Abstract
Background
Oxidative stress and inflammatory processes are responsible for the pathogenesis of AF, but their relationship with the sizes of the LA and PVs in AF patients remains unclear.
Hypothesis
Oxidative stress and inflammatory processes are associated with the sizes of the LA and PVs in AF patients.
Methods
82 AF patients were compared to 30 control patients by using a case‐control study design. Oxidative stress, inflammatory biomarkers and the sizes of the LA and PVs were detected.
Results
(1) Hs‐CRP, IL‐6, IL‐8, TNF‐α, MDA and ox‐LDL were higher, and SOD was lower in AF patients than in control patients. Hs‐CRP, MDA and ox‐LDL were higher in permanent AF patients than in paroxysmal and persistent AF patients. (2) CsA of LSPV, RSPV, RIPV, LAA and LAV were statistically higher in AF patients than in control patients. CsA of RSPV, LSPV, LIPV and LAV were higher in permanent AF patients than in paroxysmal and persistent AF patients. (3) In the AF group, hs‐CRP and TNF‐α were positively correlated with LAV; MDA was positively correlated with CsA of LAA, LSPV and LAV; SOD was passively correlated with CsA of LAA and LAV; ox‐LDL was positively correlated with CsA of LAA and LAV. Multivariate logistic regression analysis showed hs‐CRP, ox‐LDL, RSPV CsA, LIPV CsA and LAV were associated with AF.
Conclusions
Oxidative stress, inflammatory biomarkers and the sizes of the LA and PVs were significantly increased in AF patients. Hs‐CRP, ox‐LDL, RSPV CsA, LIPV CsA and LAV were associated with AF persistence.
Keywords: oxidative stress, inflammation, left atrium, pulmonary vein, atrial fibrillation
1. INTRODUCTION
Atrial fibrillation (AF) is the most prevalent arrhythmia, which has a high risk for heart failure, stroke, and cardiogenic mortality. The mechanisms underlying the initiation and development of AF are not fully understood. Many factors, including electrophysiological and structural remodeling, metabolic and ischemic disorders, and genetic alterations, could result in AF.1
Oxidative stress has been reported to have a tight relation with AF,2, 3 and antioxidants have been reported to alleviate the electrical remodeling in animal AF models. Moreover, increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX)2/4 activity in the fibrillation atria played an important role in the production of reactive oxygen species (ROS) in AF.4 Oxidative status had a significant correlation with the size of the left atrium (LA) using multivariate analysis,3 and the dilatation of the LA was the only marker that could predict the oxidative status.
Previous studies also found inflammation was associated with AF.2, 5, 6, 7 Patients with AF have elevated serum levels of C‐reactive protein (CRP), interleukin‐6 (IL‐6), IL‐8, and tumor necrosis factor‐α (TNF‐α).2 The major oxidant system in leukocytes is constituted by NOX and myeloperoxidase, which are the key enzymes in a cascade of reaction leading to ROS.8 A previous study has shown that myeloperoxidase activity was a potential factor for apocynin's function on NOX activity,9 but the key factors that contribute to oxidative stress and inflammatory processes in AF patients are not fully investigated.
In this study, we aimed to determine whether anatomical variables of the LA and pulmonary veins (PVs), evaluated by computed tomography (CT) imaging, were associated with the plasma oxidative stress and inflammatory processes in AF patients.
2. METHODS
2.1. Patient data
The study population consisted of 82 consecutive AF patients in the cardiology department. Thirty healthy age‐ and sex‐matched people at the physical examination center were chosen as the control group. Detailed medical histories and physical examinations were obtained, and routine biochemical testing was performed in the morning for all patients. A 12‐lead electrocardiogram was also obtained after admission. The transthoracic echocardiography was employed to evaluate the left ventricular ejection fraction (LVEF) and left atrial diameter (LAD). AF duration was defined as detection on the electrocardiogram of AF while an abrupt‐onset palpitation was described by the patient. The study protocol was approved by the hospital's ethics committee. Patients with coronary artery disease, valvular heart disease, chronic renal failure, and ongoing systemic inflammation (eg, infection, cancer, rheumatoid arthritis, liver fibrosis, and chronic obstructive pulmonary disease) were excluded.10 Patients who had undergone surgery within 60 days or had an LVEF <40% were also excluded.
2.2. AF classification
All AF patients were divided into paroxysmal, persistent, and permanent AF groups based on American Heart Association guidelines.11 Paroxysmal AF was defined as recurrent AF <7 days in duration that terminates spontaneously. Patients with continuous AF ≥7 days in duration that was successfully converted to sinus rhythm were considered to have persistent AF. Patients with AF ≥6 months in duration and with failure to restore normal sinus rhythm were considered to have permanent AF.10
2.3. Biochemical measurements
Blood samples were taken and centrifuged at 1000 relative centrifugal force for 10 minutes at 4°C, and blood samples were frozen at −80°C. Patients with paroxysmal AF were in sinus rhythm when biochemical analysis was performed; other patients were in AF rhythm when biochemical analysis was performed. Plasma levels of oxidized low‐density lipoprotein (ox‐LDL), high‐sensitivity CRP (hs‐CRP), IL‐6, IL‐8, and TNF‐α were detected by enzyme‐linked immunosorbent assay. Plasma levels of maleic dialdehyde (MDA) and superoxide dismutase (SOD) were detected by colorimetry.
2.4. Measurement of anatomical parameters by CT
CT angiograms of the LA and PVs were evaluated using a 64‐slice multidetector cardiac CT scanner (Siemens, Erlangen, Germany). CT data were transferred to the CARTO 3 system for analysis. The following variables were measured: LA volume (cm3); cross‐sectional area (CsA) of the PVs (cm2; left superior PV [LSPV], left inferior PV [LIPV], right superior PV [RSPV], and right inferior PV [RIPV]); and CsA of the left atrial appendage (LAA, cm2; Figure 1). Patients with common left PV and/or right‐middle PV were excluded from this measurement. The CsAs of the PVs were measured at the “ostium” level. The PV ostium was defined as the point of maximal inflection between the PV wall and the LA wall.12
Figure 1.
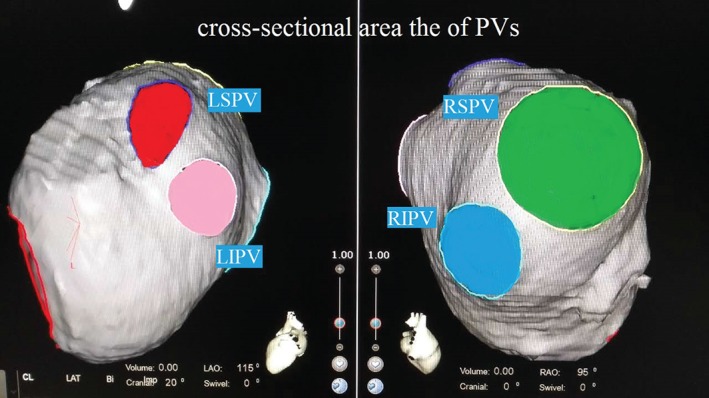
CT angiograms of the LA and PVs. Abbreviations: CT, computed tomography; LA, left atrium; LAO, left anterior oblique; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; PV, pulmonary vein; RAO, right anterior oblique; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
2.5. Statistical analysis
Continuous data were expressed as mean ± SEM. The Kolmogorov‐Smirnov test was applied to the normality of the distribution of each variable. Differences of continuous variables between groups were compared by independent samples t test. Differences of categorical variables between groups were analyzed by χ2 test. To identify factors that determine the levels of oxidative stress markers and inflammatory markers, we performed multiple regression analysis. To identify independent factors of AF, we then performed multiple logistic regression analysis. A P value of <0.05 was considered statistically significant. All data were analyzed by using SPSS software, version 13.0 (SPSS Inc., Chicago, Illinois).
3. RESULTS
3.1. Baseline characteristics of the study population
Eighty‐two AF patients (44 cases of paroxysmal AF, 25 cases of persistent AF, and 13 cases of permanent AF) and 30 control patients were included in this study. The demographic characteristics of the participants are presented in Table 1. The LVEF of patients in the AF group was statistically lower than that in the control group (Table 1). In subgroup analysis, patients with permanent AF were older and had larger LAD and lower LVEF than patients in the paroxysmal AF and persistent AF subgroups, but these differences were not statistically significant (Table 2).
Table 1.
Baseline characteristics, biochemical parameters, and CT parameters of the study population
| Control Group, n = 30 | AF Group, n = 82 | P Value | |
|---|---|---|---|
| Age, y | 64.7 ± 8.6 | 65.4 ± 10.5 | 0.7443 |
| Male sex | 17 (56.7) | 47 (57.3) | 0.9509 |
| BMI, kg/m2 | 25.7 ± 5.6 | 25.5 ± 4.8 | 0.8523 |
| Smoking | 7 (23.3) | 21 (25.6) | 0.8054 |
| DM | 2 (6.7) | 5 (6.1) | 0.9123 |
| HTN | 4 (13.3) | 11 (13.4) | 0.9911 |
| LAD, mm | 36.6 ± 8.7 | 40.3 ± 9.3. | 0.0606 |
| LVEF, % | 62.6 ± 10.4 | 57.5 ± 10.6 | 0.0254 |
| Biochemical parameters | |||
| hs‐CRP, mg/L | 1.42 ± 0.96 | 2.48 ± 1.65 | 0.0012 |
| IL‐6, pg/mL | 1.68 ± 0.92 | 3.04 ± 1.87 | 0.0002 |
| IL‐8, pg/mL | 4.45 ± 1.58 | 7.24 ± 4.21 | 0.0006 |
| TNF‐α, pg/mL | 2.02 ± 1.15 | 7.63 ± 3.42 | <0.0001 |
| MDA, nmol/mL | 3.37 ± 0.87 | 5.63 ± 2.55 | <0.0001 |
| SOD, U/mL | 103.4 ± 26.3 | 81.3 ± 20.6 | <0.0001 |
| ox‐LDL, U/L | 58.9 ± 13.7 | 67.3 ± 15.4 | 0.0098 |
| CT parameters | |||
| RSPV CsA, mm2 | 332.4 ± 37.6 | 365.3 ± 40.3 | 0.0002 |
| LSPV CsA, mm2 | 358.3 ± 30.3 | 381.4 ± 44.6 | 0.0100 |
| RIPV CsA, mm2 | 322.2 ± 35.6 | 377.6 ± 33.8 | <0.0001 |
| LIPV CsA, mm2 | 366.7 ± 36.1 | 384.2 ± 47.4 | 0.0692 |
| LAA CsA, mm2 | 1135.5 ± 226.7 | 1767.5 ± 243.5 | <0.0001 |
| LAV, cm3 | 20.42 ± 4.28 | 30.66 ± 8.32 | <0.0001 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CsA, cross‐sectional area; CT, computed tomography; DM, diabetes mellitus; hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; IL, interleukin; LAA, left atrial appendage; LAD, left atrial diameter; LAV, left atrial volume; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; LVEF, left ventricular ejection fraction; MDA, maleic dialdehyde; ox‐LDL, oxidized low‐density lipoprotein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SEM, standard error of the mean; SOD, superoxide dismutase; TNF‐α, tumor necrosis factor‐α.
Data are presented as n (%) or mean ± SEM.
Table 2.
Baseline characteristics, biochemical parameters, and CT parameters in subtypes of AF
| Paroxysmal AF, n = 44 | Persistent AF, n = 25 | Permanent AF, n = 13 | P Value | |
|---|---|---|---|---|
| Age, y | 62.2 ± 12.1 | 65.7 ± 8.8 | 67.3 ± 10.3 | 0.2314 |
| Male sex | 23 (52.3) | 17 (68) | 7 (53.8) | 0.4300 |
| BMI, kg/m2 | 25.8 ± 4.2 | 25.3 ± 5.6 | 25.1 ± 5.8 | 0.8670 |
| Smoking | 13 (29.5) | 5 (20) | 3 (23.1) | 0.6655 |
| DM | 5 (11.4) | 0 | 0 | |
| HTN | 6 (13.6) | 2 (8) | 3 (23.1) | 0.4322 |
| LAD, mm | 39.4 ± 7.7 | 40.6 ± 11.2 | 41.5 ± 10.5 | 0.7374 |
| LVEF, % | 58.8 ± 10.3 | 57.7 ± 10.6 | 57.2 ± 11.8 | 0.8565 |
| Biochemical parameters | ||||
| hs‐CRP, mg/L | 1.95 ± 0.63 | 2.84 ± 1.77 | 3.01 ± 1.74 | 0.0048 |
| IL‐6, pg/mL | 2.85 ± 1.38 | 3.11 ± 1.98 | 3.72 ± 2.02 | 0.2663 |
| IL‐8, pg/mL | 6.28 ± 3.53 | 7.01 ± 3.33 | 8.32 ± 5.21 | 0.2282 |
| TNF‐α, pg/mL | 7.14 ± 3.22 | 7.74 ± 4.81 | 8.82 ± 4.04 | 0.3867 |
| MDA, nmol/ml | 4.72 ± 1.88 | 5.58 ± 2.07 | 6.13 ± 2.16 | 0.0471 |
| SOD, U/ml | 88.8 ± 18.7 | 80.5 ± 17.6 | 77.4 ± 20.3 | 0.0739 |
| ox‐LDL, U/L | 65.2 ± 10.5 | 68.6 ± 9.6 | 75.2 ± 16.4 | 0.0226 |
| CT parameters | ||||
| RSPV CsA, mm2 | 327.7 ± 38.4 | 361.4 ± 47.5 | 373.4 ± 63.3 | 0.0013 |
| LSPV CsA, mm2 | 374.5 ± 30.2 | 389.7 ± 34.1 | 396.3 ± 36.5 | 0.0492 |
| RIPV CsA, mm2 | 364.6 ± 36.5 | 372.6 ± 31.7 | 380.4 ± 38.3 | 0.3271 |
| LIPV CsA, mm2 | 355.8 ± 36.7 | 373.3 ± 50.6 | 410.5 ± 67.3 | 0.0017 |
| LAA CsA, mm2 | 1715.5 ± 227.8 | 1722.4 ± 327.3 | 1818.3 ± 303.6 | 0.4800 |
| LAV, cm3 | 28.41 ± 5.44 | 30.32 ± 5.65 | 33.11 ± 6.89 | 0.0336 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CsA, cross‐sectional area; CT, computed tomography; DM, diabetes mellitus; hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; IL, interleukin; LAA, left atrial appendage; LAD, left atrial diameter; LAV, left atrial volume; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; LVEF, left ventricular ejection fraction; MDA, maleic dialdehyde; ox‐LDL, oxidized low‐density lipoprotein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SEM, standard error of the mean; SOD, superoxide dismutase; TNF‐α, tumor necrosis factor‐α.
Data are presented as n (%) or mean ± SEM.
3.2. Serum oxidative stresses and inflammatory markers levels in AF patients
The levels of serum hs‐CRP, IL‐6, IL‐8, TNF‐α, MDA, and ox‐LDL were significantly higher in AF patients than in control patients (Table 1). In addition, serum SOD level was significantly lower in AF patients than in control patients. In subgroup analysis, serum hs‐CRP, MDA, and ox‐LDL levels were significantly higher in permanent AF patients than in paroxysmal and persistent AF patients (Table 2).
3.3. Sizes of LA and PVs in AF patients
The CsAs of the RSPV, LSPV, RIPV, LAA, and LAV were significantly higher in AF patients than in the control patients (Table 1). In subgroup analysis, the CsA of the RSPV, LSPV, LIPV, and LAV were significantly higher in permanent AF patients than in paroxysmal and persistent AF patients (Table 2).
3.4. Serum oxidative stress and inflammatory markers levels associated with LA and PV sizes in AF patients
In the AF group, after adjusting for age, sex, and body mass index (BMI), serum hs‐CRP (r = 0.221, P = 0.046; Figure 2A) and TNF‐α (r = 0.284, P = 0.040; Figure 2B) levels were significantly correlated with LAV; serum MDA level was significantly correlated with LAA CsA (r = 0.485, P = 0.013; Figure 2C), LAV (r = 0.503, P = 0.006; Figure 2D), and LSPV CsA (r = 0.427, P = 0.026; Figure 2E); serum SOD level was significantly correlated with LAA CsA (r = −0.335, P = 0.034; Figure 2F) and LAV (r = −0.278, P = 0.041; Figure 2G); and serum ox‐LDL level was significantly correlated with LAA CsA (r = 0.358, P = 0.030; Figure 2H) and LAV (r = 0.532, P = 0.004; Figure 2I).
Figure 2.
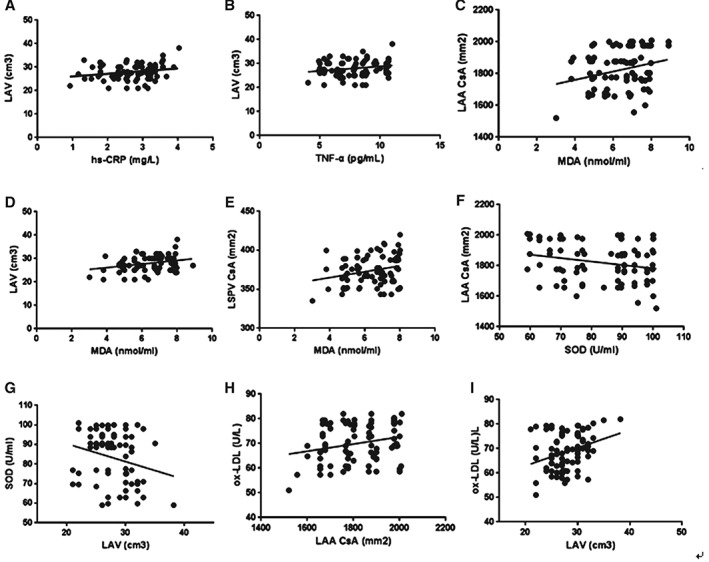
Association of serum oxidative stress and inflammatory marker levels with LA and PV sizes in AF patients. Abbreviations: AF, atrial fibrillation; CsA, cross‐sectional area; CT, computed tomography; hs‐CRP, high‐sensitivity C‐reactive protein; LA, left atrium; LAA, left atrial appendage; LAV, left atrial volume; PV, pulmonary vein; LSPV, left superior pulmonary vein; MDA, maleic dialdehyde; ox‐LDL, oxidized low‐density lipoprotein; SOD, superoxide dismutase; TNF‐α, tumor necrosis factor‐α.
3.5. Association between serum oxidative stress, inflammatory marker levels, sizes of LA and PV, and AF
Multivariate logistic regression analysis was employed in this section, including independent variables of age, sex, BMI, smoking, diabetes mellitus, and hypertension. The results show that serum hs‐CRP level (odds ratio [OR]: 1.38, 95% confidence interval [CI]: 1.04‐1.92, P = 0.037), ox‐LDL (OR: 1.45, 95% CI: 1.07‐2.08, P = 0.034), RSPV CsA (OR: 1.23, 95% CI: 1.07‐1.88, P = 0.025), LIPV CsA (OR: 1.06, 95% CI: 1.01‐1.92, P = 0.047), and LAV (OR: 1.43, 95% CI: 1.15‐1.83, P = 0.018) were associated with AF. After adjusting for age, sex, BMI, smoking, diabetes mellitus, hypertension, and the parameters of the LA and PVs, the results of multivariate regression analysis showed that hs‐CRP level (OR: 1.19, 95% CI: 1.08‐1.57, P = 0.021) and ox‐LDL level (OR: 1.15, 95% CI: 1.02‐2.08, P = 0.046) were associated with AF.
4. DISCUSSION
A close relationship between AF and oxidative stress and inflammation has been reported by several clinical and experimental studies. In the histological studies, increase of oxidative stress and inflammatory biomarkers have been observed in the atrial tissue of patients with AF, which promotes atrial structural and electrical remodeling.13, 14 In addition, the elevation of systemic inflammation and oxidative stress has been found in patients with AF. Drugs that have antioxidant and anti‐inflammatory effects, such as the statins, have been found to be useful for preventing AF and lowering the risk of AF.15 One of the characteristics of oxidative stress is ROS production that is not balanced between the generations and removed by the antioxidant defense system. Oxidative stress and inflammation are closely linked because inflammation can lead to the production of ROS, and ROS could also promote the synthesis of pro‐inflammatory cytokines.16, 17 Increased levels of ROS, such as H2O2 and superoxide, have been found to be associated with AF in myocardial tissues,18, 19 as a consequence of the decrease in nitric oxide bioavailability.20 Increased ROS causes damage to proteins, lipids, and DNA and then potentiates inflammation by activating inflammatory cells, which in turn further induces tissue damage. The involvement of ROS in cardiac structural and electrical remodeling increases the sensitivity to AF.21 Atrial RyR2 is a specific molecular target of oxidative stress, which plays a fundamental role in the initiation of AF. Mitochondrial‐derived ROS oxidizes RyR2 in atrial myocytes, which leads to an increase of intracellular Ca2+ flow. In addition, reduction of mitochondrial RO decreases atrial diastolic Ca2+ flow from the sarcoplasmic reticulum and prevents the initiation of AF.22 Moreover, oxidative status had a significant correlation with the size of the LA using multivariate analysis, and the distension of the LA was the only predictor of oxidative status.3
There is increasing evidence that oxidative stress and inflammatory processes are responsible for the pathogenesis of AF. However, it is unclear if oxidative stress and inflammatory processes are associated with the sizes of the LA and PVs in AF patients. In the present study, we investigated whether anatomical variables of the LA and PVs were associated with the oxidative stress in AF patients. We found that serum MDA and ox‐LDL levels were significantly higher in AF patients compared with controls. In subgroup analysis, serum MDA and ox‐LDL levels were significantly higher in permanent AF patients than in paroxysmal and persistent AF patients. Increase of serum MDA and ox‐LDL level, and decrease of SOD level, were significantly correlated with LAA CsA and LAV in patients with AF. Multivariate logistic regression analysis found that serum ox‐LDL level was significantly associated with AF, which suggests it could serve as a predictor for AF.
Previous studies have shown that patients with AF have an increased inflammatory state. The levels of hs‐CRP, IL‐6, and TNF‐α were significantly higher than those in controls after pharmacological cardioversion in patients with paroxysmal AF.5 Similarly, another study reported that elevated CRP was closely associated with AF.6 Patients with typical AF had significantly elevated serum concentrations of IL‐10, TNF‐α, and N‐terminal probrain natriuretic peptide when compared with those with lone AF in a case‐control study,7 suggesting a strong association between inflammation and the arrhythmia. AF leads to several changes in atrial structure and extracellular matrix composition, which is mediated through matrix metalloproteinases (MMPs).23 MMP‐9 was significantly correlated with inflammatory markers such as CRP. Multivariate analysis revealed that the only independent predictors of MMP‐9 levels were CRP and sex. Moreover, the only independent predictors of CRP were hypertension and age.3 We found that serum hs‐CRP, IL‐6, IL‐8, and TNF‐α levels were significantly higher in AF patients compared with controls. In subgroup analysis, serum hs‐CRP was significantly higher in permanent AF patients than in paroxysmal and persistent AF patients. The up‐regulation of serum hs‐CRP and TNF‐α level was significantly correlated with LAV in patients with AF. Multivariate logistic regression analysis found that serum hs‐CRP level was significantly associated with AF and also the predictor of AF, suggesting that elevated inflammatory conditions may lead to or may result in atrial remodeling.
The anatomy of the LA and PVs is important to the formation of AF. Kato et al24 evaluated 28 consecutive patients who underwent AF ablation by use of a PV approach and in whom magnetic resonance imaging was obtained. They found that PV anatomy is highly variable, with 38% of patients demonstrating variant anatomy; and that PV and LA sizes are larger in AF patients than in controls. Anselmino et al25 found that a significant difference exists in PVs ostial dimensions in patients with paroxysmal and persistent AF. Furthermore, a significant enlargement of both the LA and LAA volumes exists in patients with persistent AF compared with paroxysmal AF. In the present study, we searched for not only the sizes of the LA and PVs, but also the relationship between the inflammatory processes and oxidative stress with the remodeling of the LA and PVs in patients with AF. We found that the CsA of the RSPV, LSPV, RIPV, LAA, and LAV was significantly higher in AF patients compared with the controls. In subgroup analysis, the CsA of the RSPV, LSPV, LIPV, and LAV was significantly higher in permanent AF patients than in paroxysmal and persistent AF patients. Increased serum MDA, ox‐LDL, hs‐CRP, and TNF‐α levels, and decreased SOD level, were significantly correlated with LAV in patients with AF.
4.1. Study limitations
This study has several limitations that must be considered in further analysis. First, the study only explores the associations and precludes the determination of a causal relationship with a cross‐sectional design. Second, this is a single‐center study with a relatively small number of samples. In the future, more comprehensive research is needed with a larger study cohort. Third, patients with a common left PV and/or right‐middle PV were excluded from this study; this results in loss of some data that may reflect the correlation between inflammatory status and oxidative stress with the sizes of the LA and PVs. Finally, we have not performed a subgroup analysis for the correlation of inflammatory status and oxidative stress with the sizes of the LA and PVs in AF patients, because the sample size of the subgroup was small and may generate biased results.
5. CONCLUSION
We found a close relationship between oxidative stress and inflammatory processes and the anatomy size of LA and PVs in AF patients.
Conflicts of interest
The authors declare no potential conflicts of interest.
Acknowledgments
The authors thank Dr. Ying‐lin Wu and Jing‐bo Jiang for manuscript editing and revision.
Li J‐y, He Y, Ke H‐h, Jin Y, Jiang Z‐y and Zhong G‐q. Plasma oxidative stress and inflammatory biomarkers are associated with the sizes of the left atrium and pulmonary vein in atrial fibrillation patients, Clin Cardiol, 2017;40(2):89–94.
Jin‐yi Li and Yan He contributed equally to this work.
Funding information This study was supported by the Guangxi science and technology development project (Gui Ke Gong No. 0592007‐1D) and the scientific research project of education department of Guangxi government (No. 201106LX121).
REFERENCES
- 1. Aviles RJ Martin DO, Apperson‐Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. [DOI] [PubMed] [Google Scholar]
- 2. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. [DOI] [PubMed] [Google Scholar]
- 3. Tousoulis D, Zisimos K, Antoniades C, et al. Oxidative stress and inflammatory process in patients with atrial fibrillation: the role of left atrium distension. Int J Cardiol. 2009;136:258–262. [DOI] [PubMed] [Google Scholar]
- 4. Kim YM, Kattach H, Ratnatunga C, et al. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:68–74. [DOI] [PubMed] [Google Scholar]
- 5. Sata N, Hamada N, Horinouchi T, et al. C‐reactive protein and atrial fibrillation: is inflammation a consequence or a cause of atrial fibrillation? Jpn Heart J. 2004;45:441–445. [DOI] [PubMed] [Google Scholar]
- 6. Asselbergs FW van den Berg MP, Diercks GF, et al. C‐reactive protein and microalbuminuria are associated with atrial fibrillation. Int J Cardiol. 2005;98:73–77. [DOI] [PubMed] [Google Scholar]
- 7. Li J, Solus J, Chen Q, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies MJ. Myeloperoxidase‐derived oxidation: mechanisms of biological damage and its prevention. J Clin Biochem Nutr. 2011;48:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Almeida AC, Dos Santos Vilela MM, Condino‐Neto A, et al. The importance of myeloperoxidase in apocynin‐mediated NADPH oxidase inhibition. ISRN Inflamm. 2012;260453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han X, Chen C, Cheng G, et al. Serum fibroblast growth factor 21 levels are increased in atrial fibrillation patients. Cytokine. 2015;73:176–180. [DOI] [PubMed] [Google Scholar]
- 11. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society [published correction appears in Circulation. 2007;116:e138]. Circulation. 2006;114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 12. Kiuchi K, Yoshida A, Takei A, et al. Topographic variability of the left atrium and pulmonary veins assessed by 3D‐CT predicts the recurrence of atrial fibrillation after catheter ablation. J Arrhythm. 2015;31:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang CX, Liu Y, Xia WF, et al. Oxidative stress: a possible pathogenesis of atrial fibrillation. Med Hypotheses. 2009;72:466–467. [DOI] [PubMed] [Google Scholar]
- 14. Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. [DOI] [PubMed] [Google Scholar]
- 15. Koyama T, Tada H, Sekiguchi Y, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol. 2010;56:1463–1472. [DOI] [PubMed] [Google Scholar]
- 16. Schindhelm RK, van der Zwan LP, Teerlink T, et al. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin Chem. 2009;55:1462–1470. [DOI] [PubMed] [Google Scholar]
- 17. Khaper N, Bryan S, Dhingra S, et al. Targeting the vicious inflammation‐oxidative stress cycle for the management of heart failure. Antioxid Redox Signal. 2010;13:1033–1049. [DOI] [PubMed] [Google Scholar]
- 18. Chang JP, Chen MC, Liu WH, et al. Atrial myocardial NOX2 containing NADPH oxidase activity contribution to oxidative stress in mitral regurgitation: potential mechanism for atrial remodeling. Cardiovasc Pathol. 2011;20:99–106. [DOI] [PubMed] [Google Scholar]
- 19. Zhang J, Youn JY, Kim AY, et al. NOX4‐dependent hydrogen peroxide overproduction in human atrial fibrillation and Hl‐1 atrial cells: relationship to hypertension. Front Physiol. 2012;3:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai H, Li Z, Goette A, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106:2854–2858. [DOI] [PubMed] [Google Scholar]
- 21. Youn JY, Zhang J, Chen H, et al. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie W, Santulli G, Reiken SR, et al. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep. 2015;5:11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mukherjee R, Herron AR, Lowry AS, et al. Selective induction of matrix metalloproteinases and tissue inhibitor of metalloproteinases in atrial and ventricular myocardium in patients with atrial fibrillation. Am J Cardiol. 2006;97:532–537. [DOI] [PubMed] [Google Scholar]
- 24. Kato R, Lickfett L, Meininger G, et al. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation. 2003;107:2004–2010. [DOI] [PubMed] [Google Scholar]
- 25. Anselmino M, Blandino A, Beninati S, et al. Morphologic analysis of left atrial anatomy by magnetic resonance angiography in patients with atrial fibrillation: a large single‐center experience. J Cardiovasc Electrophysiol. 2011;22:1–7. [DOI] [PubMed] [Google Scholar]


