Abstract
Background
Cycle exercise echocardiography is a useful tool to “unmask” diastolic dysfunction; however, this approach can be limited by respiratory and movement artifacts. Isometric handgrip avoids these issues while reproducibly increasing afterload and myocardial oxygen demand.
Hypothesis
Isometric handgrip echocardiography (IHE) can differentiate normal from abnormal diastolic function.
Methods
First recruited 19 young healthy individuals (mean age, 24 ± 4 years) to establish the “normal” response. To extend these observations to a more at‐risk population, we performed IHE on 17 elderly individuals (mean age, 72 ± 6 years) with age‐related diastolic dysfunction. The change in the ratio of mitral valve inflow velocity to lateral wall tissue velocity (E/e'), a surrogate for left ventricular filling pressure, was used to assess the diastolic stress response in each group.
Results
In the young subjects, isometric handgrip increased heart rate and mean arterial pressure (25 ± 12 bpm and 26 ± 17 mmHg, respectively), whereas E/e' changed minimally (0.6 ± 0.9). In the elderly subjects, heart rate and mean arterial pressure were similarly increased with isometric handgrip (19 ± 16 bpm and 25 ± 11 mmHg, respectively), whereas E/e' increased more dramatically (2.3 ± 1.7). Remarkably, 11 of the 17 elderly subjects had an abnormal diastolic response (ΔE/e': 3.4 ± 1.1), whereas the remaining 6 elderly subjects showed very little change (ΔE/e': 0.3 ± 0.7), independent of age or the change in myocardial oxygen demand.
Conclusions
IHE is a simple, effective tool for evaluating diastolic function during simulated activities of daily living.
Keywords: Aging, Diastolic Function, Diastolic Stress Test, Isometric Handgrip, Stress Echocardiography
1. INTRODUCTION
Normal left ventricular (LV) diastole requires the coordination of several physiological processes that allow the heart to fill sufficiently under low filling pressures. As systole ends, LV elastic recoil and active relaxation give rise to an abrupt decline in ventricular pressure until the mitral valve opens and blood flows along a pressure gradient toward the apex. Upon pressure equilibration between the left atrium (LA) and the LV (ie, diastasis), the final component of ventricular filling occurs when the atrium contracts and systole resumes. Derangement of any one of these components can result in a rise in LV filling pressure that is transmitted to the LA and pulmonary veins and may be associated with pulmonary edema and dyspnea.
Doppler ultrasound has emerged as the clinical standard for measuring diastolic function, attributable to its wide accessibility, high temporal resolution, and minimal risk (noninvasive). Indeed, Doppler ultrasound provides clear and useful estimates of early (E) and late (A) transmitral flow, tissue Doppler measurement of mitral annular velocity (e' and a', respectively), and estimations of LV filling pressure (E/e').1, 2, 3 In overt heart disease (ie, acute myocardial infarction, cardiomyopathy, and heart failure [HF] with preserved and reduced ejection fraction [HFpEF and HRrEF]), both E/A and E/e' predict all‐cause mortality, cardiovascular death, and HF hospitalizations.4, 5 However, when disease is less advanced (subclinical) and/or when the diagnosis remains equivocal, diastolic stress echocardiography may be indicated to differentiate cardiac vs noncardiac pathology.
Over the last several years, diastolic stress testing has emerged as a powerful tool to enhance detection of ischemia and/or to document diastolic dysfunction as the etiological feature of exertional dyspnea. Ha et al. were among the first to combine supine bicycle exercise with Doppler ultrasound,6 demonstrating the feasibility of this approach to discriminate cardiac‐ vs noncardiac‐related exertional dyspnea. This approach has since been adopted by others.3, 7, 8, 9, 10, 11 For example, Burgess et al. were among the first to validate the use of cycle echocardiography with invasively measured LV filling pressures.3 Obokata et al demonstrated the prognostic power of supine cycle echocardiography in differentiating HFpEF vs noncardiac dyspnea.7 Accordingly, cycle exercise echocardiography is now recommended by both the American Society of Echocardiography and the European Association of Echocardiography for diastolic stress testing.12
Cycle exercise echocardiography is not without limitation. Indeed, supine cycle exercise increases both respiratory and movement artifact, which are exacerbated by the increased body adiposity and poor acoustic window often found in clinical populations.13, 14 In contrast, isometric handgrip exercise is a simple, well‐established, and highly robust tool that reproducibly increases LV afterload and myocardial oxygen demand15 without movement or respiratory artifact. Moreover, isometric handgrip exercise has long been used during invasive assessment of LV filling pressures, providing robust differentiation between many patient groups, including those at risk for or with established HF.7, 15, 16
Accordingly, we hypothesized that isometric handgrip echocardiography (IHE) could be used to differentiate normal from abnormal diastolic function while avoiding the limitations associated with dynamic whole‐body exercise. To test this hypothesis, we first established the normal diastolic stress response to isometric handgrip in a group of healthy young volunteers. Then we recruited a group of independently living seniors with age‐related diastolic dysfunction to determine if IHE could differentiate normal from abnormal diastolic functional reserve.
2. METHODS
2.1. Study population
To establish the normative response to IHE, we recruited a group of healthy young volunteers (age 20–32 years). None had any history of cardiovascular, metabolic, or neurological disease, nor were they taking any medications other than oral contraceptives. With the normative response established, we then recruited a group of independently living seniors with age‐related diastolic dysfunction to determine if IHE could differentiate normal from abnormal diastolic functional reserve. Those with an LV ejection fraction <50%, atrial or ventricular arrhythmia, valvular disease (of moderate or greater severity), or history of myocardial infarction were excluded. Some individuals had history of hypertension (n = 9) and hypercholesterolemia (n = 4). All participants were instructed to withdraw from any medication and/or supplements the day before testing. Additionally, all subjects presented to the laboratory after an overnight fast, having abstained from alcohol and caffeine for ≥24 hours.
Results also were compared with a single patient with clinically diagnosed New York Heart Association (NYHA) class III HFpEF, recruited from the local Dallas–Fort Worth community. To differentiate cardiac‐related vs noncardiac‐related exertional dyspnea, this patient underwent invasive pulmonary artery capillary wedge pressure assessment at rest and during low‐level upright cycle exercise (20 watts). To evaluate peak aerobic power, cardiopulmonary exercise stress testing was also performed on an upright cycle ergometer.
All subjects provided written informed consent before being enrolled to participate in the present study. The study was approved by the institutional review board at the University of Texas at Arlington and conformed to the standards set by the latest version of the Declaration of Helsinki.
2.2. Isometric handgrip echocardiography
Prior to data collection, all participants' heights and weights were measured using a dual‐function stadiometer and weighing scale (Professional 500KL; Health O Meter, McCook, IL). Beat‐by‐beat arterial blood pressure (BP) was measured from a small finger cuff placed around the middle finger of the participant's right hand (Finometer PRO; Finapres Medical Systems, Arnhem, The Netherlands) calibrated to an automated brachial artery BP cuff (Connex Spot Monitor 71WX‐B; Welch Allyn, Skaneateles Falls, NY). Heart rate was determined from the R‐R intervals of a single‐lead electrocardiogram (MLA 0313; ADInstruments, Colorado Springs, CO).
Two‐dimensional transthoracic echocardiography with Doppler ultrasound was performed by an experienced certified sonographer using a commercially available ultrasound machine (Vivid S6; GE Vingmed Ultrasound, Horten, Norway) and a 2.5‐MHz transducer. All subjects were studied in the left lateral position. A minimum of 5 consecutive cardiac cycles were collected and stored for offline analysis. From the apical window, standard 4‐chamber volumetric images were obtained.17 Pulsed Doppler images were also obtained with the sample volume placed at the tips of the mitral valve leaflets, along with peak lateral annular tissue velocities using the pulsed‐wave Doppler mode. All data were stored digitally, and measurements were made at the completion of each study.
After resting images were obtained, subjects performed 3 minutes of isometric handgrip exercise at 40% of their maximal voluntary contraction, determined prior to baseline imaging. Arterial BP, heart rate, and echocardiography data were recorded during the final minute of isometric handgrip exercise.
2.3. Data analysis
Heart rate and BP data were sampled at a rate of 1000 Hz, recorded with a data‐acquisition system (PowerLab 16/30; ADInstruments), and analyzed offline using associated software (LabChart Pro; ADInstruments). Hemodynamic and echocardiography data were time‐aligned using data markers. The product of heart rate and systolic pressure was used to calculate rate pressure product and is referred to as myocardial oxygen demand throughout.
Echocardiography and Doppler data were analyzed offline using commercially available software (EchoPAC version 113; GE Medical Systems, Milwaukee, WI). LV volumes were calculated and averaged over 3 cardiac cycles, according to the Simpson monoplane method, in accordance with the current recommendations for quantification of LV volumes by 2‐dimensional echocardiography.17 LA volumes were estimated using the area‐length method using the 4‐chamber image at end‐systole (where the left atrium is largest).17 Stroke volume was determined as the difference between end‐diastolic and end‐systolic volumes and was used to calculate cardiac output and ejection fraction. Left ventricular wall thickness was calculated from a mid‐ventricular short‐axis image, as previously described.17 LV mass was calculated according to the area‐length method, as previously described.17
Pulsed Doppler was used to quantify early and late diastolic inflow velocities. Tissue Doppler data were used to assess annular tissue velocities (lateral wall) during systole, early diastole, and late diastole. The ratios between early and late diastolic mitral inflow and LV lateral wall velocities were calculated as E/A and e'/a' ratios, respectively. The ratio between early diastolic mitral valve inflow velocity and early diastolic lateral wall tissue velocity (E/e' ratio) was calculated and used as a surrogate measure of diastolic filling pressure.6, 7 All Doppler and 2‐dimensional data were analyzed and averaged over 3 cardiac cycles when possible.
2.4. Statistical analysis
All dependent variables were assessed for normal distribution and homoscedasticity using the Shapiro–Wilk test. In the young healthy population, changes from rest to IHE were assessed using a 2‐way paired t test when parametric, whereas a 2‐way Wilcoxon test was used for nonparametric data. In addition, the same statistical method was adopted to test for changes from rest to exercise in the asymptomatic elderly population as a whole. Nonresponders and responders were compared for group and exercise main effects using a 2‐way repeated‐measures ANOVA, followed by a Bonferroni post hoc test if significant main effects were present. All statistical analyses were performed using GraphPad Prism for Windows, version 5.0.1 (GraphPad Prism, San Diego, CA). All data are expressed as mean ± SD unless otherwise stated, and statistical significance was considered at P ≤ 0.05.
3. RESULTS
3.1. Normative data
In total, 19 healthy young volunteers participated in this study (Table 1). By design, all had a normal body mass index and were free of cardiovascular, metabolic, or neurological disease. All had normal diastolic function at rest, with normal LV morphology and systolic function (Table 1).
Table 1.
Normative demographic, hemodynamic, and echocardiographic data at rest and in response to IHE
| Demographics | |||
| Age, y | 24 ± 4 | ||
| Female sex, % | 63 | ||
| Height, cm | 169.7 ± 7.2 | ||
| Weight, kg | 70.4 ± 15.6 | ||
| BMI, kg/m2 | 24.3 ± 4.5 | ||
| BSA, m2 | 1.80 ± 0.20 | ||
| Rest | IHE | Δ Change | |
| Hemodynamics | |||
| Heart rate, bpm | 63 ± 10 | 88 ± 12 | 25 ± 12a |
| SBP, mm Hg | 120 ± 8 | 151 ± 24 | 32 ± 20a |
| DBP, mm Hg | 75 ± 6 | 99 ± 16 | 24 ± 16a |
| MAP, mm Hg | 90 ± 6 | 116 ± 18 | 26 ± 17a |
| RPP, mm Hg·bpm | 7553 ± 1170 | 13 266 ± 2423 | 5713 ± 2514a |
| LV structure | |||
| Diastolic wall thickness, cm | 0.80 ± 0.15 | 0.80 ± 0.13 | 0.00 ± 0.07 |
| Systolic wall thickness, cm | 1.08 ± 0.14 | 1.08 ± 0.17 | 0.00 ± 0.12 |
| LV mass, g | 191.9 ± 63.4 | — | — |
| LVMI, g·m2 | 104.7 ± 26.0 | — | — |
| LV and LA volumes | |||
| EDVi, mL·m2 | 64.0 ± 13.0 | 62.1 ± 14.5 | −1.8 ± 10.1 |
| ESVi, mL·m2 | 23.6 ± 7.1 | 22.9 ± 7.3 | −0.7 ± 5.1 |
| Stroke index, mL·m2 | 40.4 ± 7.1 | 39.3 ± 8.9 | −1.2 ± 7.6 |
| LVEF, % | 63.7 ± 5.2 | 63.6 ± 6.2 | −0.1 ± 6.2 |
| Cardiac index, L·min−1·m2 | 2.53 ± 0.38 | 3.41 ± 0.60 | 0.88 ± 0.61a |
| LA volume, mL | 40.0 ± 11.8 | — | — |
| LAVI, mL·m2 | 22.0 ± 5.4 | — | — |
| LV Doppler | |||
| MV E velocity, m·s−1 | 0.83 ± 0.15 | 0.82 ± 0.16 | −0.01 ± 0.09 |
| MV deceleration time, ms | 174.3 ± 30.2 | 148.9 ± 35.7 | −25.5 ± 31.8a |
| MV deceleration slope, m·s2 | 5.1 ± 1.5 | 6.1 ± 2.3 | 1.0 ± 1.5a |
| MV A velocity, m·s−1 | 0.39 ± 0.09 | 0.62 ± 0.18 | 0.24 ± 0.14a |
| MV E/A ratio | 2.21 ± 0.42 | 1.38 ± 0.37 | −0.83 ± 0.39a |
| LV lateral s′ velocity, m·s−1 | 0.10 ± 0.03 | 0.09 ± 0.03 | 0.00 ± 0.02 |
| LV lateral e′ velocity, m·s−1 | 0.17 ± 0.04 | 0.15 ± 0.04 | −0.02 ± 0.02a |
| LV lateral a′ velocity, m·s−1 | 0.06 ± 0.02 | 0.09 ± 0.02 | 0.03 ± 0.02a |
| LV e′/a′ ratio | 3.13 ± 0.93 | 1.72 ± 0.50 | −1.36 ± 0.81a |
| LV E/e′ ratio | 5.18 ± 1.48 | 5.75 ± 1.67 | 0.56 ± 0.89a |
Abbreviations: BMI, body mass index; BSA, body surface area; DBP, diastolic blood pressure; EDVi, end‐diastolic volume index; ESVi, end‐systolic volume index; IHE, isometric handgrip echocardiography; LA, left atrial; LAVI, left atrial volume index; LV, left ventricular; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MAP, mean arterial pressure; MV, mitral valve; RPP, rate pressure product; SBP, systolic blood pressure.
Within‐group difference.
IHE caused a significant increase in arterial BP and heart rate (Figure 1), which was consistent across all subjects studied (Table 1). Despite this significant increase in LV afterload and myocardial oxygen demand, diastolic function was well preserved in these healthy normative subjects. In particular, the ratio of early mitral inflow velocity to early annular tissue velocity, a surrogate measure of left ventricular filling pressure, changed minimally from rest to exercise (ΔE/e': 0.56 ± 0.89).
Figure 1.
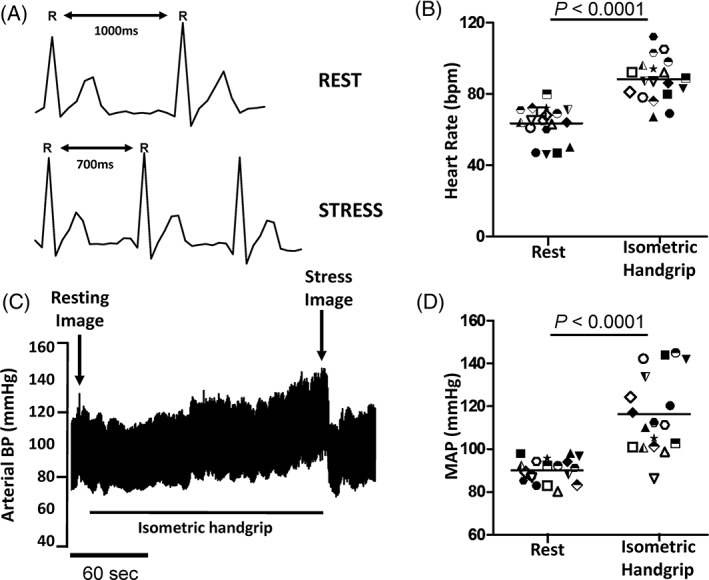
Representative heart rate and arterial BP response to IHE in young healthy individuals, showing (A) a representative ECG tracing for a healthy young individual at rest and during isometric handgrip exercise stress at 40% MVC; (B) average group heart rate response at rest and during IHE; (C) representative arterial BP tracing at rest and during IHE; and (D) average group MAP response at rest and during IHE. Grouped data shown as mean and 95% CI. Abbreviations: BP, blood pressure; CI, confidence interval; ECG, electrocardiogram; IHE, isometric handgrip echocardiography; MAP, mean arterial blood pressure; MVC, maximal voluntary contraction
3.2. IHE in the elderly
In total, 17 independently living seniors participated in this study (Table 2). As expected, some of the participants had a history of hypertension (n = 9) and hypercholesterolemia (n = 4). Likewise, we observed age‐related impairments in diastolic function at rest; however, none had overt LV hypertrophy or met clinical criteria for LV diastolic dysfunction beyond that which occurs with healthy aging,18 and all had normal systolic function (Table 2).
Table 2.
Elderly demographic, hemodynamic, and echocardiographic data at rest and in response to IHE
| Demographics | |||
| Age, y | 72 ± 6 | ||
| Female sex, % | 76 | ||
| Height, cm | 166.1 ± 9.3 | ||
| Weight, kg | 74.7 ± 11.2 | ||
| BMI, kg/m2 | 27.0 ± 3.3 | ||
| BSA, m2 | 1.8 ± 0.2 | ||
| Rest | IHE | Δ Change | |
| Hemodynamics | |||
| Heart rate, bpm | 63 ± 7 | 82 ± 19 | 19 ± 16a |
| SBP, mm Hg | 139 ± 17 | 180 ± 25 | 42 ± 17a |
| DBP, mm Hg | 75 ± 6 | 91 ± 14 | 16 ± 14a |
| MAP, mm Hg | 96 ± 8 | 121 ± 14 | 25 ± 11a |
| RPP, mm Hg·bpm | 8764 ± 1664 | 14 803 ± 3724 | 6034 ± 3081a |
| LV structure | |||
| Diastolic wall thickness, cm | 0.86 ± 0.06 | 0.85 ± 0.07 | −0.02 ± 0.06 |
| Systolic wall thickness, cm | 1.37 ± 0.13 | 1.36 ± 0.12 | −0.01 ± 0.15 |
| LV mass, g | 185.9 ± 27.1 | — | — |
| LVMI, g·m2 | 101.8 ± 11.6 | — | — |
| LV and LA volumes | |||
| EDVi, mL·m2 | 53.7 ± 6.5 | 57.3 ± 7.4 | 3.6 ± 4.6a |
| ESVi, mL·m2 | 20.6 ± 3.1 | 24.0 ± 4.4 | 3.4 ± 2.9a |
| Stroke index, mL·m2 | 33.2 ± 4.6 | 33.3 ± 4.6 | 0.1 ± 3.6 |
| LVEF, % | 61.6 ± 3.7 | 58.1 ± 4.2 | −3.5 ± 3.6a |
| Cardiac index, L·min−1·m2 | 2.08 ± 0.34 | 2.72 ± 0.64 | 0.64 ± 0.47a |
| LA volume, mL | 38.0 ± 9.8 | — | — |
| LAVI, mL·m2 | 19.64 ± 7.35 | — | — |
| LV Doppler | |||
| MV E velocity, m·s−1 | 0.66 ± 0.10 | 0.75 ± 0.20 | 0.09 ± 0.17a |
| MV deceleration time, ms | 197 ± 47 | 159 ± 37 | −38 ± 62a |
| MV deceleration slope, m·s2 | 3.5 ± 0.9 | 5.2 ± 2.5 | 1.7 ± 2.6a |
| MV A velocity, m·s−1 | 0.70 ± 0.26 | 0.90 ± 0.21 | 0.20 ± 0.21a |
| MV E/A ratio | 1.05 ± 0.38 | 0.82 ± 0.21 | −0.23 ± 0.47 |
| LV lateral s′ velocity, m·s−1 | 0.09 ± 0.03 | 0.08 ± 0.02 | −0.01 ± 0.02 |
| LV lateral e′ velocity, m·s−1 | 0.09 ± 0.03 | 0.08 ± 0.03 | −0.01 ± 0.02a |
| LV lateral a′ velocity, m·s−1 | 0.11 ± 0.03 | 0.12 ± 0.04 | 0.01 ± 0.03a |
| LV e′/a′ ratio | 0.94 ± 0.29 | 0.74 ± 0.31 | −0.20 ± 0.30a |
| LV E/e′ ratio | 7.55 ± 2.57 | 9.84 ± 3.00 | 2.29 ± 1.74a |
Abbreviations: BMI, body mass index; BSA, body surface area; DBP, diastolic blood pressure; EDVi, end‐diastolic volume index; ESVi, end‐systolic volume index; IHE, isometric handgrip echocardiography; LA, left atrial; LAVI, left atrial volume index; LV, left ventricular; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MAP, mean arterial pressure; MV, mitral valve; RPP, rate pressure product; SBP, systolic blood pressure.
Within‐group difference.
Similar to our normative data, IHE resulted in a significant increase in arterial BP and heart rate (Table 2). In contrast to our normative data, however, the ΔE/e' ratio increased significantly from rest to exercise (2.29 ± 1.74; P < 0.0001), suggestive of an exercise‐induced increase in LV filling pressure. However, this response was not universal across all of the aging participants.
To explore this “responder vs nonresponder” phenomenon further, we divided the elderly participants according to their diastolic stress response during IHE (Figure 2). Specifically, and in line with several recent cycle echocardiography publications,6, 7 a “responder” was defined as someone who changed E/e' > 1.5 with exercise stress. With this new definition, 6 elderly participants were found to be “nonresponders,” vs 11 elderly participants found to be “responders.” Remarkably, both responders and nonresponders had similar resting diastolic function (Table 3) and a similar rise in both heart rate and BP (myocardial oxygen demand) with isometric handgrip (Figure 2). No differences in age were observed between the 2 groups (Table 3). Individual E/E' data are presented in Supporting Information, Figure 1, in the online version of this article.
Figure 2.
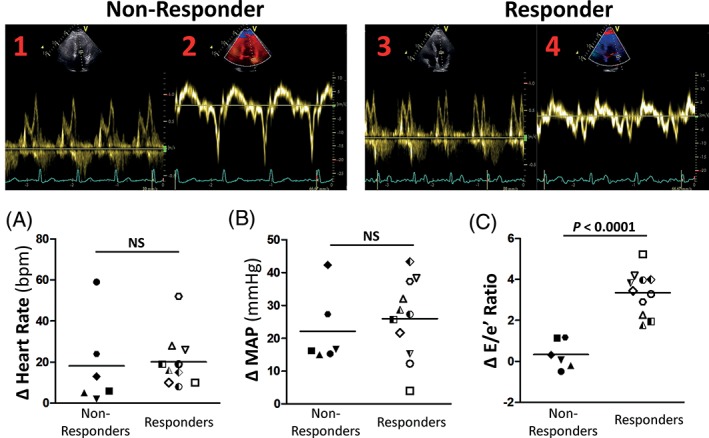
Example of a Doppler mitral inflow velocity tracing for a representative responder (1) at rest and (3) during isometric handgrip exercise stress at 40% MVC, along with tissue Doppler tracing from the lateral wall of the same individual taken (2) during rest and (4) during isometric handgrip exercise stress at 40% MVC. Also shown is change in (A) heart rate, (B) MAP, and (C) LV early mitral inflow velocity to early annular tissue velocity (E/e') from rest to IHE in nonresponders and responders. Each specific symbol represents data from a single individual and is consistent across parameters. Heart rate and BP responded similarly between both groups; however, the responders had an abnormal rise in E/e' (defined as >1.5), a surrogate measure of LV filling pressure, in response to isometric handgrip stress. Abbreviations: BP, blood pressure; IHE, isometric handgrip echocardiography; LV, left ventricular; MAP, mean arterial blood pressure; MVC, maximal voluntary contraction
Table 3.
Demographic, hemodynamic, and echocardiographic data comparing nonresponders with responders at rest and during IHE
| Nonresponders, n = 6 | Responders, n = 11 | |||
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 71 ± 7 | 73 ± 6 | ||
| Female sex, % | 100 | 64 | ||
| Height, cm | 158.8 ± 4.6 | 170.1 ± 8.9a | ||
| Weight, kg | 68.2 ± 6.6 | 78.2 ± 11.8 | ||
| BMI, kg/m2 | 27.1 ± 3.3 | 27.0 ± 3.4 | ||
| BSA, m2 | 1.70 ± 0.07 | 1.90 ± 0.17a | ||
| Rest | IHE | Rest | IHE | |
| Hemodynamics | ||||
| Heart rate, bpm | 61 ± 7 | 79 ± 25 | 64 ± 8 | 84 ± 16b |
| SBP, mm Hg | 134 ± 22 | 168 ± 27b | 141 ± 14 | 187 ± 23b |
| DBP, mm Hg | 73 ± 6 | 90 ± 11b | 76 ± 6 | 92 ± 16b |
| MAP, mm Hg | 94 ± 10 | 116 ± 14b | 98 ± 8 | 124 ± 13b |
| RPP, mm Hg·bpm | 8229 ± 1779 | 13 090 ± 3688b | 9056 ± 1608 | 15 737 ± 3559b |
| LV structure | ||||
| Diastolic wall thickness, cm | 0.85 ± 0.07 | 0.83 ± 0.04 | 0.87 ± 0.06 | 0.86 ± 0.08 |
| Systolic wall thickness, cm | 1.38 ± 0.08 | 1.32 ± 0.09 | 1.36 ± 0.16 | 1.37 ± 0.13 |
| LV mass, g | 167.80 ± 20.83 | — | 195.81 ± 25.58a | — |
| LVMI, g·m2 | 98.54 ± 10.10 | — | 103.53 ± 12.45 | — |
| LV and LA volumes | ||||
| EDVi, mL·m2 | 57.5 ± 7.3 | 62.2 ± 7.2b | 51.7 ± 5.3 | 54.6 ± 6.3a |
| ESVi, mL·m2 | 22.8 ± 3.6 | 27.2 ± 5.3b | 19.4 ± 2.2a | 22.3 ± 2.6a , b |
| Stroke index, mL·m2 | 34.7 ± 5.6 | 35.0 ± 2.6 | 32.3 ± 3.9 | 32.3 ± 5.3 |
| LVEF, % | 60.2 ± 5.2 | 56.6 ± 4.1 | 62.4 ± 2.7 | 58.9 ± 4.3b |
| Cardiac index, L·min−1·m2 | 2.11 ± 0.49 | 2.74 ± 0.79 | 2.06 ± 0.27 | 2.71 ± 0.58b |
| LA volume, mL | 37.7 ± 14.2 | — | 38.2 ± 7.0 | — |
| LAVI, mL·m2 | 21.93 ± 7.26 | — | 18.39 ± 7.43 | — |
| LV Doppler | ||||
| MV E velocity, m·s−1 | 0.67 ± 0.13 | 0.64 ± 0.21 | 0.65 ± 0.09 | 0.82 ± 0.18b |
| MV deceleration time, ms | 192 ± 26 | 164 ± 35 | 200 ± 57 | 157 ± 40 |
| MV deceleration slope, m·s2 | 3.6 ± 0.8 | 4.0 ± 1.2 | 3.5 ± 1.0 | 5.8 ± 2.9b |
| MV A velocity, m·s−1 | 0.70 ± 0.31 | 0.86 ± 0.23 | 0.70 ± 0.23 | 0.93 ± 0.21b |
| MV E/A ratio | 1.11 ± 0.49 | 0.77 ± 0.23 | 1.01 ± 0.32 | 0.85 ± 0.21 |
| LV lateral s′ velocity, m·s−1 | 0.08 ± 0.02 | 0.07 ± 0.02b | 0.09 ± 0.03 | 0.08 ± 0.02 |
| LV lateral e′ velocity, m·s−1 | 0.08 ± 0.02 | 0.07 ± 0.02 | 0.10 ± 0.03 | 0.09 ± 0.04b |
| LV lateral a′ velocity, m·s−1 | 0.10 ± 0.03 | 0.12 ± 0.05 | 0.11 ± 0.03 | 0.12 ± 0.04 |
| LV e′/a′ ratio | 0.85 ± 0.28 | 0.75 ± 0.38 | 0.99 ± 0.30 | 0.73 ± 0.29b |
| LV E/e′ ratio | 8.42 ± 2.31 | 8.76 ± 2.12 | 7.08 ± 2.69 | 10.43 ± 3.33b |
Abbreviations: BMI, body mass index; BSA, body surface area; DBP, diastolic blood pressure; EDVi, end‐diastolic volume index; ESVi, end‐systolic volume index; IHE, isometric handgrip echocardiography; LA, left atrial; LAVI, left atrial volume index; LV, left ventricular; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MAP, mean arterial pressure; MV, mitral valve; RPP, rate pressure product; SBP, systolic blood pressure.
Between‐group difference.
Within‐group difference.
3.3. IHE in HFpEF: A case study
To begin to establish the clinical significance of this novel diastolic stress test, IHE was also performed in a 78‐year‐old female with NYHA class III HFpEF (weight, 110 kg; height, 169 cm; peak VO2, 10.6 mL·kg−1·min−1; LV ejection fraction, 58%; left atrial volume index, 30.5 mL/m2). With mild upright cycle exercise (20 W), pulmonary artery capillary wedge pressure increased dramatically, from 10 mm Hg at rest to 29 mm Hg during exercise, characteristic of HFpEF exercise hemodynamics.7 With IHE, E/e' changed by 6.7 (from 12.5 at rest to 19.2 with stress), reflecting changes in cardiac filling pressures seen with exercise.
3.4. Reproducibility of measures
To determine the reproducibility of the diastolic stress response, 6 elderly participants (3 nonresponders and 3 responders) repeated IHE on a separate visit. Upon retesting, all 3 responders showed a similar rise in E/e' (3.9 at visit 1 vs 4.0 at visit 2), whereas E/e' remained <1.5 in the nonresponders during the retest visit (0.2 at visit 1 vs 1.2 at visit 2). The between‐day coefficient of variation for the change in E/e', expressed in absolute terms, was 0.97 ± 0.74. For individual data, see Supporting Information, Figure 2, in the online version of this article.
4. DISCUSSION
The data herein introduce a simple but effective diastolic stress test that easily could be implemented clinically as a noninvasive exertional diastolic discriminator. The major novel findings were 3‐fold: first, IHE is associated with a robust increase in LV afterload and myocardial oxygen demand, while maintaining an optimal acoustic window and limiting respiratory artifact. Second, in young healthy individuals, diastolic function is well preserved in response to IHE, establishing the normal healthy response. Third, IHE is able to distinguish between a normal and abnormal diastolic stress response in a group of seniors with age‐related resting diastolic impairments and clinically stable, well‐characterized HFpEF patients with severe exercise intolerance (peak VO2 36% lower than healthy age‐ and sex‐matched sedentary control).
Diastolic stress testing is becoming a popular noninvasive alternative to enhance detection of ischemia and/or to document diastolic dysfunction. Ha et al.6 were among the first to demonstrate the feasibility of using the change in E/e' in response to supine cycle exercise to discriminate cardiac‐related vs noncardiac‐related exertional dyspnea. More recently, Obokata et al demonstrated the prognostic power of supine cycle echocardiography in differentiating HFpEF vs noncardiac dyspnea.7 Importantly, this study showed a strong relationship between the change in E/e' and invasively measured pulmonary capillary wedge pressures. Of note, the change in E/e' in nonresponders from each of these studies was <1.5. Despite its growing popularity, cycle echocardiography has several important limitations to its application, especially in clinical populations. For example, resting‐image quality is often compromised by increased body adiposity, poor respiratory function, and orthopedic challenges, and cycle exercise only exacerbates these limitations by increased respiratory and movement artifacts, even during low‐intensity exercise.
In contrast to cycle exercise, however, isometric handgrip exercise is associated with a marked increase in myocardial oxygen demand while avoiding respiratory and movement artifacts. Indeed, isometric handgrip exercise has been used in the clinical setting to elicit stress for close to a century.19 The sympathetic neural response to this form of exercise is well described20 and related to local mechanical and chemical afferent stimuli, as well as central sympathetic outflow.21, 22, 23, 24, 25 As shown by our own data, these sympathetic stimuli result in formidable increases in both heart rate and arterial BP. Here, we combined this well‐established clinical approach with echocardiography to create a simple and effective stress echocardiography test. Whereas cycle exercise focuses largely on global oxygen demand (ie, central and peripheral), we believe the unique afterload challenge caused by isometric handgrip produces a more “isolated” (ie, central) diastolic stress. Indeed, the increase in end‐systolic wall stress observed during IHE in this study was entirely explained by a rise in arterial BP (see Supporting Information, Table, in the online version of this article). This increase in afterload will result in a large infiltration of calcium into the myocyte having a positive inotropic and chronotropic effect during systole, as reflected by the increase in heart rate and contractility (end‐systolic elastance) in this study. However, the inability of the myocyte to efficiently sequestrate or remove the elevated levels of cytosolic calcium during diastole will result in prolonged actin‐myosin cross‐bridge formation and subsequently impair LV active relaxation.26, 27 The result of this delayed myocardial relaxation would be a stiffer LV, giving rise to increased LV pressures.
To our knowledge, this is the first report using IHE to noninvasively discriminate normal from abnormal diastolic function in healthy older individuals. We first established the “normative” response to this unique approach in a group of young healthy individuals. As expected, diastolic function was preserved in this cohort, despite a significant rise in LV afterload and myocardial oxygen demand. That E/e' changed minimally (<0.6) with isometric handgrip in this group suggests that a healthy heart can, and should, compensate by maintaining the intraventricular pressure gradient.
To translate these normative data to a more at‐risk population, we studied a group of asymptomatic seniors (age 60–83 years) and a single patient with well‐characterized HFpEF. As expected, the majority of the seniors studied showed evidence of resting grade 1 diastolic dysfunction (impaired relaxation), consistent with healthy aging.28 Despite this age‐related change in diastolic function, however, there was a heterogeneous response to IHE. Specifically, in nearly two‐thirds of seniors studied, E/e' changed >1.5, suggestive of stress‐induced increase LV filling pressure.1, 2, 3, 7, 12 In contrast, the remaining subjects showed a minimal change in E/e' during IHE (<0.4), a finding comparable with our young normative data. That IHE is reproducible, and that it mirrors the pattern of LV filling pressure changes during exercise in HFpEF, strongly supports the validity of our results. Taken together, these proof‐of‐concept data establish the clinical utility of this simple stress test for differentiating cardiac vs noncardiac pathology. Future work is warranted to determine the predictive value of this stress test.
Our ability to differentiate between normal and abnormal diastolic reserve is entirely consistent with previous invasive7, 15 and noninvasive diastolic stress‐testing protocols.6 However, the exact mechanism for this response remains to be elucidated. Importantly, these observations do not appear to be related to hemodynamic differences, as heart rate and arterial BP (and thus myocardial oxygen demand) changed similarly in both responders and nonresponders. Because isometric handgrip produces an “isolated” afterload challenge (similar to the original A.V. Hill experiments),29 it is interesting to speculate that the group differences observed herein may be related to impaired intracellular calcium handling. Specifically, in a sick or failing myocardium (even one that is asymptomatic, as described herein), transient increases in intracellular calcium would lead to prolonged actin‐myosin cross‐bridge formation and increased myocardial stiffness.
4.1. Study limitations
The primary outcome measure in this study was E/e', which is regarded as a surrogate measure of LV filling pressure.1, 2, 3 Gold‐standard invasive pressure measurements are indeed warranted to confirm the present results; however, interindividual changes in E/e' are considered fairly robust and reflective of true changes in LV filling pressure.7 In this initial clinical investigation, we chose to study asymptomatic seniors living independently in the community, as opposed to symptomatic patients with dyspnea, limiting the prognostic application of our results. However, the fact that we observed reproducible, within‐group differences (ie, responders vs nonresponders) supports the hypothesis that IHE is a powerful discriminator of normal/abnormal diastolic dysfunction, even in subclinical populations. In addition, all data were obtained before and during isometric handgrip exercise. Future studies should consider assessing diastolic function during postexercise recovery to evaluate the sensitivity of this measurement to acute changes in physiological status. Finally, this acute proof‐of‐concept study cannot provide any insight into the predictive potential of this stress test. Future longitudinal studies are therefore warranted to address this specific limitation.
5. CONCLUSION
Despite these limitations, these initial proof‐of‐concept data demonstrate the feasibility of IHE as a simple and effective tool for evaluating diastolic function during simulated activities of daily living. Future studies are warranted to extend these observations to additional patients further along the HF continuum (eg, American Heart Association/American College of Cardiology class B and D HF).
Supporting information
Figure S1. Individual data representing the change in Doppler mitral inflow velocity‐to‐lateral wall tissue velocity ratio (E/e’) from rest to isometric handgrip at 40% MVC for both non‐responders and responders
Figure S2. Individual reproducibility data representing the change in Doppler mitral inflow velocity‐to‐lateral wall tissue velocity ratio (E/e’) from rest to isometric handgrip at 40% MVC for both non‐responders (n = 3) and responders (n = 3) during separate but identical trials
Table S1. Left ventricular end‐systolic structure and pressure used for the calculation of wall stress. Wall stress calculations was based on a previously published equation (Haykowsky et al. 2001, Chest, 119: 150‐154).
ACKNOWLEDGMENTS
The authors would like to thank the research volunteers for their time and effort. The authors also thank Wesley Tucker, PhD, Susie Chung, BSc, Ryan Rosenberry, BSc, and Madison Munson, BSc, for their assistance with data collection.
Conflicts of interest
The authors declare no potential conflicts of interest.
Jake Samuel T, Beaudry R, Haykowsky MJ, et al. Isometric handgrip echocardiography: A noninvasive stress test to assess left ventricular diastolic function. Clin Cardiol. 2017;40:1247–1255. 10.1002/clc.22818
Funding information This work was supported by American Heart Association (16SDG27260115), the Harry S. Moss Heart Trust, and the National Institutes for Health (1R15NR016826‐01). Professor Haykowsky's research is supported by the Moritz Chair in Geriatrics, College of Nursing and Health Innovation, University of Texas at Arlington.
REFERENCES
- 1. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. [DOI] [PubMed] [Google Scholar]
- 2. Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. [DOI] [PubMed] [Google Scholar]
- 3. Burgess MI, Jenkins C, Sharman JE, et al. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47:1891–1900. [DOI] [PubMed] [Google Scholar]
- 4. Aljaroudi W, Alraies MC, Halley C, et al. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125:782–788. [DOI] [PubMed] [Google Scholar]
- 5. Okura H, Takada Y, Kubo T, et al. Tissue Doppler–derived index of left ventricular filling pressure, E/E', predicts survival of patients with non‐valvular atrial fibrillation. Heart. 2006;92:1248–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ha JW, JK Oh, Pellikka PA, et al. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr. 2005;18:63–68. [DOI] [PubMed] [Google Scholar]
- 7. Obokata M, Kane GC, Reddy YN, et al. The role of diastolic stress testing in the evaluation for HFpEF: a simultaneous invasive‐echocardiographic study. Circulation. 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donal E, Lund LH, Oger E, et al. Value of exercise echocardiography in heart failure with preserved ejection fraction: a substudy from the KaRen study. Eur Heart J Cardiovasc Imaging. 2016;17:106–113. [DOI] [PubMed] [Google Scholar]
- 9. Donal E, Thebault C, Lund LH, et al. Heart failure with a preserved ejection fraction: additive value of an exercise stress echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13:656–665. [DOI] [PubMed] [Google Scholar]
- 10. Tartière‐Kesri L, Tartière JM, Logeart D, et al. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;59:455–461. [DOI] [PubMed] [Google Scholar]
- 11. Talreja DR, Nishimura RA, Oh JK. Estimation of left ventricular filling pressure with exercise by Doppler echocardiography in patients with normal systolic function: a simultaneous echocardiographic‐cardiac catheterization study. J Am Soc Echocardiogr. 2007;20:477–479. [DOI] [PubMed] [Google Scholar]
- 12. Mitter SS, Shah SJ, Thomas JD. A test in context: E/A and E/e' to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol. 2017;69:1451–1464. [DOI] [PubMed] [Google Scholar]
- 13. Haykowsky MJ, Brubaker PH, Morgan TM, et al. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Upadhya B, Haykowsky MJ, Eggebeen J, et al. Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatr Cardiol. 2015;12:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Penicka M, Bartunek J, Trakalova H, et al. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure‐volume loop analysis. J Am Coll Cardiol. 2010;55:1701–1710. [DOI] [PubMed] [Google Scholar]
- 16. Yoshikawa T, Miyazaki T, Akaishi M, et al. Diastolic pressure‐volume relationship during handgrip exercise in patients with coronary artery disease. Clin Cardiol. 1991;14:743–748. [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1e14–39e14. [DOI] [PubMed] [Google Scholar]
- 18. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868–1877. [DOI] [PubMed] [Google Scholar]
- 19. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol. 2015;5:475–512. [DOI] [PubMed] [Google Scholar]
- 21. Mark AL, Victor RG, Nerhed C, et al. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. [DOI] [PubMed] [Google Scholar]
- 22. Victor RG, Secher NH, Lyson T, et al. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ Res. 1995;76:127–131. [DOI] [PubMed] [Google Scholar]
- 23. Victor RG, Vissing SF, Urias L, et al. Central motor command activates sympathetic outflow to skin during static exercise in humans. Clin Res. 1989;37:A524. [Google Scholar]
- 24. Delaney EP, Greaney JL, Edwards DG, et al. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2010;299:H1318–H1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogoh S, Wasmund WL, Keller DM, et al. Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol. 2002;543(part 1):349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gwathmey JK, Copelas L, MacKinnon R, et al. Abnormal intracellular calcium handling in myocardium from patients with end‐stage heart failure. Circ Res. 1987;61:70–76. [DOI] [PubMed] [Google Scholar]
- 27. Hunter WC. Role of myofilaments and calcium handling in left ventricular relaxation. Cardiol Clin. 2000;18:443–457. [DOI] [PubMed] [Google Scholar]
- 28. Carrick‐Ranson G, Hastings JL, Bhella PS, et al. Effect of healthy aging on left ventricular relaxation and diastolic suction. Am J Physiol Heart Circ Physiol. 2012;303:H315–H322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond Ser B Biol Sci. 1938;126:136–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Individual data representing the change in Doppler mitral inflow velocity‐to‐lateral wall tissue velocity ratio (E/e’) from rest to isometric handgrip at 40% MVC for both non‐responders and responders
Figure S2. Individual reproducibility data representing the change in Doppler mitral inflow velocity‐to‐lateral wall tissue velocity ratio (E/e’) from rest to isometric handgrip at 40% MVC for both non‐responders (n = 3) and responders (n = 3) during separate but identical trials
Table S1. Left ventricular end‐systolic structure and pressure used for the calculation of wall stress. Wall stress calculations was based on a previously published equation (Haykowsky et al. 2001, Chest, 119: 150‐154).


