Abstract
Oral anticoagulation (OAC) is recommended in both paroxysmal atrial fibrillation (pxAF) and nonparoxysmal AF (non‐pxAF), but disagreement exists in classes of recommendation. Data on incidence/rate of stroke in pxAF are conflicting, and OAC is often underused in this population. The objectives of the meta‐analysis were to investigate different impact on outcomes of pxAF and non‐pxAF, with and without OAC. Two reviewers searched for prospective studies on risk of stroke and systemic embolism (SE) in pxAF and non‐pxAF, with and without OAC. Quality of evidence was assessed according to GRADE approach. Stroke combined with SE was the main outcome. Meta‐regression was performed to evaluate OAC effect on stroke and SE incidence rate. We identified 18 studies. For a total of 239 528 patient‐years of follow‐up. The incidence rate of stroke/SE was 1.6% (95% confidence interval [CI]: 1.3%‐2.0%) in pxAF and 2.3% (95% CI: 2.0%‐2.7%) in non‐pxAF. Paroxysmal AF was associated with a lower risk of overall thromboembolic (TE) events (risk ratio: 0.72, 95% CI: 0.65‐0.80, P < 0.00001) compared with non‐pxAF. In both groups, the annual rate of TE events decreased as proportion of patients treated with OAC increased. Non‐pxAF showed a reduction from 3.7% to 1.7% and pxAF from 2.5% to 1.2%. Major bleeding rates did not differ among groups. Stroke/SE risk is significantly lower, although clinically meaningful, in pxAF. OAC consistently reduces TE event rates across any AF pattern. As a whole, these data provide the evidence to warrant OAC irrespective of the AF pattern in most (virtually all) patients.
Keywords: Paroxysmal Atrial Fibrillation, Oral Anticoagulants, Stroke, Systemic Embolism
1. INTRODUCTION
Atrial fibrillation (AF), the most common sustained cardiac arrhythmia, predisposes patients to an increased risk of embolic stroke and has a higher mortality than sinus rhythm.1, 2, 3 Anticoagulation with international normalized ratio–adjusted warfarin4 or with non–vitamin K antagonist (non‐VKA) oral anticoagulants (NOACs) is highly effective in reducing the risk of stroke.4, 5 Whether the risk of stroke is affected by the type, duration, and frequency of AF has been debated for years.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 A general consensus has been reached on the definition based on the temporal occurrence of AF: paroxysmal AF episodes are self‐limiting and last <1 week; episodes lasting >7 days are referred to as persistent AF; and permanent AF refers to AF without any intervening sinus rhythm.1, 2 Previous studies on the relation between AF pattern and the risk of stroke have yielded conflicting results.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Although some recent trials have reported higher stroke rates in patients with permanent compared with paroxysmal AF, other studies did not.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Practice guidelines on AF management made identical recommendations regarding stroke prevention for all types of AF, based on known risk factors for stroke in this patient population.1, 2 The available data comparing stroke risk in patients with paroxysmal and permanent AF are limited by methodological issues, such as small sample sizes or differential use of anticoagulation in patients with paroxysmal AF compared with permanent AF.
The objectives of the current review and meta‐analysis were to investigate the differences, if any, in outcomes between nonanticoagulated and anticoagulated patients with persistent vs paroxysmal AF, and to determine whether there was a difference in treatment effect in these groups.
2. METHODS
2.1. Search strategy and study selection criteria
Two independent reviewers performed a combined search in 3 main databases, PubMed, Cochrane Library, and Scopus, from 1990 through September 2015 using the following keywords: “atrial fibrillation,” “thromboembolism,” “outcome,” “systemic embolism,” “embolism,” “stroke,” “cerebral embolism,” “paroxysmal,” and “intermittent.” Furthermore, all references of expert reviews and manuscripts were screened to manually add studies not identified by the automatic search. The PRISMA statement was followed as standard considering the nonintervention nature of the meta‐analysis.24 Results were exported in a dedicated database to identify and eliminate duplicate as reported in the flow diagram (see Supporting Information, Figure 1, in the online version of this article).
Figure 1.
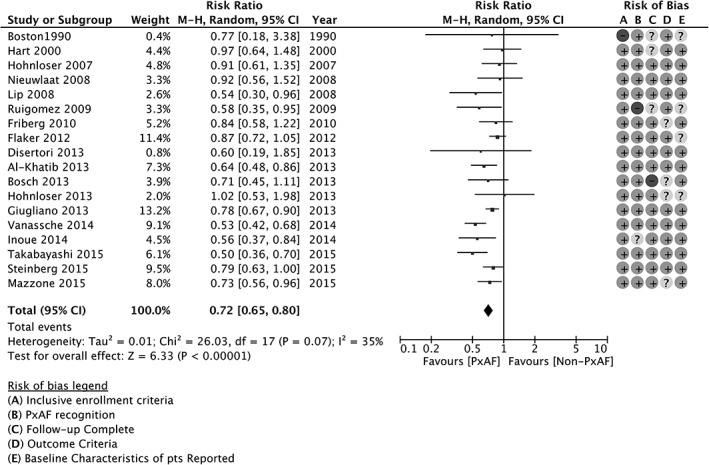
Stroke and SE by AF pattern, forest plot. Abbreviations: AF, atrial fibrillation; CI, confidence interval; df, degrees of freedom; M‐H, Mantel‐Haenszel; non‐PxAF, non‐paroxysmal AF; pts, patients; PxAF, paroxysmal AF; SE, systemic embolism.
Only English‐language studies were considered for the present analysis. The following criteria were selected by protocol for studies inclusion or exclusion: (1) longitudinal prospective randomized or observational studies including (2) unselected prespecified patients with paroxysmal/intermittent and persistent/permanent AF with (3) unrestricted utilization of medical therapy or procedures during follow‐up and (4) defined outcomes in terms of cerebral embolism and/or systemic embolism. Studies including only patients selected for ablation or cardiac procedures, acute coronary syndromes, and secondary prevention (ie, postischemic cerebral events) were excluded to avoid any significant bias in patient selection.
2.2. Outcome definition and data extraction
Ischemic or unspecified stroke combined with systemic embolism when reported were selected as the main outcome for the present analysis. Transient ischemic attack (TIA), due to its more subjective nature, has been excluded whenever possible while deemed acceptable only when indivisible from the main endpoint. Adjunctive sensitivity analysis excluding such studies was performed to avoid bias related to such subjective outcome. Two reviewers (AL and ADC) independently reviewed all selected studies to extract the absolute number or events within each group, overall patients included that completed the study, length of follow‐up, and incidence rate of events. The number of patient‐years of follow‐up for each study is then estimated. The following additional information was retrieved from studies when available (see Supporting Information, Table 1, in the online version of this article): percentage of patients taking oral anticoagulant (OAC) within each group, age, risk score when reported as CHADS score, and bleeding events (again summarized as events per 100 patient‐years).
Table 1.
Summary of findings
| Outcome | No. of Studies | Design | Events/Patient‐Years | Relative Effect (95% CI) | No. of Patients | Quality of Evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|---|---|
| PxAF | Non‐pxAF | |||||||
| Stroke and/or SE | 18 | 11 RCTs, 7 registries | 941/62 553 | 3611/176 975 | 0.72 (0.65‐0.80) | 239 528 | + + + + | |
| Guideline definition | 14 | 7 RCTs, 7 registries | 883/60 108 | 3349/166 278 | 0.70 (0.63‐0.78) | 226 386 | + + + + | |
| Stroke only | 10 | 5 RCTs, 6 registries | 298/21 059 | 955/48 314 | 0.75 (0.62‐0.89 | 69 373 | + + + − | Risk of bias in individual studies |
| Guideline definition | 8 | 2 RCTs, 6 registries | 269/20 054 | 848/44 662 | 0.72 (0.59‐0.88 | 64 716 | + + + − | Risk of bias in individual studies |
| Major bleedings | 7 | RCTs | 1100/37 550 | 3420/124 434 | 1.04 (0.92‐1.18) | 161 984 | + + − − | Data only from RCTs, inconsistency |
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCTs, randomized controlled trials; SE, systemic embolism.
Studies with assessed risk of bias showed very low weight in the main analysis, and downgrade was not justified.
2.3. Data collection and analysis
Pooled results of the meta‐analysis are presented as risk ratio (RR) with 95% confidence intervals (CI). Heterogeneity across studies was assessed by the Cochran Q statistic and the I2 statistic. Potential publication bias was assessed by visual assessment of constructed funnel plots (see Supporting Information, Figure 2, in the online version of this article). The differences in RR were combined across studies with random‐effects model. Analyses were conducted using RevMan (Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012).
Figure 2.
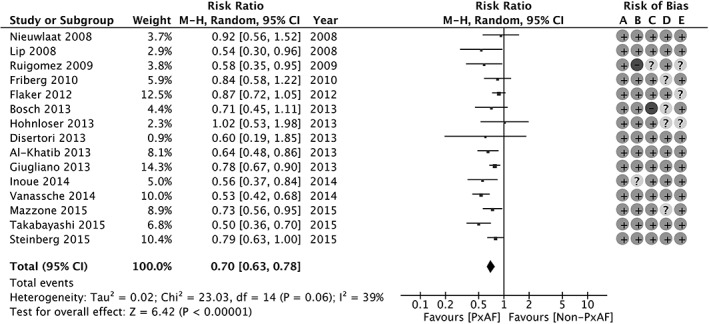
Stroke and SE by AF pattern in studies with definition of paroxysmal according to current guidelines; forest plot. Abbreviations: AF, atrial fibrillation; CI, confidence interval; df, degrees of freedom; M‐H, Mantel‐Haenszel; non‐PxAF, non‐paroxysmal AF; PxAF, paroxysmal AF; SE, systemic embolism.
2.4. Sensitivity analyses
Prespecified sensitivity analysis was performed excluding studies with TIA as indivisible outcome. Moreover, a separate analysis was planned to better understand if differences exist between randomized controlled trials (RCTs) and registries, and if adjusted data from studies with multivariable adjustment differ from unadjusted event rates (generic inverse variance methods, hazard ratio).
Due to the importance of OAC, a final analysis including only studies with completely matched OAC assumption between paroxysmal AF (pxAF) and persistent/permanent AF (non‐pxAF) was performed.
2.5. Meta‐regression analysis
A set of specific analyses was performed to better understand the weight of OAC on thromboembolic (TE) event incidence/rate within patients with pxAF or non‐pxAF. Meta‐regression graph was constructed with proportion of subjects taking OAC on the x‐axis and the TE rate on the y‐axis. A graph for each subgroup was presented. A pooled weighted incidence rate of events in the pxAF and non‐pxAF groups was derived for studies without OAC, studies with full anticoagulated population, and finally for intermediate condition. These and pooled incidence analyses were conducted with the Agency for Healthcare Research and Quality Open Meta‐Analyst.
2.6. Quality assessment and reporting
We reported risk of bias for each study included in the meta‐analysis and overall quality of evidence assessment for 3 main outcomes. Briefly, 5 main domains were considered for risk of bias for prognostic studies: (1) representativeness of the reported population; (2) recognition and definition of AF pattern; (3) completeness and length of the follow‐up; (4) reported clear and objective criteria for the outcome; and (5) recognition and recording of the other important prognostic factors associated with the outcome.
A summary‐of‐findings table was constructed for 3 main outcomes: stroke and/or SE, stroke alone, and major bleedings. Overall quality of evidence for each outcome was assessed according to the modified Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach for prognostic studies.25
3. RESULTS
3.1. Study characteristics
The literature search identified a total of 4618 studies between 1990 to 2015, of which 18 were finally included in this analysis (see Supporting Information, Figure 1, in the online version of this article).6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Characteristics of the 18 included studies are reported in Supporting Information, Table 1, in the online version of this article. The final population comprised 176 975 patient‐years in the non‐PxAF group and 62 553 patient‐years in the pxAF group. Particularly, 22 studies reported full data about AF subtype and outcome, but 4 of them were excluded due to retrospective assignment of the outcome. The study design was an RCT to assess anticoagulation in 11 studies6, 7, 8, 9, 13, 14, 15, 17, 18, 19, 22 and a prospective observational registry in the other 7 studies.10, 11, 12, 16, 21, 22, 23 SE combined with stroke was the main outcome in 13 studies, but in 4 of them stroke alone can also be considered independently. Stroke alone was the only outcome in 4 studies, and TIA could not be excluded from the combined clinical event in only 3 studies. The percentage of patients with OAC could be retrieved in the majority of the studies (16 of 18) and ranged from 0% (2 studies) to 100% (5 studies). The CHADS2 score in both non‐PxAF and PxAF was the main risk score available for the meta‐analysis in 5 studies after 2007. The follow‐up length ranged from 1 to 3.6 years.
3.2. Clinical outcomes and subgroup analysis
A total of 941 events in the pxAF group and 3611 events in the non‐PxAF group have been computed for a pooled weighted incidence rate of 1.6% (95% CI: 1.3%‐2.0%) and 2.3% (95% CI: 2.0% 2.7%), respectively. The pooled estimate RR was 0.72 (95% CI: 0.65‐0.80, P < 0.00001) for pxAF as compared with non‐PxAF, with only mild heterogeneity (I2 = 35%; P = 0.07; Figure 1). Similar results were obtained also by considering the 10 studies with evenly matched proportion of OAC therapy within pxAF and non‐pxAF groups (RR: 0.74, 95% CI: 0.65‐0.85, P < 0.00001; see Supporting Information, Figure 3, in the online version of this article).
Figure 3.
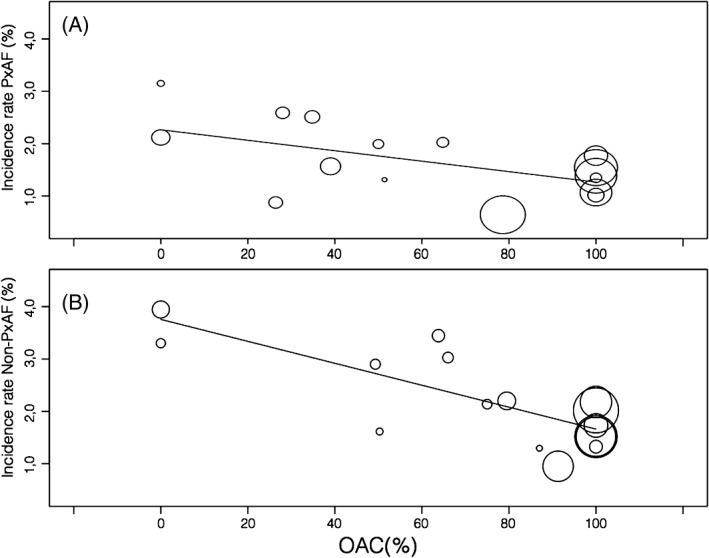
Meta‐regression analysis. Pooled incidence rate (y‐axis) and according to OAC proportion within studies (x‐axis) in paroxysmal (Panel A) and non‐paroxysmal (Panel B) AF. Abbreviations: OAC, oral anticoagulation; non‐PxAF, non‐paroxysmal AF; PxAF, paroxysmal AF.
After excluding the 3 oldest studies to fulfill the contemporary guideline definition of pxAF, the estimated RR did not change significantly (RR: 0.70, 95% CI: 0.63‐0.78, P < 0.00001; Figure 2). Similarly, the main results were confirmed also excluding studies with TIA as an indivisible part of the outcome (RR: 0.72, 95% CI: 0.65‐0.81, P < 0.00001). Nine studies for a total of 111 117 patient‐years reported multivariate analysis with baseline characteristics adjustment. The pooled estimated adjusted RR was 0.71 (95% CI: 0.51‐0.87, P < 0.0001; I2 = 52%).
Total weighted incidence rate in observational registries was 2.5% (95% CI: 1.6%‐3.5%; total patient‐years 42 789) and was slightly higher than in randomized trials (1.9%, 95% CI: 1.6%‐2.3%; total patient‐years 196 749). The RR between pxAF and non‐pxAF was modestly lower in observational registries (RR: 0.67, 95% CI: 0.57‐0.79) as compared with RCTs (RR: 0.75, 95% CI: 0.66‐0.85).
When considering stroke as the only main clinical outcome, the RR was reduced to 0.75 (95% CI: 0.62 0.89); whereas when SE was included, RR was 0.71 (95% CI: 0.63‐0.80; Table 1; see also Supporting Information, Figure 4, in the online version of this article).
Pooled‐weighted mean CHADS2 score assessed in 5 studies was 2.16 (95% CI: 1.35‐2.97) in the pxAF group and 2.45 (95% CI: 1.69 3.21) in the non‐pxAF group (P < 0.01).
Incidence of major bleeding was 2.5% (95% CI: 1.8%‐3.3%) in the pxAF group and 2.3% (95% CI: 1.6%‐3.1%) in the non‐PxAF group (RR: 1.04, 95% CI: 0.92‐1.18, P = 0.49; Table 1; see also Supporting Information, Figure 5, in the online version of this article).
3.3. The OAC effect
A meta‐regression analysis considering the percentage of patients taking OAC and the incidence rate of stroke or stroke and SE is reported in Figure 3. In both the pxAF and non‐pxAF groups, a progressive reduction of events in studies with a higher proportion of patients taking OAC is observed, the trajectory being steeper in the non‐PxAF group (Figure 3B) as compared with PxAF (Figure 3A). The pooled weighted incidence rate was more than halved in studies with anticoagulated population as compared with studies performed without OAC (Table 2). This effect was consistent in both pxAF and non‐pxAF patients.
Table 2.
Pooled estimates incidence according to OAC
| OAC, % | PxAF, % | Persistent/Permanent AF, % |
|---|---|---|
| 0 | 2.5 (1.5‐3.5) | 3.7 (3.1‐4.3) |
| 25–75 | 1.8 (1.3‐2.4) | 2.8 (2.2‐3.5) |
| 75–100 | 1.2 (0.8‐1.5) | 1.4 (1.4‐2.0) |
Abbreviations: AF, atrial fibrillation; OAC, oral anticoagulation; pxAF, paroxysmal atrial fibrillation.
4. DISCUSSION
Current guidelines from Europe and North America recommend the selection of antithrombotic therapy based on the individual risk of TE, regardless of the AF pattern.1, 2 Major potential threats to the robustness of the data supporting such an approach could be inferred by the different grading of the level of evidence and the class of recommendation attributed.1, 2 Previous randomized clinical studies7, 8 and observational registries10 have reported similar risk of stroke/SE events in patients with pxAF and non‐pxAF. Conversely, in 2 large post‐hoc analyses of randomized trials19, 22 and 2 large Asian registries20, 21 published in 2014 and 2015, an incremental TE risk was found in patients with non‐pxAF being 2‐fold of that observed in pxAF. A recent meta‐analysis systematically evaluated the outcomes of different types of AF,26 suggesting a significantly higher risk in non‐pxAF patterns without providing information about the relative OAC effect.
Our aim was to systematically review available data, trying to bridge the aforementioned gap in the evidence. To the best of our knowledge, this was the first comprehensive meta‐analysis pooling together all the available data systematically assessing the risk of TE with the relative effect of OAC as a function of AF patterns. The main findings indicated that pxAF is associated with a non‐negligible RR reduction of stroke/SE as compared with non‐pxAF despite retaining a clinically meaningful event rate. As a consequence, OAC therapy, as warranted in the guidelines, was consistently shown to halve the risk in any AF pattern.
The major strength of the meta‐analysis, the highest level of evidence, is to pool results from RCTs, which are strictly selective, and from registries, which, conversely, are more representative of the real‐life population but with lower quality of follow‐up and data. In line with several previous observations, the present meta‐analysis showed a 28% lower stroke/SE RR in patients with pxAF. The risk reduction was even smaller considering only stroke as the major outcome. To avoid potential bias due to the different definitions of “paroxysmal” used in the analyzed studies (covering a time period of nearly 25 years, from 1990 to 2015), a sensitivity analysis was performed including only 15 studies that strictly fulfilled the guideline definition of pxAF. The subanalysis confirmed and reinforced the findings of the main dataset, suggestive of a modestly increased TE ischemic risk in non‐pxAF.
Since the release of the 2010 European Society of Cardiology guidelines,1 the paradigm of stroke and SE risk stratification has shifted toward the identification of the truly low‐risk patients (whose annual risk is virtually 0%), confirming the previous indication to consider, in presence of risk factors, the stroke risk of paroxysmal similar to that of persistent and permanent AF.
In parallel, the availability of non‐VKA oral anticoagulants (NOACs)5 has extended the potential net clinical benefit threshold of OAC therapy down to subjects with CHA2DS2‐VASc score 1.27 In a recent analysis of nonanticoagulated AF patients, yearly ischemic stroke rates were 2.1%, 3.0%, and 4.2% for paroxysmal, persistent, and permanent AF, respectively, with adjusted hazard ratio of 1.83 for permanent vs paroxysmal and 1.44 for persistent vs paroxysmal.18 In light of such a difference in the event rates, the potential role of a risk refinement based on the only AF pattern would be of pivotal interest particularly in low‐risk patients, in whom the risk‐benefit ratio of anticoagulation is less clear. Unfortunately, very few data, if any, are available for this subset of low‐risk patients (eg, no CHADS‐VASc score 0 and only 10% with a CHADS‐VASc score 1).
In our meta‐analysis, the pooled weighted mean CHADS2 score (available in 5 out of 18 studies only) was 2.16 and 2.45 in pxAF and non‐pxAF patients, respectively, potentially raising concerns about the generalizability of our findings to the whole AF population. Nevertheless, sensitivity analysis with adjusted outcome from 9 studies confirmed main observation. Moreover, patients with a CHADS‐VASc score ≥1 (deducible by the mean patient age >65) were analyzed, virtually excluding only patients with CHADS‐VASc score 0. In this subset of PxAF patients, we found a yearly event rate for stroke/SE of 1.7%, warranting OAC therapy in line with current guidelines.1, 2 A nonparoxysmal pattern further identifies an increased stroke/SE risk for any incremental point added to the score but should not be regarded as a stand‐alone stroke risk. Whether this statement applies to younger patients, age <65 years, without risk factors, remains to be addressed.
As expected, in the present meta‐analysis, OAC use dramatically reduced the incidence of stroke and SE. The rate of TE events decreased as the proportion of patients on OAC increased, the greater magnitude of reduction being achieved in non‐pxAF patients systematically treated with OAC. Of note, the residual risk of stroke/SE in anticoagulated patients with non‐pxAF (1.4%) was significantly lower than the risk of untreated pxAF patients (2.5%), again emphasizing the marginal role of the AF pattern in most patients in the decision‐making on antithrombotic therapy. In other words, these data reinforce the concept that it is unacceptable to withhold OAC therapy based only on the nonparoxysmal pattern of AF.
Recent findings from a subanalysis of the Global Study to Assess the Safety and Effectiveness of Edoxaban (DU‐176b) vs Standard Practice of Dosing With Warfarin in Patients With Atrial Fibrillation (ENGAGE AF) trial further confirmed this statement being also suggestive of a greater beneficial effect attained with edoxaban as compared with warfarin, consistently seen across the different AF pattern populations.28 Remarkably, edoxaban significantly reduced all‐cause death, even in lower‐risk patients with pxAF. Unfortunately, data on the potentially diverse impact of VKA and non‐VKA agents were not available for analysis and have not been included in our study. Yet, overall data from RCTs univocally showed that NOACs are at least as effective as warfarin regardless of any individual variables.13, 15, 17, 22
Regrettably, data on major bleeding events were scarce, probably because of methodological heterogeneity in event definition and study reports. However, the event rate did not seem to differ among pxAF and non‐pxAF patients. As a whole, these results were consistent with a net clinical benefit in favor of OAC throughout the analyzed patient population of >200 000 patient‐years. Confidently assuming a comparable major bleeding event rate in pxAF and non‐pxAF, also drawing from confirmatory data from a recent RCT,28 it could be inferred that, when engaged in decision‐making, physicians might be more keen on prescribing OAC by means of NOACs in patients at lower risk of TE events, such as non‐pxAF subjects, given the extensively shown lower intrinsic major bleeding risk of NOACs.13, 15, 17, 22 Nevertheless, there were insufficient data to assess the bleeding risk in most of the studies included in the meta‐analysis so that no HAS‐BLED score could be calculated, and speculations on a more favorable net clinical benefit of NOACs, especially in non‐pxAF, despite being highly plausible, are hypotheses‐generating only.
4.1. Study limitations
We acknowledge several limitations to the present meta‐analysis. First, a general operative definition of “paroxysmal” is likely to cover many phenotypes of pxAF, with respect to episode duration, number, and overall arrhythmic burden. As a consequence, different subtypes of pxAF have been necessarily included and studied as a whole in the present meta‐analysis (still, we do not foresee this as a factor potentially jeopardizing the consistency of our results, but rather as a subject for future studies). Reasonably enough, the potential clinical meaning of an episode of AF lasting 30 seconds and an episode lasting 24 hours is different.
Second, the revision of the available studies highlighted unmatched, and often unbalanced, distribution of factors implicated in the genesis of TE risk (eg, diabetes, hypertension, age, prior stroke). Future individual patient meta‐analysis may try to address this issue. Otherwise direct matched comparison appears by now unfeasible in patients without anticoagulation. Our aim was then to understand the entity of TE with the available unrepeatable data. Selected studies showed good quality with risk factors adequately reported. This may allow the reader to put the results in the correct context.
5. CONCLUSION
Our results have an important implication for clinical practice. Several reports highlighted that pxAF is one of the main factors precluding OAC prescription in clinical practice.29 Current guidelines discourage this practice.1, 2 Our pooled large analysis confirms that even if some difference exists between paroxysmal and persistent/permanent AF, it should be considered clinically negligible, at least in intermediate‐ to high‐risk subjects. Indeed, our analysis highlighted that OAC consistently halves the embolic events rate across any type of AF. As a whole, these data provide the evidence to warrant OAC irrespective of the AF pattern in most (virtually all) patients.
5.1. Author Contributions
All authors participated sufficiently in the work, with substantial contributions to the conception and design; acquisition, analysis, and interpretation of data; and drafting or revising the article critically. They approved the final version to be submitted.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Figure S1. Study flow diagram
Figure S2. Funnel plot
Figure S3. Stroke and Systemic Embolism by AF pattern considering only studies with completely matched OAC Therapy between paroxysmal and non‐paroxysmal AF, Forest Plot. PxAF: paroxysmal AF, non‐PxAF: non‐Paroxysmal AF.
Figure S4. Subgroups analysis stroke vs stroke/SE
Figure S5. Major Bleedings
Table S1. Included study
Table S2. Risk Of Bias Table
Lilli A, Di Cori A and Zacà V. Thromboembolic risk and effect of oral anticoagulation according to atrial fibrillation patterns: A systematic review and meta‐analysis, Clin Cardiol, 2017;40:641–647. 10.1002/clc.22701
REFERENCES
- 1. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) [published correction appears in Eur Heart J. 2011;32:1172]. Eur Heart J . 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society [published correction appears in Circulation. 2014;130:e272–e274]. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026. [DOI] [PubMed] [Google Scholar]
- 4. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 5. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomized trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 6. The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators . The effect of low‐dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med . 1990;323:1505–1511. [DOI] [PubMed] [Google Scholar]
- 7. Hart RG, Pearce LA, Rothbart RM, et al; Stroke Prevention in Atrial Fibrillation Investigators . Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. J Am Coll Cardiol . 2000;35:183–187. [DOI] [PubMed] [Google Scholar]
- 8. Hohnloser SH, Pajitnev D, Pogue J, et al; ACTIVE W Investigators . Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol. 2007;50:2156–2161. [DOI] [PubMed] [Google Scholar]
- 9. Lip GY, Frison L, Grind M; SPORTIF Investigators . Stroke event rates in anticoagulated patients with paroxysmal atrial fibrillation. J Intern Med. 2008;264:50–61. [DOI] [PubMed] [Google Scholar]
- 10. Nieuwlaat R, Prins MH, Le Heuzey JY, et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow‐up of the Euro Heart Survey on atrial fibrillation. Eur Heart J. 2008;29:1181–1189. [DOI] [PubMed] [Google Scholar]
- 11. Ruigómez A, Johansson S, Wallander MA, et al. Risk of cardiovascular and cerebrovascular events after atrial fibrillation diagnosis. Int J Cardiol. 2009;136:186–192. [DOI] [PubMed] [Google Scholar]
- 12. Friberg L, Hammar N, Rosenqvist M. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation. Eur Heart J. 2010;31:967–975. [DOI] [PubMed] [Google Scholar]
- 13. Flaker G, Ezekowitz M, Yusuf S, et al. Efficacy and safety of dabigatran compared to warfarin in patients with paroxysmal, persistent, and permanent atrial fibrillation: results from the RE‐LY (Randomized Evaluation of Long‐Term Anticoagulation Therapy) study . J Am Coll Cardiol. 2012;59:854–855. [DOI] [PubMed] [Google Scholar]
- 14. Disertori M, Franzosi MG, Barlera S, et al; GISSI‐AF Investigators . Thromboembolic event rate in paroxysmal and persistent atrial fibrillation: data from the GISSI‐AF trial. BMC Cardiovasc Disord. 2013;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al‐Khatib SM, Thomas L, Wallentin L, et al. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J . 2013;34:2464–2471. [DOI] [PubMed] [Google Scholar]
- 16. Bosch RF, Kirch W, Theuer JD, et al. Atrial fibrillation management, outcomes and predictors of stable disease in daily practice: prospective non‐interventional study. Int J Cardiol. 2013;167:750–756. [DOI] [PubMed] [Google Scholar]
- 17. Giugliano RP, Ruff CT, Braunwald E, et al; ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 18. Hohnloser SH, Shestakovska O, Eikelboom J, et al. The effects of apixaban on hospitalizations in patients with different types of atrial fibrillation: insights from the AVERROES trial. Eur Heart J. 2013;34:2752–2759. [DOI] [PubMed] [Google Scholar]
- 19. Vanassche T, Lauw MN, Eikelboom JW, et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin‐treated patients in ACTIVE‐A and AVERROES. Eur Heart J . 2015;36:281a–287a. [DOI] [PubMed] [Google Scholar]
- 20. Inoue H, Atarashi H, Okumura K, et al. Thromboembolic events in paroxysmal vs. permanent non‐valvular atrial fibrillation. Subanalysis of the J‐RHYTHM Registry. Circ J . 2014;78:2388–2393. [DOI] [PubMed] [Google Scholar]
- 21. Takabayashi K, Hamatani Y, Yamashita Y, et al. Incidence of stroke or systemic embolism in paroxysmal versus sustained atrial fibrillation: the Fushimi Atrial Fibrillation Registry. Stroke. 2015;46:3354–3361. [DOI] [PubMed] [Google Scholar]
- 22. Steinberg BA, Hellkamp AS, Lokhnygina Y, et al; ROCKET‐AF Steering Committee and Investigators . Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET‐AF Trial. Eur Heart J . 2015;36:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazzone C, Carriere C, Grande E, et al. Nonvalvular atrial fibrillation: data from the Observatory of Cardiovascular Diseases in the province of Trieste (Italy) [article in Italian]. G Ital Cardiol (Rome) . 2015;16:361–372. [DOI] [PubMed] [Google Scholar]
- 24. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. [DOI] [PubMed] [Google Scholar]
- 26. Ganesan AN, Chew DP, Hartshorne T, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta‐analysis. Eur Heart J. 2016;37:1591–1602. [DOI] [PubMed] [Google Scholar]
- 27. Banerjee A, Lane DA, Torp‐Pedersen C, et al. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a ‘real world’ atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb Haemost. 2012;107:584–589. [DOI] [PubMed] [Google Scholar]
- 28. Link MS, Giugliano RP, Ruff CT, et al; ENGAGE AF‐TIMI 48 Investigators . Stroke and Mortality Risk in Patients With Various Patterns of Atrial Fibrillation: results from the ENGAGE AF‐TIMI 48 Trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation‐Thrombolysis in Myocardial Infarction 48). Circ Arrhythm Electrophysiol . 2017;10:pii:e004267. doi:10.1161/CIRCEP.116.004267. [DOI] [PubMed] [Google Scholar]
- 29. Kakkar AK, Mueller I, Bassand JP, et al; GARFIELD Registry Investigators . Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One . 2013;8:e63479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study flow diagram
Figure S2. Funnel plot
Figure S3. Stroke and Systemic Embolism by AF pattern considering only studies with completely matched OAC Therapy between paroxysmal and non‐paroxysmal AF, Forest Plot. PxAF: paroxysmal AF, non‐PxAF: non‐Paroxysmal AF.
Figure S4. Subgroups analysis stroke vs stroke/SE
Figure S5. Major Bleedings
Table S1. Included study
Table S2. Risk Of Bias Table


