Abstract
Background
Excessive daytime sleepiness is a frequent symptom of obstructive sleep apnea (OSA) and has been proposed as a motivator for adherence to continuous positive airway pressure (CPAP) therapy. However, excessive daytime sleepiness is absent in many patients with coronary artery disease (CAD) and concomitant OSA. We evaluated long‐term use of CPAP and predictors of CPAP use in nonsleepy and sleepy OSA patients from a CAD cohort.
Hypothesis
Long‐term CPAP use is lower in CAD patients with nonsleepy OSA vs sleepy OSA.
Methods
Nonsleepy (Epworth Sleepiness Scale [ESS] score < 10) OSA patients randomized to CPAP (n = 122) and sleepy (ESS ≥10) OSA patients offered CPAP (n = 155) in the RICCADSA trial in Sweden were included in this substudy. The median follow‐up was 4.8 years for the main trial, with a predefined minimum follow‐up of 2 years.
Results
The probability of remaining on CPAP at 2 years was 60% in nonsleepy patients and 77% in sleepy patients. Multivariate analyses indicated that age and hours of CPAP use per night at 1 month were independently associated with long‐term CPAP use in nonsleepy patients. In the sleepy phenotype, body mass index, acute myocardial infarction at baseline, and hours of CPAP use per night at 1 month were predictors of long‐term CPAP use.
Conclusions
Long‐term use of CPAP is likely to be challenging for CAD patients with nonsleepy OSA. Early CPAP use is an important predictor of continued long‐term use of CPAP, so optimizing patients' initial experience with CPAP could promote adherence.
Keywords: Adherence, Coronary Artery Disease, Obstructive Sleep Apnea, Positive Airway Pressure, Sleepiness
1. INTRODUCTION
Obstructive sleep apnea (OSA), characterized by repetitive upper‐airway obstruction during sleep, is highly prevalent in coronary artery disease (CAD) and is associated with poor long‐term prognosis in this patient population.1, 2, 3, 4, 5 Continuous positive airway pressure therapy (CPAP) is effective in eliminating apneas and hypopneas, improving quality of life, and reducing potential cardiovascular (CV) consequences when used consistently.6, 7, 8, 9, 10 Excessive daytime sleepiness (EDS), a frequent symptom of OSA, has been proposed as a factor motivating CPAP adherence, given the reductions in daytime sleepiness with CPAP treatment.11, 12 On **the other hand, those without EDS may perceive a lack of benefit that could jeopardize CPAP adherence. Almost two‐thirds of CAD patients with OSA do not report EDS,2 which can contribute to lack of diagnosis and treatment of OSA and potentially poor adherence among those receiving treatment.
A recent meta‐analysis found CPAP to have no overall beneficial effect on systolic blood pressure or risk for CV events and minimal reductions in diastolic blood pressure in OSA patients without EDS.13 Similarly, findings from randomized controlled trials of CPAP in CAD patients with minimal sleepiness reported no reduction in long‐term CV event rates.14, 15 Poor CPAP adherence may partially account for these negative findings in OSA patients without EDS, given subgroup analyses revealing reductions in CV events in those with CPAP use of ≥4 hours per night.15, 16 Consequently, it is clinically important to evaluate CPAP usage and potential correlates of use among CAD patients with OSA, for whom CV benefits of CPAP are particularly important. The aims of this study were to examine long‐term use of CPAP in nonsleepy and sleepy OSA patients from a CAD population and evaluate predictors of long‐term CPAP use in this cohort.
2. METHODS
2.1. Study population
Patients were part of a larger randomized controlled trial, Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA (RICCADSA), conducted to determine whether CPAP treatment will reduce the risk of cardiovascular events in patients with CAD and nonsleepy OSA. Patients from Skaraborg County, West Sweden, were recruited between December 2005 and November 2010, and follow‐up was completed in May 2013.
Methodological details and patient characteristics have been previously described.17 Briefly, a total of 511 patients with angiography‐verified CAD who had undergone percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) and had an apnea‐hypopnea index (AHI) of <5 or ≥15 were enrolled. Patients with existing OSA, an AHI of 5 to 14, and predominantly central apneas with Cheyne‐Stokes respiration were excluded. For the purpose of this substudy, analyses were conducted on 122 CAD patients with nonsleepy OSA (AHI ≥15, Epworth Sleepiness Scale [ESS] <10) randomized to CPAP, and 155 CAD patients with sleepy OSA (AHI ≥15, ESS ≥10) who were offered CPAP (for the flow diagram for patient recruitment and randomization, see Supporting Information, Figure, in the online version of this article). This study conformed to the principles defined in the Declaration of Helsinki. Local ethics committee approval was obtained, and all patients provided written informed consent.
2.2. Procedures
OSA diagnosis was based on a portable, limited sleep study performed with the Embletta PDS (Portable Digital System) device (Embla Systems, Broomfield, CO). Details of sleep recording procedures have been previously described.15 Apneas were defined as an almost complete (≥90%) cessation of airflow. Hypopneas were defined as a ≥ 50% reduction in thoracoabdominal movement and/or a ≥ 50% decrease in the nasal pressure amplitude for ≥10 seconds. Events with a ≥ 30% reduction in thoracoabdominal movement and/or with a nasal pressure amplitude of ≥30% for ≥10 seconds were also scored as hypopneas if there was significant oxygen desaturation (≥4%).18 Patients with an AHI ≥15 per hour of estimated sleep time, independent of symptom occurrence, were defined as having OSA. All CAD patients with OSA diagnosis during home sleep testing underwent an unattended overnight polysomnography in hospital at baseline. Details of the in‐hospital polysomnography have been previously described.15
EDS was assessed using the ESS.19 The ESS contains 8 items evaluating the chance of dozing off in 8 scenarios in the past month. Each item is rated on a scale of 0 to 3 (0 for would never doze, 1 for slight chance of dozing, 2 for moderate chance of dozing, and 3 for high chance of dozing). Total scores range from 0 to 24. EDS was defined as an ESS score ≥ 10.
Nonsleepy OSA patients allocated to CPAP treatment and sleepy OSA patients offered CPAP treatment were provided autotitrating CPAP (S8 or S9; ResMed, San Diego, CA) by trained staff. All patients were evaluated at 1 month, 3 months, 6 months, 12 months, and yearly to end of main study. At each follow‐up visit, necessary adjustments of the CPAP device and mask fittings were done and hours of CPAP use per night were obtained from the device and recorded. The CPAP data used in this study were from the start of treatment to the 24‐month follow‐up, date of death, CPAP dropout, or loss to follow‐up.
2.3. Outcome measure
The endpoints of interest were continued use of CPAP treatment and variables associated with continued CPAP use. Whenever a patient returned the device, CPAP dropout was recorded along with reasons for stopping CPAP. A CPAP dropout was recorded whenever a patient discontinued treatment. Data was censored at the date of the last visit for patients who died, were lost to follow‐up, or dropped out, and the data collected up to that point were used to analyze adherence.
2.4. Statistical analysis
Categorical variables were summarized as counts and percentages, and continuous variables were presented as median (interquartile range). Comparisons of variables between sleepy and nonsleepy patients were performed using the Mann–Whitney U test or χ2 test. Kaplan–Meier survival analyses were used to estimate the proportion of patients still on CPAP. The log‐rank test was used to compare rates of continuation of CPAP treatment between sleepy and nonsleepy patients. Univariate Cox regression analysis was used to test the association between continued use of CPAP treatment and clinically important baseline factors (age, sex, body mass index [BMI], smoking, ESS, AHI, percentage of nighttime spent with oxygen saturation < 90%, hypertension [HTN], diabetes mellitus [DM], acute myocardial infarction [AMI], type of revascularization, previous revascularization, and average hours of CPAP use per night at 1 month). Age, sex, and variables found on univariate analyses to have a P value <0.20 were included in a multivariate Cox regression model to evaluate independent associations with continued use of CPAP treatment. The proportional hazards assumption was tested by the distribution of Schoenfeld residuals. Separate univariate and multivariate Cox regression analyses were conducted for sleepy and nonsleepy patients. A P value of <0.05 was considered statistically significant. SPSS 24 for Windows (IBM Corp., Armonk, NY) was used to conduct the statistical analyses.
3. RESULTS
3.1. Study population
Baseline characteristics of the 122 nonsleepy and 155 sleepy patients are detailed in Table 1. Groups were similar in sex, smoking habit, AHI, % nighttime spent with oxygen saturation < 90%, comorbidities, type of revascularization, and previous revascularization. Nonsleepy patients had a higher median age (66.2 years; IQR, 60.4–71.4 years), lower median BMI (27.9 kg/m2; IQR, 25.7–30.2 kg/m2), and lower oxygen desaturation index (ODI; median, 13.6; IQR, 9.1–21.7) than did sleepy patients (median age, 62.0 years [IQR, 57.5–68.0 years]; median BMI, 29.0 kg/m2 [IQR, 26.3–32.2 kg/m2]; median ODI, 15.8 [IQR, 8.8–27.4], respectively).
Table 1.
Baseline characteristics of nonsleepy and sleepy OSA patients
| Nonsleepy OSA, n = 122 | Sleepy OSA, n = 155 | Total, N = 277 | P Value | |
|---|---|---|---|---|
| Age, y | 66.2 (60.4–71.4) | 62.0 (57.5–68.0) | 63.9 (58.9–69.5) | 0.001 |
| Female sex | 22 (18.0) | 17 (11.0) | 39 (14.1) | 0.09 |
| BMI, kg/m2 | 27.9 (25.7–30.2) | 29.0 (26.3–32.2) | 28.9 (25.9–31.3) | 0.01 |
| Obesity | 34 (27.9) | 64 (41.3) | 98 (35.4) | 0.02 |
| Current smoker | 22 (18.0) | 27 (17.4) | 49 (17.7) | 0.89 |
| AHI | 23.7 (18.0–36.6) | 27.9 (18.7–40.2) | 26.0 (18.4–37.7) | 0.09 |
| ODI | 13.6 (9.1–21.7) | 15.8 (8.8–27.4) | 15.1 (8.8–23.5) | 0.02 |
| % of nighttime spent with oxygen saturation < 90% | 1.3 (0.3–5.3) | 2.5 (0.4–7.9) | 1.8 (0.3–6.7) | 0.05 |
| ESS score | 6.0 (3.0–8.0) | 11.0 (10.0–14.0) | 10.0 (6.0–12.0) | <0.001 |
| Treated HTN | 84 (68.9) | 89 (57.4) | 173 (62.5) | 0.05 |
| History of AF | 15 (12.3) | 29 (18.7) | 44 (15.9) | 0.15 |
| AMI at baseline | 65 (53.3) | 76 (49.0) | 141 (50.9) | 0.48 |
| CABG at baseline | 33 (27.0) | 40 (25.8) | 73 (26.4) | 0.82 |
| Previous PCI or CABG | 27 (22.1) | 31 (20.0) | 58 (20.9) | 0.67 |
| DM | 34 (27.9) | 39 (25.2) | 73 (26.4) | 0.61 |
| CPAP hours/night at 1 montha | 3.9 (2.2–6.2) | 5.2 (3.0–6.4) | 4.9 (2.5–6.3) | 0.06 |
Abbreviations: AF, atrial fibrillation; AHI, apnea‐hypopnea index; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; DM, diabetes mellitus; ESS, Epworth Sleepiness Scale; HTN, hypertension; IQR, interquartile range; MI, myocardial infarction; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; PCI, percutaneous coronary intervention.
Data are presented as n (%) or median (IQR).
Data available for n = 105 nonsleepy and n = 147 sleepy patients.
During the 24‐month follow‐up period, of the 122 nonsleepy patients allocated to CPAP at baseline, 49 (40.2%) patients were CPAP dropouts, 2 (1.6%) died, and 1 (0.8%) was lost to follow‐up. As for the 155 sleepy patients provided CPAP at baseline, 37 (23.9%) patients were CPAP dropouts, 2 (1.3%) died, and 1 (0.7%) was lost to follow‐up. The Figure shows the Kaplan–Meier plot of the proportion of sleepy and nonsleepy patients who were still on CPAP therapy over 24‐month follow‐up. As shown, the proportion still on CPAP was significantly lower in the nonsleepy patients, with 86% still on CPAP at 1 month, 63% at 12 months, and 60% at 24 months (compared with 95% at 1 month, 78% at 12 months, and 77% at 24 months among sleepy patients; log‐rank P = 0.001). The median use of CPAP at 1 month, 3 months, 6 months, 12 months, and 24 months was not significantly different between nonsleepy and sleepy patients (Table 2).
Table 2.
Nightly CPAP use in nonsleepy and sleepy OSA patients
| Month 1 | Month 3 | Month 6 | Month 12 | Month 24 | |
|---|---|---|---|---|---|
| Nonsleepy OSA | |||||
| No. of patients | 105 | 92 | 83 | 77 | 70 |
| Nightly CPAP use, h | 4.1 (2.4) | 4.9 (2.2) | 5.4 (2.0) | 5.7 (1.8) | 5.9 (1.9) |
| Nightly CPAP use, h | 3.9 (2.2–6.2) | 5.2 (3.3–6.8) | 5.8 (4.1–6.8) | 5.9 (4.8–7.0) | 6.1 (5.1–7.2) |
| Sleepy OSA | |||||
| No. of patients | 147 | 137 | 127 | 121 | 115 |
| Nightly CPAP use, h | 4.7 (2.4) | 5.0 (2.1) | 5.3 (1.9) | 5.5 (1.7) | 5.7 (1.8) |
| Nightly CPAP use, h | 5.2 (3.0–6.4) | 5.5 (3.6–6.7) | 5.8 (4.1–6.8) | 5.8 (4.3–6.9) | 6.0 (4.8–7.0) |
Abbreviations: CPAP, continuous positive airway pressure; IQR, interquartile range; OSA, obstructive sleep apnea; SD, standard deviation.
Data are presented first as mean (SD) and then as median (IQR).
Figure 1.
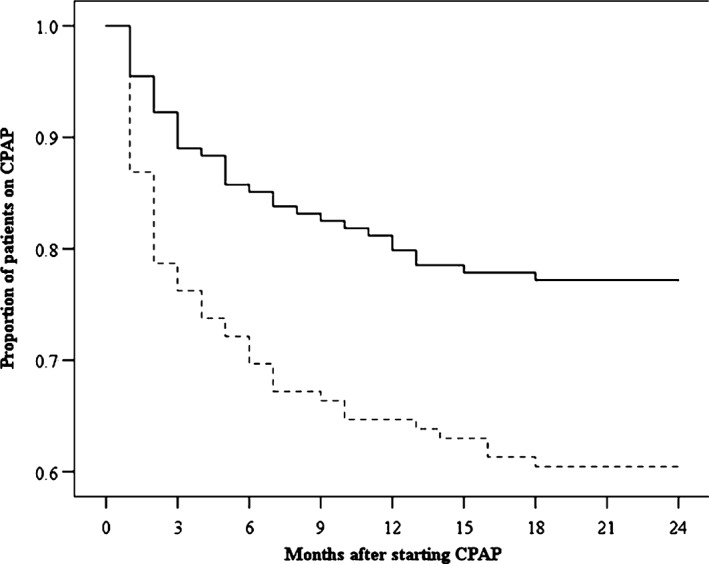
Kaplan–Meier plot showing the proportion of sleepy and nonsleepy patients using CPAP therapy vs time. Abbreviations: CPAP, continuous positive airway pressure
3.2. Predictors of long‐term CPAP use in sleepy patients
In univariate analyses, BMI, AHI, % nighttime spent with oxygen saturation < 90%, AMI at baseline, and CPAP usage at 1 month were associated with continued CPAP use (Table 3). Higher BMI, higher AHI, greater % nighttime spent with oxygen saturation < 90%, AMI at baseline, and higher hours of CPAP use at 1 month were associated with continued CPAP use. Age, sex, current smoking at baseline, ESS score, HTN, DM, type of revascularization, and previous revascularization were not associated with continued CPAP use (Table 3).
Table 3.
Univariate and multivariate Cox regression analyses for association between baseline variables and continued CPAP use in CAD patients with sleepy OSA
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, y | 1.03 (0.98‐1.08) | 0.22 | 1.03 (0.98‐1.08) | 0.20 |
| Female sex | 0.93 (0.33‐2.63) | 0.89 | 0.49 (0.13‐1.87) | 0.30 |
| BMI, kg/m2 | 0.93 (0.85‐1.01) | 0.08 | 0.87 (0.77‐0.99) | 0.04 |
| Current smoker | 1.00 (0.41‐2.40) | 0.99 | — | — |
| AHI | 0.98 (0.96‐1.01) | 0.16 | 1.00 (0.97‐1.03) | 0.90 |
| % time spent with oxygen saturation < 90% | 0.94 (0.88‐1.01) | 0.07 | 0.99 (0.94‐1.04) | 0.62 |
| ESS score | 1.06 (0.94‐1.19) | 0.35 | — | — |
| Treated HTN | 1.26 (0.65‐2.45) | 0.50 | — | — |
| DM | 1.02 (0.49‐2.12) | 0.97 | — | — |
| CABG at baseline | 1.40 (0.70‐2.79) | 0.35 | — | — |
| AMI at baseline | 0.28 (0.13‐0.60) | 0.001 | 0.34 (0.14‐0.81) | 0.02 |
| Previous CABG or PCI | 1.45 (0.68‐3.09) | 0.34 | — | — |
| CPAP hours/night at 1 month | 0.61 (0.52‐0.73) | <0.001 | 0.56 (0.46‐0.69) | <0.001 |
Abbreviations: AHI, apnea‐hypopnea index; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CI, confidence interval; CPAP, continuous positive airway pressure; DM, diabetes mellitus; ESS, Epworth Sleepiness Scale; HR, hazard ratio; HTN, hypertension; OSA, obstructive sleep apnea; PCI, percutaneous coronary intervention.
Multivariate analyses (including age and sex) indicated that BMI (hazard ratio [HR]: 0.87, 95% confidence interval [CI]: 0.77‐0.99), AMI at baseline (HR: 0.34, 95% CI: 0.14‐0.81), and hours of CPAP use at 1 month (HR: 0.56, 95% CI: 0.46‐0.69)—but not AHI or % nighttime spent with oxygen saturation < 90%—remained significantly associated with continued CPAP use (Table 3).
3.3. Predictors of long‐term CPAP use in nonsleepy patients
In univariate analyses, the only variables associated with continued CPAP use were previous revascularization and hours of CPAP use at 1 month (Table 4). Having a previous CABG or PCI and higher hours of CPAP use at 1 month were associated with continued CPAP use. Age, sex, BMI, current smoking at baseline, AHI, % nighttime spent with oxygen saturation < 90%, ESS score, HTN, DM, type of revascularization, and AMI were not associated with continued CPAP use (Table 4).
Table 4.
Univariate and multivariate Cox regression analyses for association between baseline variables and continued CPAP use in CAD patients with nonsleepy OSA
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, y | 1.01 (0.98‐1.05) | 0.44 | 1.07 (1.02‐1.12) | 0.009 |
| Female sex | 1.12 (0.52‐2.39) | 0.78 | 0.64 (0.19‐2.19) | 0.48 |
| BMI, kg/m2 | 1.00 (0.94‐1.08) | 0.91 | — | — |
| Current smoker | 0.80 (0.37‐1.70) | 0.56 | — | — |
| AHI | 1.00 (0.98‐1.03) | 0.82 | — | — |
| % time spent with oxygen saturation < 90% | 1.01 (0.97‐1.04) | 0.72 | — | — |
| ESS score | 0.93 (0.82‐1.05) | 0.23 | — | — |
| Treated HTN | 1.10 (0.59‐2.04) | 0.78 | — | — |
| DM | 1.01 (0.53‐1.90) | 0.98 | — | — |
| CABG at baseline | 1.37 (0.75‐2.52) | 0.31 | — | — |
| AMI at baseline | 0.94 (0.54‐1.65) | 0.83 | — | — |
| Previous CABG or PCI | 0.61 (0.29‐1.30) | 0.20 | 0.80 (0.33‐1.94) | 0.61 |
| CPAP hours/night at 1 month | 0.64 (0.53‐0.77) | <0.001 | 0.57 (0.47‐0.69) | <0.001 |
Abbreviations: AHI, apnea‐hypopnea index; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CI, confidence interval; CPAP, continuous positive airway pressure; DM, diabetes mellitus; ESS, Epworth Sleepiness Scale; HR, hazard ratio; HTN, hypertension; OSA, obstructive sleep apnea; PCI, percutaneous coronary intervention.
Multivariate analyses (including age and sex) indicated that age (HR: 1.07, 95% CI: 1.02‐1.12) and hours of CPAP use at 1 month (HR: 0.57, 95% CI: 0.47‐0.69)—but not previous revascularization—remained significantly associated with continued CPAP use (Table 4).
4. DISCUSSION
This study compared long‐term use of CPAP between sleepy and nonsleepy OSA patients from a CAD cohort and identified predictors of long‐term CPAP use within these groups. The main findings of this study are as follows: (1) a significantly lower proportion of nonsleepy patients was continuing to use CPAP at 2 years as compared with sleepy patients; (2) younger age and hours of CPAP use at 1 month predicted long‐term CPAP use in nonsleepy patients; and (3) BMI, AMI at baseline, and hours of CPAP use at 1 month predicted long‐term CPAP use in sleepy patients.
CPAP adherence is presumed to be problematic in OSA patients without EDS due to a lack of subjective benefits from treatment. Given the risk of recurrent CV events among patients with cardiovascular disease and OSA, CPAP treatment has been recommended in this population, although many do not have EDS.20 In our cohort of CAD patients with OSA, a significantly lower proportion of nonsleepy patients was still using CPAP at 2‐year follow‐up compared with sleepy patients, and 13% of nonsleepy patients had discontinued CPAP by 1 month (vs 5% of sleepy patients). It should be noted that although a lower proportion of nonsleepy patients was still using CPAP at 2 years, a significant proportion of nonsleepy (60%) and sleepy patients (77%) was still using CPAP at 2‐year follow‐up. No significant difference in median CPAP hours of use was found between nonsleepy and sleepy patients (5.3 vs 5.5 hours, respectively), consistent with usage reported in prior studies.21, 22, 23 However, in contrast to our study, CPAP use was lower in patients with cardiovascular disease and OSA with minimal sleepiness from the Sleep Apnea Cardiovascular Endpoints (SAVE) trial (median, 3.3 hours per night at 4 years).14 Our findings suggest that cardiologists managing CAD patients with nonsleepy OSA could be challenged with the task of getting these patients to accept and adhere to CPAP. Educating this population about the seriousness of the CV risk of CPAP nonadherence may help motivate patients to use their CPAP more consistently.
Greater hours of CPAP use during the first month of treatment independently predicted long‐term continued use of CPAP in both sleepy and nonsleepy patients, which is consistent with previous studies.11, 24, 25, 26 In our CAD population, >2× nonsleepy OSA patients (13%) had discontinued treatment by 1 month compared with sleepy patients (5%). Patterns of CPAP use have been shown to be established very early, within the first few days of use.27, 28 Our results suggest that CAD patients with OSA, particularly those without EDS, should have their early CPAP usage monitored and be seen soon after CPAP initiation to address potential problems that may deter continued use.
In contrast to other studies,11, 21, 22, 24, 29 we found higher BMI to be a predictor of long‐term CPAP use among sleepy patients and younger age to be associated with continued CPAP use in nonsleepy patients. Younger patients in our study population may have been motivated to continue usage of CPAP treatment given their recent and potentially first CV event and the potential CV benefits of treating their OSA. The sleepy group had a significantly higher BMI than the nonsleepy group, which also was associated with increased ESS. Thus, decreasing ESS in the sleepy group could have been a motivator for continued CPAP use. Other baseline variables including OSA severity and ESS score did not predict long‐term CPAP use in nonsleepy or sleepy patients. These findings are consistent with previous investigations.22, 24, 29, 30, 31, 32
AMI at baseline was an independent predictor of continued long‐term CPAP use in our sleepy patients. Among the nonsleepy patients, previous revascularization was associated with long‐term CPAP use in univariate analyses but was no longer associated with long‐term use when other confounders were adjusted for. Prior studies examining CV comorbidities as predictors of CPAP adherence in nonsleepy and sleepy OSA patients have found conflicting results.21, 22, 24 Patients with previous cardiac events may have received more direct education from their medical provider about cardiac risk factors and/or may be more concerned about their cardiac condition and are therefore more motivated to engage in risk‐reducing behaviors.
4.1. Study limitations
A limitation of our study is that the ESS was used to categorize patients as “nonsleepy” based on ESS threshold. This approach does not provide objective measurement of daytime sleepiness, as do other methods such as the Multiple Sleep Latency Test.33 However, the ESS is a widely used subjective measure of sleepiness. Additionally, this was a Swedish cohort, so the findings may not be generalizable to other geographic areas.
5. CONCLUSION
This study found that CAD patients with nonsleepy OSA are more likely to discontinue treatment than sleepy patients and that a greater proportion will discontinue CPAP therapy within the first year of treatment. Long‐term use of CPAP treatment may be particularly challenging for cardiac populations with nonsleepy OSA. Thus, interventions aimed at optimizing patients' early experience with CPAP may help promote CPAP acceptance and establish optimal usage patterns that can be maintained long‐term.
Conflicts of interest
PJS has received personal fees from ResMed, Inspire Medical Systems, and Philips Respironics; has served as a consultant for Jazz Pharmaceuticals and Itamar Medical; has been a member of the NFL General Medical Committee; and has given expert testimony for Harris v Emory. ET received lecture fees from ResMed and Pfizer. YP received institutional grants and lecture fees from ResMed and consultant fees from BresoTec, outside the submitted work. The authors declare no other potential conflicts of interest.
Supporting information
Supplemental Figure 1 Flow diagram for patient recruitment and randomization
CAD, coronary artery disease; CSA‐CSR, central sleep apnea‐Cheyne Stokes respiration; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnea
Luyster FS, Strollo PJ, Thunström E, Peker Y Long‐term use of continuous positive airway pressure therapy in coronary artery disease patients with nonsleepy obstructive sleep apnea. Clin Cardiol. 2017;40:1297–1302. 10.1002/clc.22827
Funding information This study was supported by grants from the Swedish Research Council, the Swedish Heart‐Lung Foundation, the “Agreement concerning research and education of doctors” of Vastra Gotalandsregionen, the Heart Foundation of Karnsjukhuset, ResMed Foundation, and ResMed Ltd.
REFERENCES
- 1. De Torres‐Alba F, Gemma D, Armada‐Romero E, et al. Obstructive sleep apnea and coronary artery disease: from pathophysiology to clinical implications. Pulm Med. 2013;2013:768064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glantz H, Thunström E, Herlitz J, et al. Occurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohort. Ann Am Thorac Soc. 2013;10:350–356. [DOI] [PubMed] [Google Scholar]
- 3. Mooe T, Rabben T, Wiklund U, et al. Sleep‐disordered breathing in men with coronary artery disease. Chest. 1996;109:659–663. [DOI] [PubMed] [Google Scholar]
- 4. Shahar E, Whitney CW, Redline S, et al. Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;63:19–25. [DOI] [PubMed] [Google Scholar]
- 5. Peker YK, Hedner J, Kraiczi H, et al. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162:81–86. [DOI] [PubMed] [Google Scholar]
- 6. Campos‐Rodriguez F, Martinez‐Garcia MA, Reyes‐Nuñez N, et al. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189:1544–1550. [DOI] [PubMed] [Google Scholar]
- 7. Fava C, Dorigoni S, Dalle Vedove F, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea: a systematic review and meta‐analysis. Chest. 2014;145:762–771. [DOI] [PubMed] [Google Scholar]
- 8. Fu Y, Xia Y, Yi H, et al. Meta‐analysis of all‐cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath. 2016;21:181–189. [DOI] [PubMed] [Google Scholar]
- 9. George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–613. [DOI] [PubMed] [Google Scholar]
- 11. McArdle N, Devereux G, Heidarnejad H, et al. Long‐term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(4 part 1):1108–1114. [DOI] [PubMed] [Google Scholar]
- 12. Sin DD, Mayers I, Man GC, et al. Long‐term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population‐based study. Chest. 2002;121:430–435. [DOI] [PubMed] [Google Scholar]
- 13. Zhang D, Luo J, Qiao Y, et al. Continuous positive airway pressure therapy in non‐sleepy patients with obstructive sleep apnea: results of a meta‐analysis. J Thorac Dis. 2016;8:2738–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. [DOI] [PubMed] [Google Scholar]
- 15. Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194:613–620. [DOI] [PubMed] [Google Scholar]
- 16. Barbé F, Durán‐Cantolla J, Sánchez‐de‐la‐Torre M, et al; Spanish Sleep and Breathing Network. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–2168. [DOI] [PubMed] [Google Scholar]
- 17. Peker Y, Glantz H, Thunström E, et al. Rationale and design of the Randomized Intervention with CPAP in Coronary Artery Disease and Sleep Apnoea—RICCADSA trial. Scand Cardiovasc J. 2009;43:24–31. [DOI] [PubMed] [Google Scholar]
- 18. Flemons W, Buysse D, Redline S, et al. Sleep‐related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 19. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 20. Hedner J, Grote L. The link between sleep apnea and cardiovascular disease: time to target the nonsleepy sleep apneics? Am J Respir Crit Care Med. 2001;163:5–6. [DOI] [PubMed] [Google Scholar]
- 21. Campos‐Rodriguez F, Martinez‐Alonso M, Sanchez‐de‐la‐Torre M, et al. Long‐term adherence to continuous positive airway pressure therapy in non‐sleepy sleep apnea patients. Sleep Med. 2016;17:1–6. [DOI] [PubMed] [Google Scholar]
- 22. Campos‐Rodriguez F, Martinez‐Garcia MA, Reyes‐Nuñez N, et al. Long‐term continuous positive airway pressure compliance in females with obstructive sleep apnoea. Eur Respir J. 2013;42:1255–1262. [DOI] [PubMed] [Google Scholar]
- 23. Gagnadoux F, Le Vaillant M, Paris A, et al; IRSR Sleep Cohort Group. Adherence to positive airway pressure in non‐sleepy patients with obstructive sleep apnoea. Eur Respir J. 2013;42:863–866. [DOI] [PubMed] [Google Scholar]
- 24. Chai‐Coetzer CL, Luo YM, Antic NA, et al. Predictors of long‐term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep. 2013;36:1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Popescu G, Latham M, Allgar V, et al. Continuous positive airway pressure for sleep apnoea/hypopnoea syndrome: usefulness of a 2‐week trial to identify factors associated with long‐term use. Thorax. 2001;56:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turnbull CD, Bratton DJ, Craig SE, et al. In patients with minimally symptomatic OSA, can baseline characteristics and early patterns of CPAP usage predict those who are likely to be longer‐term users of CPAP? J Thorac Dis. 2016;8:276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aloia MS, Arnedt JT, Stepnowsky C, et al. Predicting treatment adherence in obstructive sleep apnea using principles of behavior change. J Clin Sleep Med. 2005;1:346–353. [PubMed] [Google Scholar]
- 28. Weaver T, Kribbs N, Pack A, et al. Night‐to‐night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–283. [DOI] [PubMed] [Google Scholar]
- 29. Kohler M, Smith D, Tippett V, et al. Predictors of long‐term compliance with continuous positive airway pressure. Thorax. 2010;65:829–832. [DOI] [PubMed] [Google Scholar]
- 30. Sampol G, Rodés G, Romero O, et al. Adherence to nCPAP in patients with coronary disease and sleep apnea without sleepiness. Respir Med. 2007;101:461–466. [DOI] [PubMed] [Google Scholar]
- 31. Sucena M, Liistro G, Aubert G, et al. Continuous positive airway pressure treatment for sleep apnoea: compliance increases with time in continuing users. Eur Respir J. 2006;27:761–766. [DOI] [PubMed] [Google Scholar]
- 32. Wolkove N, Baltzan M, Kamel H, et al. Long‐term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wise MS. Objective measures of sleepiness and wakefulness: application to the real world? J Clin Neurophysiol. 2006;23:39–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Flow diagram for patient recruitment and randomization
CAD, coronary artery disease; CSA‐CSR, central sleep apnea‐Cheyne Stokes respiration; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnea


