CVD.
Keywords: Preventive cardiology, coronary artery calcium score, General clinical cardiology/adult, computed tomography < Imaging, cardiovascular guidelines
1. INTRODUCTION
Statin therapy remains one of the most effective interventions and risk‐factor modifications to reduce future atherosclerotic cardiovascular disease (ASCVD) events and mortality.1, 2 Identifying persons at high risk for future ASCVD events, especially those without known disease (ie, primary prevention), remains a challenge. Accordingly, guidelines have been issued to help identify high‐risk groups (persons with class I recommendation) for statin/lipid‐lowering therapy (LLT).3, 4, 5
The 2004 National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults,4 the 2016 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) Guidelines for the Management of Dyslipidemias,5 and the 2013 American College of Cardiology/American Heart Association (ACC/AHA) Guidelines for the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk3 are the predominant guidelines used in the United States and Europe for ASCVD risk assessment and statin/LLT recommendations. With the initial debates surrounding the ACC/AHA guidelines recommendations,6, 7, 8 and with more recent publications looking at risk‐prediction equations and guideline discrimination,9, 10 the respective accuracy of each guideline's ability to identify respective future cardiovascular (CV) events and potentially identify early subclinical disease remains unclear.
Each guideline uses different risk prediction and assessments to recommend statin/LLT for primary prevention3, 4, 5 and targets the reduction of different composite cardiovascular disease (CVD) outcomes. However, each composite outcome shares the common underlying pathophysiology of atherosclerosis,11 a continuum of vascular pathology/dysfunction that manifests as subclinical and clinical disease.12 Coronary artery calcium (CAC) score is a measure of subclinical atherosclerosis and has been shown to be an independent predictor of clinical ASCVD events.13, 14, 15, 16 The ability of cholesterol guidelines to identify those at higher risk for subclinical disease may provide more information on the accuracy of each guideline's statin/LLT assignment and better inform the patient‐clinician dialogue on ASCVD risk reduction.
In this report, we use data of persons in the Multi‐Ethnic Study of Atherosclerosis (MESA) to compare the accuracy of class I recommendations for statin/LLT by each of the 3 cholesterol guidelines in identifying persons with future clinical atherosclerotic disease and CAC.
2. METHODS
MESA (study design previously published17) is a prospective cohort study investigating the prevalence, correlates, and progression of ASCVD in persons free of ASCVD at baseline. The cohort contains 6814 participants age 45 to 84 years, representing 6 communities in the United States: Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; northern Manhattan, New York; and St. Paul, Minnesota. The adult participants are 38% Caucasian, 28% African American, 22% Hispanic, and 12% Chinese, defined by self‐reported race/ethnicity.
Data were taken from the first examination (July 2000–August 2002). Type 2 diabetes mellitus (DM) was defined as a self‐reported history, DM medication use, or fasting glucose ≥126 mg/dL. Current smoking was defined as having smoked a cigarette in the last 30 days. The average of the second and third readings of resting blood pressure was recorded. Hypertension was defined as a systolic blood pressure of ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medication. Body mass index was calculated as weight (kg) divided by height squared (m2). Total cholesterol and high‐density lipoprotein cholesterol were measured after a 12‐hour fast. Low‐density lipoprotein cholesterol (LDL‐C) was estimated by the Friedewald equation.18 Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD) equation, where sCr is serum creatinine: eGFR_MDRD = 175 × sCr−1.154 × age−0.203 (×1.212 if patient is black) (×0.742 if female).
We excluded persons with missing covariates for ASCVD risk prediction (n = 169), those taking statins at baseline exam (n = 1009), and those age >75 years (n = 634). After these exclusions, 5002 of the total 6814 participants remained.
The MESA study was approved by the institutional review boards of each site, and written informed consent was obtained from all participants.
Computed tomography scanning/interpretation methods in MESA have been previously reported.19 All participants were scanned twice over phantoms of known calcium concentration. Scans were read at a central reading center (Los Angeles Biomedical Research Institute at Harbor–UCLA, Torrance, California). Mean Agatston score for the 2 scans was used.20 Intraobserver and interobserver agreement were excellent (κ = 0.93 and κ = 0.90, respectively).
CV events, including fatal and nonfatal myocardial infarction (MI), coronary heart disease (CHD) death, fatal and nonfatal stroke, and sudden cardiac death, were adjudicated by MESA study committee members, including cardiologists, epidemiologists, and neurologists. A detailed description of the adjudication process has been published (http://www.mesa.nhlbi.org).21
For cross‐sectional analysis, baseline coronary atherosclerosis was defined using CAC score at initial examination. Significant subclinical coronary atherosclerosis was defined as CAC score ≥300 Agatston units and/or ≥75th percentile for age, sex, and ethnicity, as specifically mentioned in the ACC/AHA guidelines as a factor influencing ASCVD risk.3 Additionally, CAC >0 and CAC ≥100 were examined, as these scores have been shown to modulate risk.15, 22
Incident CV events were defined by designations in each guideline. For the NCEP ATP III, outcomes were nonfatal/fatal MI and fatal CHD. For ESC/EAS, outcomes were all fatal ASCVD events, including MI, stroke, occlusive atherosclerotic disease, and sudden cardiovascular death. For ACC/AHA, outcomes were all ASCVD events, including fatal/nonfatal MI, fatal/nonfatal stroke, and fatal CHD. Outcomes were ascertained through 10 years from baseline exam (October 2012), as this corresponds to the 10‐year risk‐prediction tools for future CV events found in each guideline.
For the NCEP ATP III, class I statin recommendations for statin/LLT were defined as persons with (1) 2+ risk factors with calculated 10‐year CHD risk of ≥20% and LDL‐C ≥100 mg/dL; (2) 2+ risk factors with 10‐year risk 10% to 20% and LDL‐C ≥130 mg/dL; (3) 2+ risk factors with 10‐year risk <10% and LDL‐C ≥160 mg/dL; (4) LDL‐C ≥190 mg/dL; and (5) DM.4 CHD risk was assessed using the Framingham Risk Score.23
For the ESC/EAS, class I recommendations for statin therapy/LLT were defined as persons with (1) 10‐year ASCVD mortality risk of ≥10% and LDL‐C ≥70 mg/dL; (2) 10‐year ASCVD risk of 5% to 10% and LDL‐C ≥100 mg/dL.5 ASCVD mortality risk was calculated using the Systematic Coronary Risk Evaluation (SCORE) equation.24
For the ACC/AHA, class I recommendations for statin/LLT were defined as persons with (1) 10‐year risk of ASCVD ≥7.5%; (2) DM; and (3) LDL‐C ≥190 mg/dL.3 ASCVD risk was estimated by the ASCVD pooled cohort equation.3
The Framingham Risk Score and SCORE risk prediction equations were applied to all persons in this cohort regardless of ethnicity. The SCORE risk prediction equation for low‐risk regions in Europe was used given that MESA is a low‐ASCVD‐risk population. Separate pooled cohort equation from the ACC/AHA guidelines are recommended for non‐Hispanic Whites and non‐Hispanic African Americans and were applied to persons in this cohort as indicated. The ACC/AHA guidelines recommends using the “Caucasian” calculator in other racial and ethnic populations, and this was done as indicated in persons from our cohort. However, ASCVD risk may be lower in Hispanics and persons of East Asian ancestry.25
2.1. Statistical analysis
Baseline characteristics were compared between groups with class I recommendations for statin/LLT by analysis of variance or χ2 tests. Kaplan‐Meier analysis was used to compare the event‐free survivals between those meeting statin/LLT (class I indication) and those who did not. The sensitivity (SN), specificity (SP), negative predictive value (NPV), positive predictive value (PPV), positive likelihood ratio (LR),26 and negative LR were calculated for statin/LLT‐eligible persons and the presence or absence of the following CAC categories (0 vs >0, <100 vs ≥100, and <300 vs ≥300). Additionally, CAC >300 or ≥75th percentile for age, sex, and ethnicity vs CAC <300 or <75th percentile for age, sex, and ethnicity was examined given the specific mention in the ACC/AHA guidelines. SN represents each guideline's ability to help identify those without a specific CAC score and/or CV event, whereas SP represents the ability to help identify those with a specific CAC score and/or future CV event. PPV and NPV represent the predictive value of statin/LLT‐qualifying persons having a designated CAC score and/or CV event in the MESA population. Positive LR represents the increased likelihood of a given CAC or CV event in statin/LLT‐qualifying persons and negative LR represents the decreased likelihood of a specific CAC score and/or CV event if persons do not qualify for statin/LLT. Accuracy measures were compared across guidelines using the McNemar test.
Kaplan‐Meier analysis was used to plot event‐free survival of participants with and without a class I indication for statin/LLT for the corresponding CVD outcomes for each guideline.
Analysis for CAC categories was repeated for each guideline and corresponding CVD outcomes. Area under the curve (AUC) analyses were used to compare the discriminative ability/c‐statistics of class I indications for statin/LLT and corresponding CVD outcomes. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, North Carolina).
3. RESULTS
We analyzed 5002 MESA participants. Of these, 1297 (26%), 1381 (28%), and 2538 (51%) had a class I recommendation for statin/LLT under the NCEP ATP III, ESC/EAS, and ACC/AHA guidelines, respectively. After mean follow‐up of 10.2 years, 117/1297 (9.0%), 50/1381 (3.6%), and 195/2538 (7.7%) persons with class I recommendation by NCEP ATP III, ESC/EAS, and ACC/AHA cholesterol guidelines had an adjudicated incident CHD, ASCVD death, and ASCVD event, respectively. Table 1 shows the baseline characteristics of all persons and those with class I recommendations for statin/LLT by each guideline.
Table 1.
Clinical characteristics of the study cohort (N = 5002)
| Characteristics | Entire Cohort, N = 5002 | 2004 NCEP ATP III, n = 1297 | 2016 ESC/EAS, n = 1381 | 2013 AHA/ACC, n = 2538 |
|---|---|---|---|---|
| Mean age, y | 59.2 ± 8.7 | 61.7 ± 8.1 | 65.2 ± 7.6 | 64.4 ± 7.4 |
| Male sex | 47.3 | 58.4 | 55.0 | 60.4 |
| Race/ethnicity | ||||
| Caucasian | 37.6 | 31.3 | 34.0 | 33.5 |
| African American | 27.6 | 29.1 | 31.3 | 33.5 |
| Chinese | 12.0 | 10.1 | 10.9 | 10.6 |
| Hispanic | 22.8 | 27.6 | 28.8 | 22.4 |
| DM | 11.8 | 45.5 | 42.7 | 23.2 |
| Antihypertensive medication use | 30.5 | 40.6 | 48.2 | 44.7 |
| Family history | 40.7 | 42.1 | 42.1 | 42.6 |
| Smoking | ||||
| Current | 15.0 | 18.7 | 17.0 | 19.0 |
| Former | 35.5 | 37.5 | 37.5 | 38.1 |
| Never | 49.5 | 43.9 | 45.5 | 42.9 |
| SBP, mm Hg | 124.3 ± 20.6 | 130.6 ± 21.4 | 136.3 ± 22.2 | 133.3 ± 20.8 |
| DBP, mm Hg | 72.3 ± 10.4 | 73.9 ± 10.6 | 74.7 ± 10.8 | 74.8 ± 10.4 |
| LDL‐C, mg/dL | 119.6 ± 31.3 | 141.0 ± 36.8 | 122.8 ± 31.6 | 122.6 ± 33.8 |
| HDL‐C, mg/dL | 50.9 ± 15.0 | 45.6 ± 11.0 | 48.3 ± 13.6 | 48.3 ± 14.2 |
| Total cholesterol, mg/dL | 196.2 ± 35.6 | 215.9 ± 41.0 | 199.1 ± 36.8 | 198.5 ± 38.5 |
| Glucose, mg/dL | 103.2 ± 30.5 | 127.0 ± 50.5 | 125.4 ± 49.4 | 112.7 ± 39.5 |
| BMI, kg/m2 | 28.4 ± 5.6 | 29.6 ± 5.7 | 29.4 ± 5.7 | 28.8 ± 5.5 |
| Incident ASCVD | 4.9 | 8.0 | 8.2 | 7.7 |
| Incident ASCVD mortality | 2.0 | 3.1 | 3.6 | 3.3 |
| Incident CHD | 3.3 | 5.7 | 5.8 | 5.0 |
| CAC, Agatston units | ||||
| >0 | 42.2 | 57.0 | 59.7 | 58.1 |
| ≥100 | 17.5 | 27.1 | 30.7 | 28.6 |
| ≥300 | 8.5 | 14.5 | 16.6 | 13.4 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CAC, coronary artery calcium; CHD, coronary heart disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
Table 1 shows prevalence of CAC by threshold in the entire cohort and by statin/LLT eligibility. Notably, >40% of statin/LLT‐eligible persons by all 3 guidelines have a CAC score of zero. Table 2 shows measures of accuracy of statin/LLT recommendations and defined CAC ≥300, CAC ≥100, and CAC >0 Agatston units. The ACC/AHA had a significantly higher SN for CAC ≥300 (87.2%), CAC ≥100 (83.1%), and CAC >0 (69.8%) than both the ESC/EAS and NCEP ATP III guidelines (P < 0.000001). Additionally, the ACC/AHA had a significantly lower specificity for CAC ≥300, ≥100, and >0 (52.6%, 56.1%, and 63.2%) compared with ESC/EAS and NCEP ATP III guidelines (P < 0.05). Similar trends in SN and SP were seen across all 3 guidelines when CAC ≥300 or ≥75th percentile for age, sex, and ethnicity was examined (Table 2). NPV and PPV are also shown and were similar across all 3 guidelines.
Table 2.
SN, SP, PPV, and NPV of statin/LLT eligibility criteria defined by 3 cholesterol treatment guidelines for CAC values
| CAC Score, Agatston Units | 2004 NCEP ATP III | 2016 ESC/EAS | 2013 ACC/AHA | |
|---|---|---|---|---|
| CAC ≥300 | SN | 41.1%1 | 54.1%1 | 87.2%1 |
| SP | 75.5% | 74.8% | 52.6%1 | |
| NPV | 93.3% | 94.6% | 97.8% | |
| PPV | 13.4% | 16.6% | 14.5% | |
| Negative LR (95% CI) | 0.78 (0.72‐0.85) | 0.61 (0.55‐0.68) | 0.24 (0.19‐0.31) | |
| Positive LR (95% CI) | 1.68 (1.48‐1.90) | 2.15 (1.95‐2.38) | 1.84 (1.76‐1.93) | |
| CAC ≥100 | SN | 40.2%1 | 48.5%1 | 83.1%1 |
| SP | 77.1% | 76.8% | 56.1%1 | |
| NPV | 85.9% | 87.5% | 94.0% | |
| PPV | 27.1% | 30.7% | 28.6% | |
| Negative LR (95% CI) | 0.78 (0.73‐0.82) | 0.67 (0.63‐0.72) | 0.30 (0.26‐0.35) | |
| Positive LR (95% CI) | 1.75 (1.59‐1.94) | 2.09 (1.91‐2.28) | 1.89 (1.81‐1.98) | |
| CAC >0 | SN | 35.0%1 | 39.1%1 | 69.8%1 |
| SP | 80.7% | 80.8% | 63.2%1 | |
| NPV | 62.9% | 64.5% | 74.2% | |
| PPV | 57.0% | 59.7% | 58.1% | |
| Negative LR (95% CI) | 0.81 (0.78‐0.84 | 0.75 (0.73‐0.78) | 0.47 (0.44‐0.51) | |
| Positive LR (95% CI) | 1.81 (1.65‐1.99) | 2.03 (1.85‐2.22) | 1.90 (1.80‐2.01) | |
| CAC ≥300 + 75% | SN | 36.3%1 | 39.4%1 | 66.3%1 |
| SP | 77.2% | 75.9% | 53.9%1 | |
| NPV | 80.1% | 80.6% | 84.2% | |
| PPV | 32.4% | 33.0% | 30.2% | |
| Negative LR (95% CI) | 0.82 (0.79‐0.86) | 0.80 (0.76‐0.84) | 0.62 (0.57‐0.68) | |
| Positive LR (95% CI) | 1.59 (1.46‐1.75) | 1.62 (1.50‐1.79) | 1.44 (1.36‐1.52) |
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; CAC, coronary artery calcium; CI, confidence interval; ESC/EAS, European Society of Cardiology/European Atherosclerosis Society; LLT, lipid‐lowering therapy; LR, likelihood ratio; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; NPV, negative predictive value; PPV, positive predictive value; SN, sensitivity; SP, specificity.
P < 0.05 compared with the other 2 guidelines.
Table 2 also shows the likelihood ratios for each guideline's recommendation for statin/LLT and CAC ≥300, ≥100, and >0. ESC/EAS guidelines had a significantly higher positive LR (2.15, 95% confidence interval [CI]: 1.95‐2.38) compared with the ACC/AHA and NCEP ATP III guidelines (1.84, 95% CI: 1.76‐1.93 and 1.68, 95% CI: 1.48‐1.90, respectively). However, the ACC/AHA guidelines had a significantly lower negative LR (0.24, 95% CI: 0.19‐0.31) compared with the 2 other guidelines (Table 2).
The AUC (95% CIs) for a class I indication for statin/LLT and CAC ≥300 for the NCEP ATP III, ESC/EAS, and ACC/AHA guidelines were 0.57 (0.55‐0.60), 0.67 (0.65‐0.70), and 0.70 (0.68‐0.71), respectively.
In Kaplan‐Meier analyses, participants eligible for statin/LLT under each guideline had a significantly higher event rate compared with those ineligible for therapy (hazard ratios and 95% confidence intervals: NCEP ATP III: 2.24 [1.74‐2.88]; ESC/EAS: 2.41 [1.87‐3.10]; ACC/AHA: 4.10 [3.01‐5.60]; Figure 1). Additionally, those ineligible for statin therapy by the ACC/AHA guidelines had a lower risk for future CV events compared with those ineligible by the NCEP ATP III and ESC/EAS (Figure 1).
Figure 1.
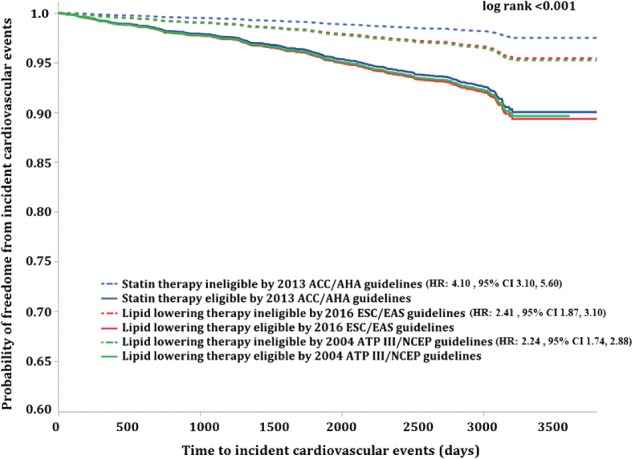
Time to incident respective CV event analysis for statin/LLT eligible vs ineligible by each guideline. Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; ATP III NCEP, Adult Treatment Panel III National Cholesterol Education Program; CI, confidence interval; CV, cardiovascular; ESC/EAS, European Society of Cardiology/European Atherosclerosis Society; HR, hazard ratio; LLT, lipid‐lowering therapy.
Table 3 shows the ACC/AHA statin/LLT eligibility had the highest SN (79.6%) and lowest SP (50.7%) for respective CV outcomes compared with NCEP ATP III and ESC/EAS. Additionally, the ACC/AHA had a significantly lower negative LR (0.40, 95% CI: 0.31‐0.52) compared with the 2 other guidelines for future CV events. The NCEP ATP III and ESC/EAS did not have significantly different positive LR for future cardiovascular events (1.84, 95% CI: 1.60‐2.13 and 1.86, 95% CI: 1.52‐2.27). Though the ACC/AHA also had a lower positive LR [1.61, 95% CI: 1.50‐1.73) compared with the other guidelines, this did not reach statistical significance. NPV and PPV for each guideline and respective CV outcome are also shown, with the ESC/EAS having a lower PPV compared with the other 2 guidelines (Table 3).
Table 3.
Sensitivity, specificity, positive predictive value and negative predictive values of statin eligibility criteria defined by three cholesterol treatment guidelines for respective cardiovascular outcomes
| 2004 NCEP/ATP III | 2016 ESC/EAS | 2013 ACC/AHA | |
|---|---|---|---|
| Outcome Event | CHD | Incident ASCVD mortality | Incident ASCVD |
| Sensitivity | 45.8% | 50.5% | 79.6% |
| Specificity | 75.1% | 72.9% | 50.7% |
| Negative Predictive Value | 96.3% | 98.7% | 98.0% |
| Positive Predictive Value | 8.9% | 3.6% | 7.7% |
| Positive Likelihood Ratio | 1.84 (1.60 – 2.13) | 1.86 (1.52 – 2.27) | 1.61 (1.50 – 1.73) |
| Negative Likelihood Ratio | 0.72 (0.64 – 0.81) | 0.68 (0.56 – 0.83) | 0.40 (0.31 – 0.52) |
The analysis is stratified by sex, age, and race/ethnicity in Supporting Information, Table 1, in the online version of this article. Notably, the ACC/AHA guidelines had a low specificity (19.5%) for older adults (age ≥60 years). In comparing accuracy in males vs females, the ACC/AHA had a higher SN and lower SP in males compared with females, in which SN and SP were similar. In the majority of cases, eligibility for statin/LLT therapy under the ACC/AHA guidelines for primary prevention of ASCVD was based on the risk of 10‐year predicted ASCVD ≥7.5% (n = 2388). The 10‐year predicted ASCVD ≥7.5% criteria had a higher SN and lower SP compared with statin eligibility based on LDL‐C ≥190 mg/dL and DM (see Supporting Information, Table 2, in the online version of this article).
The AUC (95% CIs) for statin/LLT eligibility and CV outcomes under the NCEP ATP III, ESC/EAS, and ACC/AHA guidelines are 0.59 (0.56‐0.62), 0.63 (0.60‐0.66), and 0.66 (0.63‐0.68), respectively.
4. DISCUSSION
We compared the accuracy of class I indications for statin/LLT eligibility by the 3 major cholesterol guidelines for (1) prevalent CAC (a measure of subclinical coronary atherosclerosis) and (2) 10 years of adjudicated corresponding composite CV outcomes in the most ethnically diverse, prospectively followed cohort in the United States. Our study showed an increased SN of the 2013 ACC/AHA class I indications for statin/LLT eligibility compared with the 2004 NCEP ATP III and 2016 ESC/EAS guidelines for prevalent CAC and incident future CV events. Additionally, the ACC/AHA had similar NPV compared with the 2 other guidelines and improved negative LR. Thus, despite the controversy surrounding these cholesterol guidelines, the ACC/AHA guidelines appear to be a better screening tool for statin/LLT recommendation compared with the other 2 cholesterol guidelines.
Class I indications of each of the cholesterol guidelines are screening tools for identifying asymptomatic individuals at high risk for CVD. Ideal screening tools have 2 major objectives: (1) ability to detect disease at a stage when treatment can be effective, rather than waiting for clinical presentations; and (2) ability to identify individuals with risk factors that increase the likelihood of developing the disease to modify disease risk.27 In this analysis, the ACC/AHA class I indications have a better SN for both the presence of CAC (early‐stage atherosclerosis) and also for future CVD. This study shows that the ACC/AHA class I indications may be a better screening tool and therefore supports the replacement of the 2 other guidelines with the ACC/AHA guidelines for ASCVD risk assessment in asymptomatic adults and for determining statin/LLT eligibility for primary prevention of future ASCVD events.
Additionally, our results show that persons in whom statins/LLT are not recommended by ACC/AHA have a lower risk of future CV events, compared with the NCEP ATP III and ESC/EAS guidelines. This further informs the clinician‐patient dialogue on statin/LLT and primary prevention of CV disease. A “statin not recommended” designation by the ACC/AHA guidelines provides better reassurance toward freedom from future ASCVD events.
Despite the higher SN of the ACC/AHA class I indications for statin/LLT, this guideline has a lower SP for future ASCVD events and prevalent CAC. This finding is consistent with the criticism leveled against the ACC/AHA guidelines: its use for ASCVD risk assessment likely would significantly increase the number of individuals inappropriately treated with statin/LLT therapy. This has additionally been shown in populations outside the United States.10, 28
Low SP may not be important if the therapy under consideration to reduce the risk is cost‐effective and has insignificant side effects.29 Statins are relatively inexpensive but have well‐publicized side effects. These side effects, generating significant debate in their use for primary prevention, are clinically significant and affect patient compliance.30, 31, 32, 33 Additionally, statin intolerance and side effects definitions are not universally agreed upon,34 and re‐challenging with statin therapy may be successful.35 Whether observed side effects represent a true statin intolerance or not (innovative study designs have cast doubt on this36, 37), adherence rates can be quite variable.38 Some data on incidence/prevalence of statin side effects may be underestimated because many statin trials exclude participants with side effects during the single blinded run‐in phase.39 Thus, the incidence of side effects from statins may be quite variable and difficult to identify. The lower SP of the ACC/AHA class I indications for statin/LLT becomes significantly relevant in the lifelong statin treatment discussion between clinicians and patients. Improvement of the SP of the ACC/AHA class I indications with other markers (such as CAC) could mitigate this issue.22
4.1. Study limitations
The strengths of this study include the large sample size, the multiethnic nature of this cohort, baseline screening of CAC, and the use of observed 10‐year adjudicated CV events. However, our study had several limitations. MESA is a low‐risk population that may have increased health awareness and interest, and low CV event rates may affect certain measures of accuracy affected by event prevalence. Although we excluded participants taking statins at baseline, approximately 25% of the cohort took statins for variable periods during follow‐up. This may affect the incidence of CV events in this population. However, crude adjustment for statin use or elimination of this subcohort did not significantly change our point estimates for CV event rates or our overall conclusions.
MESA is an observational study, and residual confounding from an observational design may influence our results. Finally, although MESA is one of the most ethnically diverse prospective studies in the United States, the ethnic constituents and their proportions do not perfectly match those of the overall US population. Notably, MESA lacks a significant South Asian population, which may have higher ASCVD events and mortality.40
5. CONCLUSION
The 2013 ACC/AHA class I indications for statin eligibility are an improved screening tool for the identification of asymptomatic individuals with subclinical coronary atherosclerosis (CAC) and also those at risk for future clinical CVD, compared with the 2004 NCEP ATP III and 2016 ESC/EAS class I indications for statins/LLT. The lower SP coupled with the known side effects of statins suggest that additional testing to improve the SP of the ACC/AHA statin eligibility criteria may be needed.
Supporting information
Supplemental Table 1: Accuracy of statin/lipid lowering therapy eligibility criteria defined by three cholesterol treatment guidelines for cardiovascular outcomes by sex, age, and race/ethnicity
Supplemental Table 2: Sensitivity, specificity, positive predictive value and negative predictive values of individual statin eligible subgroups defined by 2013 ACC/AHA cholesterol guidelines for incident ASCVD*
ACKNOWLEDGMENTS
The authors thank Karen P. Klein, M.A., for editing this work (Biomedical Research Services and Administration, Wake Forest School of Medicine). The authors also thank the investigators, the staff, and the participants of the MESA study for their valuable contributions.
Conflicts of interest
The authors declare no potential conflicts of interest.
Flueckiger P, Qureshi W, Michos ED, Blaha M, Burke G, Sandfort V, Herrington D and Yeboah J. Guideline‐based statin/lipid‐lowering therapy eligibility for primary prevention and accuracy of coronary artery calcium and clinical cardiovascular events: The Multi‐Ethnic Study of Atherosclerosis (MESA). Clin Cardiol. 2017;40:163–169. 10.1002/clc.22642
Funding information This research was supported by contracts N01‐HC‐95159 through N01‐HC‐95167. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
REFERENCES
- 1. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naci H, Brugts JJ, Fleurence R, et al. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all‐cause mortality: a network meta‐analysis of placebo‐controlled and active‐comparator trials. Eur J Prev Cardiol. 2013;20:641–657. [DOI] [PubMed] [Google Scholar]
- 3. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(25 suppl 2):S46–S48]. Circulation . 2014;129(25 suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 4. Grundy SM, Cleeman JI, Merz CN, et al; American College of Cardiology Foundation and American Heart Association . Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines [published correction appears in Circulation. 2004;110:763]. Circulation . 2004;110:227–239. [DOI] [PubMed] [Google Scholar]
- 5. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) . Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J . 2016. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 6. Pencina MJ, Navar‐Boggan AM, D'Agostino RB Sr, et al. Application of new cholesterol guidelines to a population‐based sample. N Engl J Med. 2014;370:1422–1431. [DOI] [PubMed] [Google Scholar]
- 7. Nissen SE. Prevention guidelines: bad process, bad outcome. JAMA Intern Med. 2014;174:1972–1973. [DOI] [PubMed] [Google Scholar]
- 8. Alagona P Jr, Ahmad TA. Cardiovascular disease risk assessment and prevention: current guidelines and limitations. Med Clin North Am. 2015;99:711–731. [DOI] [PubMed] [Google Scholar]
- 9. DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kavousi M, Leening MJ, Nanchen D, et al. Comparison of application of the ACC/AHA guidelines, Adult Treatment Panel III guidelines, and European Society of Cardiology guidelines for cardiovascular disease prevention in a European cohort. JAMA. 2014;311:1416–1423. [DOI] [PubMed] [Google Scholar]
- 11. Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol . 2006;47(8 suppl):C7–C12. [DOI] [PubMed] [Google Scholar]
- 12. Shaw LJ, Raggi P, Berman DS, et al. Coronary artery calcium as a measure of biologic age. Atherosclerosis. 2006;188:112–119. [DOI] [PubMed] [Google Scholar]
- 13. Church TS, Levine BD, McGuire DK, et al. Coronary artery calcium score, risk factors, and incident coronary heart disease events. Atherosclerosis. 2007;190:224–231. [DOI] [PubMed] [Google Scholar]
- 14. Gibson AO, Blaha MJ, Arnan MK, et al. Coronary artery calcium and incident cerebrovascular events in an asymptomatic cohort: the MESA Study. JACC Cardiovasc Imaging. 2014;7:1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 16. Breuckmann F, Olligs J, Hinrichs L, et al. Coronary artery calcium as an independent surrogate marker in the risk assessment of patients with atrial fibrillation and an intermediate pretest likelihood for coronary artery disease admitted to a German chest pain unit. Clin Cardiol. 2016;39:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bild DE, Bluemke DA, Burke GL, et al. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 18. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19. Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of Multi‐Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 20. Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 21. Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow‐mediated dilation for incident cardiovascular events in a population‐based study: the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2009;120:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66:1657–1668. [DOI] [PubMed] [Google Scholar]
- 23. Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 24. Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten‐year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 25. Roger VL, Go AS, Lloyd‐Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association [published correction appears in Circulation. 2012;125:e1002]. Circulation. 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valenti V, Ó Hartaigh B, Heo R, et al. Long‐term prognosis for individuals with hypertension undergoing coronary artery calcium scoring. Int J Cardiol . 2015;187:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herman CR, Gill HK, Eng J, et al. Screening for preclinical disease: test and disease characteristics. AJR Am J Roentgenol. 2002;179:825–831. [DOI] [PubMed] [Google Scholar]
- 28. Bittencourt MS, Staniak HL, Pereira AC, et al. Implications of the new US cholesterol guidelines in the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). Clin Cardiol. 2016;39:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pandya A, Sy S, Cho S, et al. Cost‐effectiveness of 10‐year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease [note: incorrect data in the Abstract and Conclusion]. JAMA. 2015;314:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study‐level network meta‐analysis of 246 ;955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390–399. [DOI] [PubMed] [Google Scholar]
- 31. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet. 2010;375:735–742. [DOI] [PubMed] [Google Scholar]
- 32. Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–467. [DOI] [PubMed] [Google Scholar]
- 33. Hobbs FD, Banach M, Mikhailidis DP, et al. Is statin‐modified reduction in lipids the most important preventive therapy for cardiovascular disease? A pro/con debate. BMC Med. 2016;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahmad Z. Statin intolerance. Am J Cardiol. 2014;113:1765–1771. [DOI] [PubMed] [Google Scholar]
- 35. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moriarty PM, Thompson PD, Cannon CP, et al; ODYSSEY ALTERNATIVE Investigators. Efficacy and safety of alirocumab vs ezetimibe in statin‐intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol . 2015;9:758–769. [DOI] [PubMed] [Google Scholar]
- 37. Nissen SE, Stroes E, Dent‐Acosta RE, et al; GAUSS‐3 Investigators. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle‐related statin intolerance: the GAUSS‐3 randomized clinical trial. JAMA . 2016;315:1580–1590. [DOI] [PubMed] [Google Scholar]
- 38. Slejko JF, Ho M, Anderson HD, et al. Adherence to statins in primary prevention: yearly adherence changes and outcomes. J Manag Care Pharm. 2014;20:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bitzur R, Cohen H, Kamari Y, et al. Intolerance to statins: mechanisms and management. Diabetes Care . 2013;36(suppl 2):S325–S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Palaniappan L, Wang Y, Fortmann SP. Coronary heart disease mortality for six ethnic groups in California, 1990–2000. Ann Epidemiol. 2004;14:499–506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Accuracy of statin/lipid lowering therapy eligibility criteria defined by three cholesterol treatment guidelines for cardiovascular outcomes by sex, age, and race/ethnicity
Supplemental Table 2: Sensitivity, specificity, positive predictive value and negative predictive values of individual statin eligible subgroups defined by 2013 ACC/AHA cholesterol guidelines for incident ASCVD*


