Abstract
Background
Lowering low‐density lipoprotein cholesterol with statins reduces risk of cardiovascular events. We examined patterns and predictors of filled prescriptions for lipid‐lowering therapy (LLT) in subgroups of patients with atherosclerotic cardiovascular disease (ASCVD) and/or diabetes mellitus (DM).
Hypothesis
Statin treatment remains underutilized across subgroups of high CV risk patients.
Methods
Patients in the Optum Research Database with these criteria were included: age ≥20 years, 2 years continuous enrollment, and ASCVD and/or DM. Patients were hierarchically classified by the presence of recent acute coronary syndrome, other coronary heart disease, ischemic stroke, peripheral arterial disease (PAD), or only DM. Predictors of filled LLT regimens were examined using multinomial logistic regression.
Results
A total of 1 055 932 individuals met all inclusion criteria. Evidence by point‐in‐time analysis of filled (not only written) statin prescriptions was 45% for the overall cohort. By subgroups, this was 62%, 52%, 43%, 36%, and 40% for recent acute coronary syndrome, other coronary heart disease, ischemic stroke, PAD, and only DM, respectively. Predictors of higher rates of any statin regimen included age 50 to 69 years, male sex, absence of comorbidities, and filled prescriptions of other standard‐of‐care therapies.
Conclusions
In 2014, only 49% of patients with ASCVD and 40% with only DM had evidence for a filled statin prescription. Those with indications of ischemic stroke, PAD, and DM were less likely to receive statins than those with coronary conditions. Other characteristics such as advanced age, female sex, and noncardiac conditions predicted less statin utilization, thereby representing good targets for quality improvement.
Keywords: coronary disease, stroke, peripheral vascular disease, diabetes mellitus, lipid‐lowering therapy
1. INTRODUCTION
Reducing low‐density lipoprotein cholesterol (LDL‐C) with statin therapy has been demonstrated to decrease all‐cause mortality and cardiovascular (CV) outcomes such as CV death, coronary death, myocardial infarction (MI), unstable angina, coronary revascularization procedures, and ischemic stroke in populations with prior atherosclerotic cardiovascular disease (ASCVD) as well as in certain primary‐prevention populations.1, 2 The high tolerability and safety of statins has also been established across these subgroups.1, 2, 3 Although guideline‐recommended strategies may differ for populations at high risk, consensus exists that both higher utilization of statins and reduction of LDL‐C levels remain a major public health priority.
Studies based on survey, administrative, and registry databases in the United States have highlighted suboptimal statin use in populations at high risk.4, 5, 6, 7, 8, 9, 10, 11 Our study adds to this literature by providing a comprehensive analysis of the predictors and actual filled prescription patterns of statin and nonstatin lipid‐lowering therapies (LLT) in subgroups of ASCVD and diabetes mellitus (DM) patients collected from a contemporary, generalizable database of US patients.
2. METHODS
This was a retrospective, cross‐sectional, observational study. Data were de‐identified in accordance with established privacy guidelines under the Health Insurance Portability and Accountability Act; therefore, separate institutional review board approval was not sought.
2.1. Database and cohort selection
We utilized the Optum Research Database, a large (>14 million unique members in 2014) administrative database of medical and pharmacy claims. All inpatient and outpatient medical and pharmacy encounters were captured in the database during the time patients remained enrolled in the plan. The following inclusion criteria were used in this analysis: ≥20 years of age, 2 years of continuous enrollment prior to the index date, and ≥1 high‐CV‐risk condition. Continuous enrollment for 2 years prior to the index date was required to ensure complete capture of pertinent demographic and clinical characteristics and to assess for evidence of previously filled prescriptions for LLT in those not currently treated with LLT. For patients who had ≥1 valid lipid profile from 2014 in the database, the index date was defined as the date of the last lipid profile during that year. For patients without a valid lipid profile in 2014, index dates were randomly assigned by probabilistic matching to the known distribution of index dates in those with a valid lipid profile. The index date was assigned in this manner (as opposed to selecting a random date in 2014) to facilitate further analysis of lipid level achievement (data not presented in this analysis).
High‐CV‐risk conditions were defined as follows: (1) recent acute coronary syndrome (ACS; MI or unstable angina with documented hospitalization within 12 months of index date); (2) other coronary heart disease (CHD; eg, ACS >12 months prior to index date, any coronary revascularization, stable angina, or ischemic cardiomyopathy); (3) ischemic stroke; (4) peripheral arterial disease (PAD; eg, significant limb, visceral, or aortic atherosclerosis with or without revascularization/repair as well as precerebral/cerebral artery atherosclerosis without ischemic stroke); and (5) only DM (type 1 or type 2, with or without antiglycemic therapy). The authors reviewed codes used in the analysis (International Classification of Diseases, Ninth Revision; Current Procedural Terminology; and Healthcare Common Procedure Coding System codes) to ensure greater specificity for representing a condition in which statins are indicated (see Supporting Information, Table 1, in the online version of this article). Codes felt to represent conditions or events for which statins might not be indicated by current guidelines (eg, ischemic stroke in a participant with atrial fibrillation) were excluded. Patients were categorized by their underlying high‐CV‐risk condition(s) using 2 methods. In the first method, each patient was classified hierarchically into the highest mutually exclusive CV‐risk condition (using the order above) for which he/she qualified (termed “hierarchical” subgroups). In the second method, each patient was classified into each CV‐risk condition for which he/she qualified (termed “prevalent” subgroups); thus, a patient could be included in ≥1 CV condition under prevalent categorization. As an example, an individual with a history of elective coronary revascularization and DM would be hierarchically classified as “other CHD,” but as both “other CHD” and “DM” by prevalent classification. International Classification of Diseases, Ninth Revision, Current Procedural Terminology, and Healthcare Common Procedure Coding System diagnosis and procedure codes were also used to identify non‐CV comorbidities.
Table 1.
Baseline characteristics for the overall cohort and hierarchical subgroups
| Recent ACS (n = 23 040, 2%) | Other CHD (n = 419 010, 40%) | Ischemic Stroke (n = 37 309, 4%) | PAD (n = 123 960, 12%) | Only DM (n = 452 613, 43%) | Total Cohort (N = 1 055 932) | |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Mean age, y | 70.3 | 70.3 | 69.4 | 70.1 | 61.3 | 66.4 |
| Age ≥75y, % | 39.9 | 39.7 | 40.6 | 40.6 | 18.0 | 30.5 |
| Male sex, % | 59.5 | 59.2 | 43.1 | 43.3 | 48.3 | 52.1 |
| Medicare Advantage insurance plan, % 1 | 66.9 | 63.7 | 64.9 | 64.3 | 40.9 | 54.1 |
| Region, % | ||||||
| South | 36.6 | 39.4 | 37.4 | 36.9 | 41.7 | 40.0 |
| Northeast | 20.8 | 19.8 | 16.5 | 21.2 | 17.1 | 18.7 |
| Midwest | 32.0 | 30.4 | 33.5 | 29.8 | 27.5 | 29.2 |
| West/other | 10.6 | 10.4 | 12.6 | 12.0 | 13.7 | 12.1 |
| Baseline clinical characteristics, % | ||||||
| Recent ACS | 100 | 0 | 0 | 0 | 0 | 2.2 |
| Other CHD | 56.1 | 100 | 0 | 0 | 0 | 40.9 |
| Ischemic stroke | 12.4 | 6.6 | 100 | 0 | 0 | 6.4 |
| PAD | 38.2 | 29.2 | 37.0 | 100 | 0 | 25.5 |
| DM | 50.1 | 42.7 | 35.7 | 36.0 | 100 | 66.4 |
| Other comorbidities of interest, % | ||||||
| HTN | 93.9 | 88.9 | 83.9 | 79.3 | 78.0 | 83.0 |
| History of CHF | 52.9 | 26.8 | 14.3 | 10.8 | 4.7 | 15.6 |
| CKD stage III | 19.4 | 13.8 | 12.2 | 11.4 | 7.2 | 10.8 |
| CKD stage IV–V 2 | 8.1 | 3.6 | 2.9 | 2.9 | 1.6 | 2.7 |
| Dementia | 5.8 | 4.0 | 9.2 | 5.1 | 1.3 | 3.2 |
| COPD | 47.1 | 34.7 | 28.5 | 29.5 | 19.3 | 27.5 |
| MSK pain 3 | 79.8 | 75.7 | 77.9 | 79.7 | 65.4 | 71.9 |
| Moderate/severe liver disease | 1.2 | 0.6 | 0.7 | 0.6 | 0.5 | 0.6 |
| Concomitant filled medications, % 4 | ||||||
| β‐Blockers | 62.5 | 43.6 | 25.7 | 21.7 | 16.7 | 29.3 |
| ACEIs/ARBs | 43.7 | 36.1 | 30.8 | 27.6 | 32.9 | 33.7 |
| Antiplatelets 5 | 41.6 | 13.9 | 12.5 | 4.0 | 0.7 | 7.6 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin II receptor blocker; CHD, coronary heart disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; MSK, musculoskeletal; PAD, peripheral arterial disease.
Medicare Advantage insurance plans are offered by private companies that contract with Medicare to provide patients with hospital and medical insurance benefits.
Includes dialysis.
Diagnosis associated with MSK pain.
Filled medications on index date.
Clopidogrel, ticagrelor, prasugrel.
2.2. Determination of medication treatment
For any particular medication, patients were considered to have been treated on the index date if medication supply via a filled prescription was present on or within 30 days prior to the index date, regardless of the duration of the prescription (Figure 1). Those not treated, but with evidence of a prior filled prescription, were considered to have had a history of being treated. Those without any evidence of a filled prescription for a medication within the 2 years prior to the index date were considered to have no documented history of being treated. LLT was categorized into 4 types: high‐, moderate‐, and low‐intensity statin therapy, as well as nonstatin therapy (see Supporting Information, Table 2, in the online version of this article). Statin categories included statins administered as either monotherapy or in combination with a nonstatin drug(s). Nonstatin therapies included ezetimibe, niacin, and bile acid sequestrants. Fibric acid derivatives were not considered due to their modest impact on LDL‐C lowering.12
Figure 1.
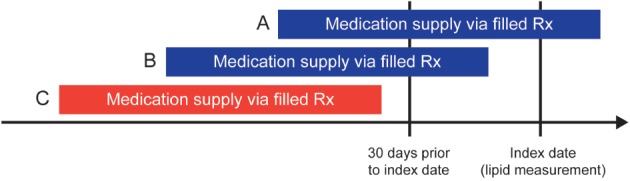
Determination of treatment status as of the index date. Blue bars representing medication supply via (A) filled Rx on the index date or (B) filled Rx within 30 days prior to the index date define the patient as being treated as of the index date. The red bar, representing medication supply via (C) filled Rx >30 days prior to the index date, defines the patient as not being treated as of the index date. Abbreviations: Rx, prescription.
Table 2.
Filled LLT prescriptions in the overall cohort and hierarchical subgroups
| Recent ACS (n = 23 040, 2%) | Other CHD (n = 419 010, 40%) | Ischemic Stroke (n = 37 309, 4%) | PAD (n = 123 960, 12%) | Only DM (n = 452 613, 43%) | Total Cohort (N = 1 055 932) | |
|---|---|---|---|---|---|---|
| High‐intensity statin, % 1 | 27.0 | 16.5 | 9.4 | 6.4 | 7.4 | 11.4 |
| Monotherapy | 96.2 | 90.4 | 96.7 | 93.8 | 94.5 | 92.2 |
| Plus ezetimibe | 2.6 | 6.7 | 2.3 | 4.1 | 3.5 | 5.3 |
| Plus other nonstatin LLT | 1.2 | 3.0 | 1.0 | 2.1 | 2.0 | 2.5 |
| Moderate‐intensity statin, % 1 | 30.1 | 30.9 | 28.1 | 24.8 | 27.3 | 28.5 |
| Monotherapy | 96.2 | 93.9 | 97.4 | 96.0 | 96.0 | 95.1 |
| Plus ezetimibe | 2.6 | 4.1 | 1.6 | 2.7 | 2.6 | 3.2 |
| Plus other nonstatin LLT | 1.2 | 2.0 | 1.0 | 1.4 | 1.4 | 1.6 |
| Low‐intensity statin, % 1 | 4.5 | 4.7 | 5.9 | 5.2 | 5.2 | 5.0 |
| Monotherapy | 97.7 | 96.2 | 98.3 | 97.5 | 97.4 | 97.0 |
| Plus ezetimibe | 1.5 | 2.1 | 0.7 | 1.3 | 1.2 | 1.5 |
| Plus other nonstatin LLT | 0.9 | 1.7 | 1.0 | 1.2 | 1.3 | 1.4 |
| Nonstatin LLT only, % 1 | 0.7 | 1.1 | 0.8 | 1.0 | 0.9 | 1.0 |
| Ezetimibe | 55.0 | 55.8 | 47.8 | 48.4 | 47.3 | 51.3 |
| Other nonstatin LLT | 45.0 | 44.2 | 52.2 | 51.6 | 52.7 | 48.7 |
| Not currently treated by LLT, % 1 | 37.8 | 46.9 | 55.8 | 62.6 | 59.2 | 54.1 |
| Previously on high‐intensity statin | 13.7 | 7.3 | 4.5 | 2.8 | 3.6 | 4.9 |
| Previously on medium‐intensity statin | 22.2 | 17.4 | 16.4 | 11.5 | 14.2 | 15.1 |
| Previously on low‐intensity statin | 4.1 | 3.7 | 4.3 | 3.0 | 3.6 | 3.6 |
| Previously on nonstatin LLT | 1.4 | 1.4 | 1.1 | 1.2 | 1.2 | 1.3 |
| No previous LLT | 58.6 | 70.1 | 73.8 | 81.4 | 77.4 | 75.0 |
Abbreviations: ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; DM, diabetes mellitus; LLT, lipid‐lowering therapy; PAD, peripheral arterial disease.
Numbers in this row are absolute percentages and add up to 100% vertically. All other numbers are relative percentages of the absolute percentages.
2.3. Statistical analysis
Demographics, clinical characteristics, and LLT utilization were summarized descriptively using proportions and mean ± SD as appropriate. A multivariate model was developed for estimating patient factors that predicted LLT treatment. Multinomial logistic regression was used to compare each of the 4 LLT types to no LLT (referent). Model coefficients (relative risk ratios [RRs]) summarized the relative likelihood for each LLT type. Covariates included patient demographic characteristics, CV and non‐CV comorbidities, and other CV medications. All analyses were conducted with SAS software version 9.2 (SAS Institute, Inc., Cary, North Carolina).
3. RESULTS
The inclusion criteria were met by 1 055 932 patients (Figure 2). After classification by hierarchical subgroups, 2% had recent ACS, 40% had other CHD, 4% had ischemic stroke, 12% had PAD, and 43% had only DM. Baseline characteristics for the overall cohort and hierarchical subgroups are provided in Table 1. See Supporting Information, Table 3, in the online version of this article for the proportion of patients and baseline characteristics by prevalent subgroups.
Figure 2.
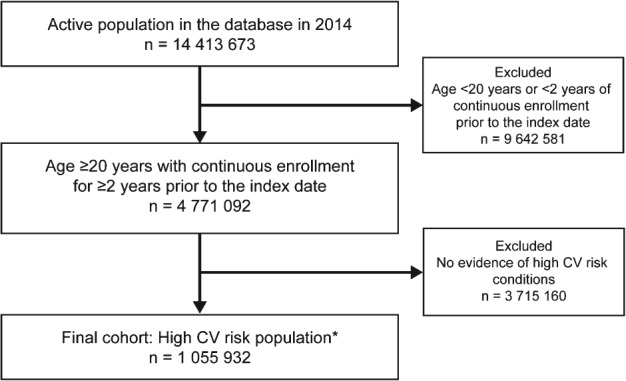
Flowchart of the cohort selection for the study. *High‐CV‐risk conditions: ACS, other CHD, ischemic stroke, PAD, DM. Abbreviations: ACS, acute coronary syndrome; CHD, coronary heart disease; CV, cardiovascular; DM, diabetes mellitus; PAD, peripheral arterial disease.
Table 3.
Multivariate analysis of LLT use by participant characteristics
| Relative RR | ||||
|---|---|---|---|---|
| High‐Intensity Statin vs No Current | Moderate‐Intensity Statin vs No Current | Low‐Intensity Statin vs No Current | Nonstatin LLT vs No Current | |
| Demographic characteristics | ||||
| Age categories, y | ||||
| 20–39 | Ref | Ref | Ref | Ref |
| 40–49 | 2.85 | 2.66 | 2.23 | 2.47 |
| 50–59 | 4.79 | 4.29 | 3.23 | 4.76 |
| 60–69 | 5.55 | 5.35 | 4.31 | 5.91 |
| 70–79 | 4.03 | 5.22 | 4.97 | 5.17 |
| ≥80 | 2.04 | 3.99 | 4.62 | 3.52 |
| Male sex | 1.41 | 1.14 | 0.97 | 0.92 |
| Region | ||||
| South | Ref | Ref | Ref | Ref |
| Northeast | 0.97 | 1.07 | 0.97 | 0.82 |
| Midwest | 1.22 | 1.10 | 1.08 | 0.94 |
| West/other | 1.14 | 1.05 | 1.07 | 0.89 |
| Baseline clinical characteristics | ||||
| Recent ACS | 1.69 | 0.94 | 0.84 | 0.62 |
| Other CHD | 2.02 | 1.17 | 0.91 | 1.30 |
| Ischemic stroke | 1.15 | 1.09 | 1.13 | 0.91 |
| PAD | 1.06 | 0.99 | 0.99 | 1.09 |
| DM | 1.17 | 1.29 | 1.31 | 1.34 |
| HTN | 1.26 | 1.23 | 1.20 | 1.33 |
| History of CHF | 0.70 | 0.74 | 0.82 | 0.72 |
| CKD | ||||
| No CKD | Ref | Ref | Ref | Ref |
| Stage III | 1.12 | 1.09 | 1.11 | 1.12 |
| Stage IV–V 1 | 0.91 | 0.95 | 1.10 | 0.94 |
| Dementia | 0.56 | 0.75 | 0.94 | 0.70 |
| COPD | 0.86 | 0.94 | 1.01 | 0.99 |
| MSK pain, % 2 | 0.86 | 0.92 | 0.98 | 1.23 |
| Moderate/severe liver disease | 0.25 | 0.35 | 0.50 | 1.19 |
| Concomitant medication use 3 | ||||
| β‐Blockers | 3.00 | 2.39 | 2.15 | 2.22 |
| ACEIs/ARBs | 2.73 | 2.56 | 2.66 | 2.06 |
| Antiplatelets, % 4 | 4.20 | 2.77 | 2.13 | 2.78 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin II receptor blocker; CHD, coronary heart disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; LLT, lipid‐lowering therapy; MSK, musculoskeletal; PAD, peripheral arterial disease; Ref, reference; RR, risk ratio.
Overall P values for each covariate were <0.001.
Includes dialysis.
Diagnosis associated with MSK pain.
Filled medications on index date.
Clopidogrel, ticagrelor, prasugrel.
In the overall cohort, 46% of patients had filled a prescription for LLT as of the index date (Table 2). By LLT regimen, this was 11% for high‐intensity statin, 29% for moderate‐intensity statin, 5% for low‐intensity statin, and 1% for only nonstatin LLT. In the overall cohort, statins were used with nonstatin medications in 2.4%. In those with an ASCVD condition (not including the DM‐only subgroup), 50% of patients had filled a prescription for LLT as of the index date. Use was 14% for high‐intensity statin, 29% for moderate‐intensity statin, 5% for low‐intensity statin, and 1% for only nonstatin LLT. When classified by hierarchical subgroups, 62% of those with a recent ACS, 52% with other CHD, 43% with ischemic stroke, 36% with PAD, and 40% with only DM had a filled statin prescription. Of those with filled statins, the relative proportion on high‐intensity was 44% for recent ACS, 32% for other CHD, 22% for ischemic stroke, 18% for PAD, and 18% for only DM. (See Supporting Information, Table 4, in the online version of this article for a summary of these metrics by prevalent subgroups.)
In the overall cohort, 2% of patients had filled a prescription for ezetimibe (either as monotherapy or combination therapy) and 1% for all other nonstatins. Of those with filled statins, use of ezetimibe was 4% and other nonstatins 2%. Table 2 and Supporting Information, Table 4, in the online version of this article provide further details on the patterns for ezetimibe and other nonstatin LLTs by background statin intensity for hierarchical and prevalent subgroups.
In the overall cohort, 75% of patients without a filled LLT prescription had no evidence of a prior filled LLT prescription during the previous 2 years. By hierarchical subgroups, this proportion was 59% in patients with recent ACS, 70% with other CHD, 74% with ischemic stroke, 81% with PAD, and 77% with only DM. Data by hierarchical and prevalent subgroups are shown in Table 2 and Supporting Information, Table 4, in the online version of this article, respectively.
We evaluated the predictors of each type of filled LLT regimen, defined by the presence of a high‐, moderate‐, and low‐intensity statin as well as nonstatin LLT, with each regimen compared with no LLT using a multinomial logistic‐regression model (Table 3). Younger patients and the very elderly were less likely to receive any type of LLT. Women were less likely than men to be prescribed more intensive statin regimens. Presence of coronary conditions was associated with higher use of more intensive statin regimens. Presence of liver disease and diagnoses associated with musculoskeletal pain were associated with lower levels of statins. In addition, a higher level of nonstatin LLT use was observed for musculoskeletal pain. Dementia and history of congestive heart failure were associated with less of any type of LLT. Concomitant use of other standard‐of‐care therapies was positively associated with every type of LLT.
An analysis also was performed to assess whether filled statin prescriptions were more frequent for those with ≥1 high‐CV‐risk condition. For patients with only coronary disease (ie, recent ACS and/or other CHD), 48% had filled prescriptions. For those with coronary disease and DM, it was 56%. For those with coronary disease and either ischemic stroke or PAD, it was 52%. For patients with coronary disease, DM, and ischemic stroke and/or PAD, 59% had filled statin prescriptions.
4. DISCUSSION
We sought to provide contemporary data on LLT utilization in a large, insured US population with ASCVD and/or DM. The analysis addressed some limitations in the literature. First, it was based on a current, large (N > 1 million), and generalizable patient sample. Second, it evaluated treatment with specific statin regimens with and without nonstatin therapies for cardiac and noncardiac indications, including recent ACS, CHD, ischemic stroke, PAD, and DM. Third, the analysis was conducted using filled prescription data and a point‐in‐time assessment, which is expected to better incorporate the impact of factors such as primary nonadherence (ie, written prescription never filled) and discontinuation over time compared with analyses of written prescriptions. Finally, a multivariate regression analysis was conducted to determine the predictors of various LLT regimens.
In the overall cohort, only 49% of participants with a history of ASCVD and 40% with only DM had filled prescriptions for statins as of the index date in 2014. Of those treated with a statin, the proportion treated with a high‐intensity statin was only 30% and 18%, respectively. Of those without a filled statin prescription, 74% and 79%, respectively, had no evidence of a previously filled statin during the preceding 2 years. Limited treatment with nonstatin LLTs was observed. Filled prescriptions for LLT were less frequent in patients with ischemic stroke and PAD compared with those with coronary conditions. The findings underscore a major gap in addressing public health and CV disease burden in a population considered to be at high risk for CV events.13
Data from the 2011–2012 National Health and Nutrition Examination Survey (NHANES) database demonstrated patient‐reported statin use was 64% in those with clinical ASCVD.5, 14 Statin use was reported as 58% among patients with CHD in the Medical Expenditure Panel Survey (MEPS) database in 2010 (n = 16 712).6 In a recent study of Medicare beneficiaries who filled a statin prescription after hospital hospitalization due to a coronary condition, only 27% of first post‐discharge fills were for a high‐intensity statin (35% of any post‐discharge fills within 1 year).4 Our findings are comparable with estimates from these studies representing clinical ASCVD, CHD, and recent ACS populations,4, 5, 6 but they are lower than those reported from registries,7, 11 which often represent a more select group of institutions with a quality‐improvement focus and often more selective patient‐inclusion criteria.
Less is known about statin use in the United States for ischemic stroke. An analysis of data from the American Heart Association (AHA) Get With The Guidelines–Stroke registry representing patients hospitalized with transient ischemic attack or stroke reported that statins were prescribed at discharge to 79% of patients between 2005 and 2007.10 This finding is substantially higher than in the current analysis and likely reflects the unique nature of registry hospitals, the discrepancy between written vs filled prescriptions, and discontinuation over time. In contrast, an analysis of 2009–2010 NHANES patient‐reported data found LLT utilization in 42% of stroke survivors.15 We found filled statin prescriptions for 43% of ischemic stroke patients without coronary disease and for 49% of the overall ischemic stroke population.
There are also few studies of statin use in US patients with PAD. Analyses from a NHANES survey representing 1999–2004 found that only 18% of patients with an ankle‐brachial index ≤0.90 (measured by the survey team) and no history of CHD or ischemic stroke reported taking statins.9 This finding likely represents not only underdiagnosis, but also insufficient treatment of diagnosed PAD. In contrast, Reduction of Atherothrombosis for Continued Health (REACH), a worldwide registry of atherothrombotic patients enrolled in 2004, reported statin use in 64% of patients previously diagnosed with PAD.11 As discussed earlier, registries typically report a substantially higher use of standard‐of‐care therapies; US‐specific results were not reported in REACH. Our findings reveal filled statin prescriptions in 36% of those with PAD but without CHD or ischemic stroke, and in 47% in the prevalent PAD population (more comparable to the REACH population).
Patient‐reported statin use in those with only DM (no ASCVD) and age 40 to 75 years was 46% from 2011–2012 NHANES data5 and ranged from 52% to 56% in 2 studies of DM patients with or without ASCVD.6, 8 Compared with these patient‐reported findings and filled‐prescription data, our data suggest lower rates of filled prescriptions in broad groups of people with DM: 40% of the only‐DM subgroup and 45% in the DM with and without ASCVD subgroup.
Statin utilization is reported to be dependent upon both provider behavior (eg, prescribing correctly) and patient behavior (eg, prescription filling and adherence). Evidence demonstrates suboptimal initiation and uptitration of statins by healthcare providers.16, 17, 18, 19 Factors related to healthcare providers include workflow constraints, reluctance to re‐evaluate long‐standing treatments (termed “clinical inertia”), incorrect assignment of “intolerance or allergy” designations, suboptimal education, and lack of acceptance of guideline recommendations. Patient‐level factors include poor understanding of the benefits of statins, presence of psychological and cognitive disorders, complex medication regimens, lifelong treatment of an asymptomatic condition, perceived or real side effects, healthcare costs, and challenges due to the healthcare system (eg, travel to appointments, insurance coverage changes).20, 21 These factors help explain why true adherence to a daily regimen may be even lower than typically reported in large, generalizable studies. As an example, whereas 94% of patients with evidence of filled LLT prescriptions also recalled being told to take LLT medications (sensitivity), only 54% of those who recalled being told to take LLT medications had evidence of filled LLT prescriptions (positive predictive value).22 These findings suggest that filled‐prescription data provide a more realistic estimate of actual exposure to LLT. An even stricter criterion for measuring medication exposure is daily adherence, which accounts for factors after a prescription is filled. In a recently reported trial studying provider‐directed medication‐adherence interventions in high‐CV‐risk patients without statin contraindications, daily statin adherence was only 20% to 40%.23
The 2013 American College of Cardiology (ACC)/AHA guidelines,24 along with the availability of generic atorvastatin (November 2011)25 and, more recently, generic rosuvastatin (July 2016),26 are anticipated to increase the initiation of high‐intensity as well as overall statin use.27, 28 In addition, particular healthcare systems such as the Veterans Affairs Healthcare System have been demonstrated the ability to increase statin utilization above others.29, 30 Since the publication of the 2013 ACC/AHA guidelines, which recommended treatment with high‐ and moderate‐intensity statins in 4 benefit groups, additional evidence has emerged for nonstatin‐based therapies. In 2014, Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE‐IT) reported CV risk reduction from addition of ezetimibe to statin therapy in patients with recent ACS.31 In 2015, the US Food and Drug Administration approved 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor–based therapies for LDL‐C lowering in individuals with ASCVD or heterozygous familial hypercholesterolemia. The PCSK9 inhibitor–based therapies are currently being assessed in CV outcomes trials, and the ACC recently published an expert consensus decision pathway regarding the use of ezetimibe and PCSK9 inhibitor–based therapies, which may increase their use in clinical practice when additional LDL‐C lowering is required.32
4.1. Study limitations
The study cohort represented a commercial and Medicare Advantage insured population in the United States with medical and pharmacy coverage. Thus, the findings may not be representative of non‐US patients, the uninsured, or those receiving insurance via Medicaid. Determination of LLT utilization was optimized using a point‐in‐time assessment via evidence of medication supply from filled prescriptions. Assessment of true adherence to a daily LLT regimen was not possible. The prevalence of certain conditions was low; however, it is well recognized that some conditions (eg, PAD) are underdiagnosed in routine clinical practice. This should not compromise our findings, as our objective was not to include all possible participants for whom a statin might be indicated, but to narrow the cohort to those for whom a clear statin indication was present. It should also be noted that the ACC/AHA guidelines on the treatment of elevated blood cholesterol were released in November 2013, and as there is an anticipated time lag in uptake and implementation of most guidelines, this analysis likely does not fully reflect the total impact of these guidelines on clinical practice patterns.
5. CONCLUSION
In an insured US population representing routine clinical practice in 2014, only 49% of patients with ASCVD and 40% with only DM had evidence for a filled statin prescription using a point‐in‐time analysis. Our study contributes to the scientific literature by providing up‐to‐date data on the utilization rates and predictors of specific LLT regimens across ASCVD subgroups and only DM. Underutilization of LLTs in these populations, which are considered to be at elevated risk for CV events, identifies an opportunity for quality improvement and underscores the continued need to develop and implement novel strategies to reduce atherogenic cholesterol.
Supporting information
Table S1. Diagnosis and Procedure Codes for Disease Categorization
Table S2. Categorization of Lipid Lowering Therapy (LLT)
Table S3. Baseline Characteristics for the Overall Cohort and Prevalent Subgroups
Table S4. Filled LLT Prescriptions in the Overall Cohort and Prevalent Subgroups
ACKNOWLEDGMENTS
Medical‐writing support was provided by Betty Thompson, Ph.D., from Prime Medica and Jeff Alexander from SNELL Medical Communication, supported by Sanofi and Regeneron Pharmaceuticals, Inc.
Conflicts of interest
D.L. Steen receives modest consultant fees from Sanofi and Regeneron. I. Khan and K. Gorcyca are employees of Sanofi. L. Becker is an employee of Optum. J.M. Foody was a consultant to Sanofi, Pfizer, Merck, and AstraZeneca at the time of initiating this analysis; she is now an employee of Merck and Co. R. Sanchez is an employee of Regeneron Pharmaceuticals, Inc. R.P. Giugliano discloses that his institution (Brigham and Women's Hospital) has received significant research‐grant support from Amgen and Merck to conduct clinical trials of lipid‐lowering therapies and that he has received modest honoraria for CME lectures and/or consulting from Amgen, Merck, Regeneron, and Sanofi related to lipid‐lowering therapies. The authors declare no other potential conflicts of interest.
Steen DL, Khan I, Becker L, Foody JM, Gorcyca K, Sanchez RJ and Giugliano RP. Patterns and predictors of lipid‐lowering therapy in patients with atherosclerotic cardiovascular disease and/or diabetes mellitus in 2014: Insights from a large US managed‐care population. Clin Cardiol. 2017;40:155–162. 10.1002/clc.22641
Funding information This study was supported by Sanofi and Regeneron Pharmaceuticals, Inc. Responsibility for all opinions, conclusions, and data interpretation lies with the authors.
REFERENCES
- 1. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mihaylova B, Emberson J, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788–2797. [DOI] [PubMed] [Google Scholar]
- 4. Rosenson RS, Kent ST, Brown TM, et al. Underutilization of high‐intensity statin therapy after hospitalization for coronary heart disease. J Am Coll Cardiol. 2015;65:270–277. [DOI] [PubMed] [Google Scholar]
- 5. Wong ND, Young D, Zhao Y, et al. Prevalence of the American College of Cardiology/American Heart Association statin eligibility groups, statin use, and low‐density lipoprotein cholesterol control in US adults using the National Health and Nutrition Examination Survey 2011–2012. J Clin Lipidol. 2016;10:1109–1118. [DOI] [PubMed] [Google Scholar]
- 6. Johansen ME, Green LA, Sen A, et al. Cardiovascular risk and statin use in the United States. Ann Fam Med. 2014;12:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maddox TM, Borden WB, Tang F, et al. Implications of the 2013 ACC/AHA cholesterol guidelines for adults in contemporary cardiovascular practice: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;64:2183–2192. [DOI] [PubMed] [Google Scholar]
- 8. Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36:2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pande RL, Perlstein TS, Beckman JA, et al. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ovbiagele B, Schwamm LH, Smith EE, et al. Recent nationwide trends in discharge statin treatment of hospitalized patients with stroke. Stroke. 2010;41:1508–1513. [DOI] [PubMed] [Google Scholar]
- 11. Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 12. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient‐centered management of dyslipidemia: part 1—full report. J Clin Lipidol. 2015;9:129–169. [DOI] [PubMed] [Google Scholar]
- 13. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association [published corrections available at http://circ.ahajournals.org/content/131/4/e29.full]. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 14. Adedinsewo D, Taka N, Agasthi P, et al. Prevalence and factors associated with statin use among a nationally representative sample of US adults: National Health and Nutrition Examination Survey, 2011–2012. Clin Cardiol. 2016;39:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah NS, Huffman MD, Ning H, et al. Trends in vascular risk factor treatment and control in US stroke survivors: the National Health and Nutrition Examination Surveys (1999–2010). Circ Cardiovasc Qual Outcomes. 2013;6:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abdallah MS, Kosiborod M, Tang F, et al. Patterns and predictors of intensive statin therapy among patients with diabetes mellitus after acute myocardial infarction. Am J Cardiol. 2014;113:1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnold SV, Kosiborod M, Tang F, et al. Patterns of statin initiation, intensification, and maximization among patients hospitalized with an acute myocardial infarction. Circulation. 2014;129:1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arnold SV, Spertus JA, Masoudi FA, et al. Beyond medication prescription as performance measures: optimal secondary prevention medication dosing after acute myocardial infarction [published correction appears in J Am Coll Cardiol. 2013;63:944]. J Am Coll Cardiol. 2013;62:1791–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Javed U, Deedwania PC, Bhatt DL, et al. Use of intensive lipid‐lowering therapy in patients hospitalized with acute coronary syndrome: an analysis of 65 396 hospitalizations from 344 hospitals participating in Get With The Guidelines (GWTG). Am Heart J. 2010;160:1130–1136. [DOI] [PubMed] [Google Scholar]
- 20. Choudhry NK, Avorn J, Glynn RJ, et al; MI‐FREEE Trial . Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. [DOI] [PubMed] [Google Scholar]
- 21. Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long‐term statin therapy? Curr Atheroscler Rep. 2013;15:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown DW, Anda RF, Felitti VJ. Self‐reported information and pharmacy claims were comparable for lipid‐lowering medication exposure. J Clin Epidemiol. 2007;60:525–529. [DOI] [PubMed] [Google Scholar]
- 23. Asch DA, Troxel AB, Stewart WF, et al. Effect of financial incentives to physicians, patients, or both on lipid levels: a randomized clinical trial. JAMA. 2015;314:1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in J Am Coll Cardiol. 2014;63(25 part B):3024–3025 and 2015;66:2812]. J Am Coll Cardiol. 2014;63(25 part B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 25.FDA approves first version of cholesterol‐lowering drug lipitor [press release]. Silver Spring, MD: US Food and Drug Administration; March 10, 2014. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm281817.htm. Accessed September 2016.
- 26. US Food and Drug Administration . First generic drug approvals. http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/drugandbiologicapprovalreports/andagenericdrugapprovals/default.htm. Accessed September 2016.
- 27. Tran JN, Caglar T, Stockl KM, et al. Impact of the new ACC/AHA guidelines on the treatment of high blood cholesterol in a managed care setting [published correction appears in Am Health Drug Benefits. 2014;7:487–488]. Am Health Drug Benefits. 2014;7:430–443. [PMC free article] [PubMed] [Google Scholar]
- 28. Pencina MJ, Navar‐Boggan AM, D'Agostino RB Sr, et al. Application of new cholesterol guidelines to a population‐based sample. N Engl J Med. 2014;370:1422–1431. [DOI] [PubMed] [Google Scholar]
- 29. Pokharel Y, Akeroyd JM, Ramsey DJ, et al. Statin use and its facility‐level variation in patients with diabetes: insight from the Veterans Affairs National Database. Clin Cardiol. 2016;39:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Virani SS, Woodard LD, Akeroyd JM, et al. Is high‐intensity statin therapy associated with lower statin adherence compared with low‐ to moderate‐intensity statin therapy? Implications of the 2013 American College of Cardiology/American Heart Association Cholesterol Management Guidelines. Clin Cardiol. 2014;37:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cannon CP, on behalf of IMPROVE‐IT Investigators . A comparison of ezetimibe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes after acute coronary syndromes. Circulation. 2014;130:2105–2126. [Google Scholar]
- 32. Lloyd‐Jones DM, Morris PB, Ballantyne CM, et al. 2016 ACC Expert Consensus decision pathway on the role of non‐statin therapies for LDL‐cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68:92–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Diagnosis and Procedure Codes for Disease Categorization
Table S2. Categorization of Lipid Lowering Therapy (LLT)
Table S3. Baseline Characteristics for the Overall Cohort and Prevalent Subgroups
Table S4. Filled LLT Prescriptions in the Overall Cohort and Prevalent Subgroups


