Abstract
Background:
Colon tumor is generated and maintained by a small subset of chemo-resistant cancer cells known as Cancer Stem-like Cells (CSCs) that are able to self-renew and differentiate into various cell types within the cancer milieu. CSCs are identified through expression of CD133 that is the most important surface marker of these cells. Epithelial Cell Adhesion Molecule (EpCAM) is another colon CSCs marker. Other markers that are probably involved in colon tumorigenesis are Leucine-rich repeat-containing G-protein-coupled Receptor 5 (LGR5), B cell-specific Moloney murine leukemia virus insertion site 1 (BMI1) and Ten-Eleven Translocations (TETs).
Methods:
Here, mRNA expression rates of LGR5, BMI1 and TETs were surveyed by real-time PCR. After collection and digestion, colon samples were used to isolate CD133 and EpCAM positive CSCs through evaluation of AC133 EpCAM by Magnetic Activated Cell Sorting (MACS) and flow cytometry. Real-time PCR was carried out for assessing expressions of LGR5, BMI1 and TETs.
Results:
High expressions for LGR5, BMI1, TET1 and TET2 in the CD133 and EpCAM positive CSCs (p≤0.05 vs. non-CSCs) were found. TET3, however, showed no significant changes for mRNA expression in the CSCs.
Conclusion:
In conclusion, high mRNA expressions for LGR5, BMI1, TET1 and TET2 in the CD133 and EpCAM positive CSCs may be a useful criterion for better identification of the cells involved in colon cancer in order to specify therapeutic targets against this type of cancer.
Keywords: Colon, Flow cytometry, Molony murine leukemia virus, Neoplastic stem cells
Introduction
Colorectal Cancer (CRC) is the second most common cancer diagnosed in women and the third most common cancer in men 1. CRC is identified to have subpopulation of highly resistant Cancer Stem-like Cells (CSCs) 2; CSCs are the minority and undifferentiated population located at the top of the tumor and involved in the re-establishment of tumor heterogeneity, while their progeny are the majority and terminal differentiated cells located at the base of the tumor and they do not contribute to tumor growth 3,4. Therefore, targeting CSCs may provide a therapeutic approach for managing metastatic disease 5.
CD133 (also called prominin-1) is the most important surface marker of CSCs 6 that is related to the tumorigenicity, poor prognosis and disease progression 2. Epithelial Cell Adhesion Molecule (EpCAM) is another colon CSCs marker that has been reported to be overexpressed in CRC and has an essential role in cancer prognosis and pathogenesis 1.
B cell-specific Moloney murine leukemia virus insertion site 1 (BMI1) is a marker of intestinal stem cells able to label quiescent stem cells. G-protein-coupled receptor 5 (LGR5) is also upregulated in CRCs 7. LGR5 may play an essential role in the prognosis and progression of CRC, and may be considered as a potential new therapeutic approach for targeting CRC. It may also be regarded as a potential marker for colorectal CSCs 8. Melo et al found that selective LGR5+ cell ablation restricts primary colon tumor growth, but does not result in tumor regression 5.
Variation of Ten-Eleven Translocation (TET) proteins (TET1, TET2 and TET3) is common in human cancers 9. TET enzymes affect cancer cell activity and presumably alter genomic 5hmC and 5mC patterns. In addition, 5mC oxidation seems to be a step for various pathways of activating DNA demethylation. This is probably essential for induction of global hypomethylation that occurs during cancer development and progression. Much has been documented regarding this interesting pathway for modification of DNA in recent years, but there is a need for more research so as to identify possible roles for TET proteins in gene regulation during carcinogenesis 10. Moreover, possible differences in the rate of expressions between various types of TETs remain to be clarified. Here, mRNA expressions were analyzed for TETs, LGR5 and BMI1 in CSCs isolated from human colon cancer samples.
Materials and Methods
Specimen preparation
Tumor samples and their matched normal tissues were collected from 14 patients diagnosed with colon adenocarcinoma. Samples were thoroughly washed three to four times in cold Phosphate-Buffered Saline (PBS pH=7.4, Gibco, Carlsbad, CA, USA) containing penicillin/streptomycin and amphotericin B (Invitrogen, Carlsbad, CA, USA) followed by incubation in Dulbecco's Modified Eagle's Medium (DMEM)/F12 (Invitrogen, Grand Island, NY) overnight at 4°C in dark. Patients exposed to chemo/radio therapy prior to surgery were not allowed to be registered for this experiment. Additional tumor and matched normal tissue fragments were kept in liquid nitrogen. For mechanical and enzymatic digestion, tissue specimens were first minced into 2 mm2 fragments, and then digested using 1.5 mg/ml collagenase IV (Invitrogen, Carlsbad, CA, USA), 20 μg/ml hyaluronidase and 40 μg/ml DNase I (Sigma Chemical Co., St. USA) for 1 hr at 37°C with pipetting every 10 min. Prior to inclusion in the study, all patients received informed consent that meets Research Ethics criteria of Tehran University of Medical Sciences and the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines.
Magnetic activated cell sorting (MACS)
AC133 and EpCAM isolation kits (Miltenyi Biotec, Bergisch-Gladbach, Germany) were used for cell isolation according to the manufacturer's protocol. AC133 antibody is frequently used for isolation of CSCs through detecting a glycosylated epitope of CD133 on these cells 11. In brief, up to 108 cells were isolated from colon cancer samples and their matched normal specimens. Samples were labeled with mouse anti-AC133 microbeads conjugated antibody (1:10) for 30 min at 4°C and washed in the MACS buffer. The cells were loaded onto the MACS MS column placed on the magnetic cell separator. AC133+ cells were attached to the column, while AC133- passed through it as negative fraction. Positive cells were then resuspended in the MACS buffer. The same procedure was performed to isolate EpCAM+ cells by using mouse anti-EpCAM microbeads conjugated antibody (1:10).
Flow cytometry
105 cells were suspended in 50 μl PBS containing 4% bovine serum albumin (BSA, Sigma Chemical Co., St. Louis, MO, USA) and labeled with mouse anti-AC133-APC and anti-EpCAM antibodies (1:10, 30 min, 4°C). The cells were washed with PBS and fixed in 4% paraformaldehyde (PFA, Sigma, CA, USA) at room temperature. Cells were then resuspended in PBS and examined by FACS Calibur Flow Cytometer (Becton Dickinson, San Jose, Canada).
Real-time PCR
Total RNA was extracted using extraction kit (GeneAll Biotechnology, Seoul Republic of Korea), and RNase-free DNase I (Cinnagen, Tehran, Iran) was added to thermal cycler for 30 min at 37°C in order to remove genomic DNA contamination. Then, 1 μg of extracted mRNA was reverse transcripted to cDNA using cDNA Synthesis Kit (Thermo Scientific, Vilnius, Lithuania). Real-time PCR was performed using specific gene primers, cDNA and additional PCR reagents (dNTP, polymerase, magnesium and buffer; 5×HOT FIREPol® EvaGreen® qPCR Mix Plus [ROX] 1 ml 08-24-00001 Solis Bio Dyne, Tartu, Estonia) on three-color real-time PCR machine (Applied Biosystems Step One, CA, USA). Firstly, samples were incubated for initial activation of polymerase at 95°C for 15 min. Samples were then denatured at 95°C for 15 s, annealed at 60°C for 20 s and elongated at 72°C for 20 s. Relative quantification was carried out using the 2−∆CT technique that includes normalization of the data to β-actin and further comparison of the fold change calculation to the non-CSCs.
Primers were as follows: LGR5, F “GGAAATCATGC CTTACAGAGC” and R “CCTGGGGAAGGTGAAC ACT”; BMI1, F “CTGGTTGCCCATTGACAGC” and R “CAGAAAATGAATGCGAGCCA”; TET1, F “AAT GGAAGCACTGTGGTTTG” and R “ACATGGAGC TGCTCATCTTG”; TET2, F “TTGGACTTCTGTGCT CATGC” and R “CATCCTCAGGTTTTCCTCCA”; TET3, F “TCGGAGACACCCTCTACCAG” and R “C TTGCAGCCGTTGAAGTACA”; and β-actin, F “TC CCTGGAGAAGAGCTACG” and R “GTAGTTTCG TGGATGCCACA”. F=Forward and R=Reverse.
Statistical analysis
Statistical analysis was carried out using SPSS 16 and independent samples T-Test to evaluate significant differences between groups. Quantitative variables were presented as mean±standard deviation (SD), and p≤ 0.05 was considered statistically significant.
Results
MACS and flow cytometry findings
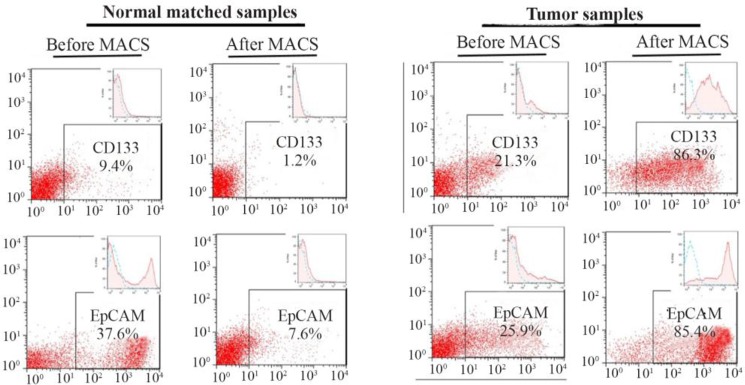
CD133 and EpCAM markers were assessed by MACS and flow cytometry. Tumor cells showed high rates of CD133 and EpCAM expressions with 85.8±0.8 and 85.1±0.4, respectively. Normal cells from the same patients, on the other hand, had low rates of expressions for CD133 and EpCAM with 1.3±0.1 and 8.1± 0.9, respectively. The rates of expressions for both markers were significant in the tumor cells compared to their matched normal cells (p≤0.05) (Figure 1).
Figure 1.
Flow cytometry assay of tumor cells and their matched normal cells isolated using a Magnetic-Activated Cell Sorter (MACS). Tumor cells showed high rates of expressions for cancer-stem like cell markers (i.e. CD133 and EpCAM). Results are presented as mean±SD (n=8).
Real-time PCR
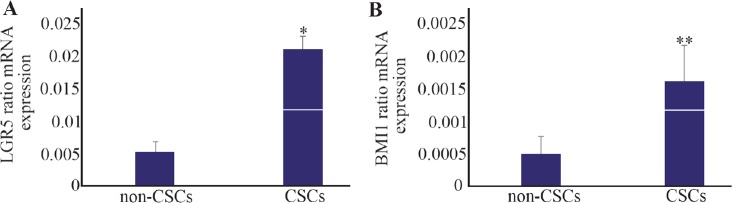
Real-time PCR was performed to evaluate expression rates for various CSCs markers. LGR5 showed a higher rate of expression with 4-fold increase in the CSCs compared with the non-CSCs. The mRNA levels for CSCs and non-CSCs were 0.02±0.002 and 0.005± 0.001, respectively (p≤0.001) (Figure 2A).
Figure 2.
Relative mRNA expressions for leucine-rich repeat-containing G-protein-coupled receptor (LGR5) and B cell-specific Moloney murine leukemia virus insertion site 1 (BMI1) analyzed by real-time RT-PCR. A) LGR5 had high level of expression in the cancer stem-like cells (CSCs). B) BMI1 showed a similar pattern with higher rate of expression in the CSCs compared with the non-CSCs. * p≤0.001 and ** p≤0.03 vs. non-CSCs. Results are presented as mean±SD (n=14).
Similarly, BMI1 had a higher rate of expression with 4-fold increase in the CSCs compared with the non-CSCs. The mRNA expressions for CSCs and non-CSCs were 0.0016±0.0005 and 0.0005±0.0002, respectively. The levels were significant in the CSCs compared with non-CSCs (p≤0.03) (Figure 2B).
Expression patterns of TET1, TET2 and TET3 were evaluated in the CSCs. TET1 showed about 7-fold increase in the rate of expression in the CSCs with 0.0036±0.0007. The expression rate of TET1 for non-CSCs was 0.0005±0.0004. The levels were significant in the CSCs compared with non-CSCs (p≤0.004) (Figure 3A).
Figure 3.
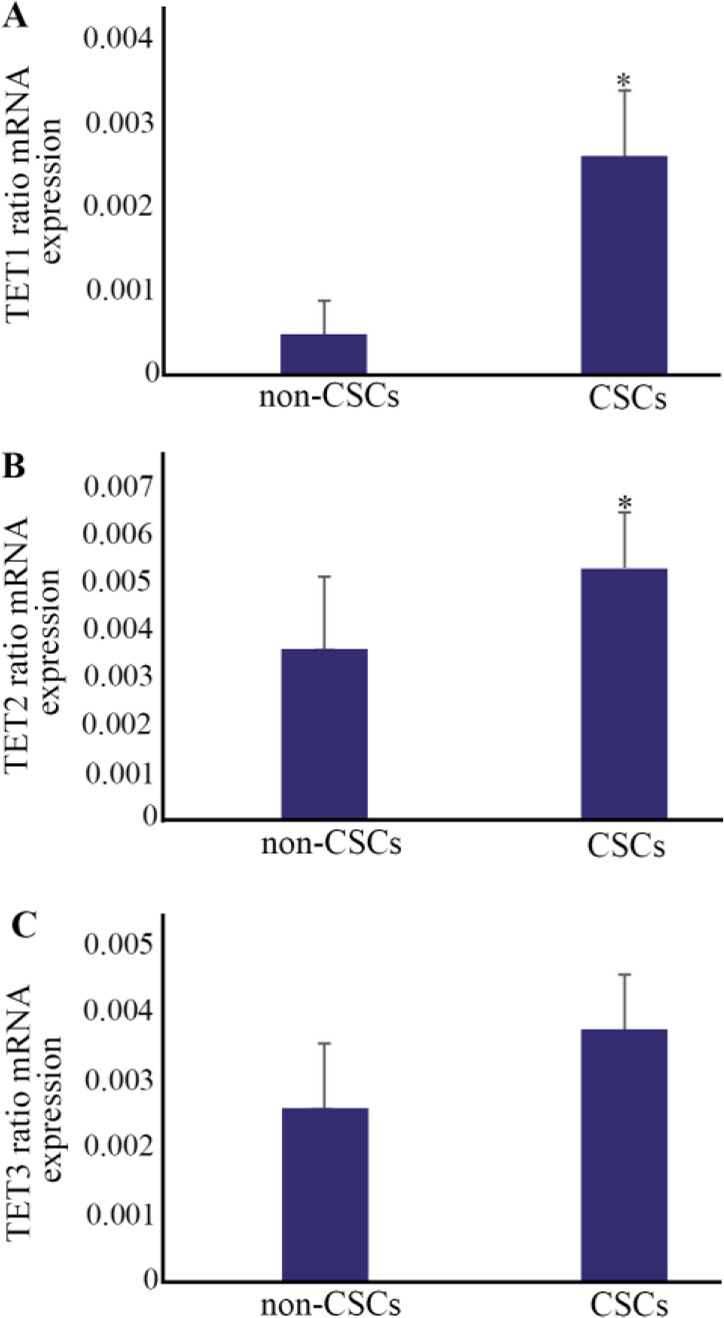
Relative mRNA expressions of Ten-Eleven Translocation (TET) enzymes (TET1, TET2 and TET3) assessed by real-time RT-PCR. A) There was a noticeable increase for TET1 expression in cancer stem-like cells (CSCs). *p≤0.004 vs. non-CSCs. B) TET2 showed significant changes in levels of mRNA expression between CSCs and non-CSCs. *p≤0.02 vs. non-CSCs. C) TET3, however, showed no considerable differences in levels of mRNA expression between CSCs and non-CSCs. Results are presented as mean±SD (n=14).
TET2 showed about 1.5-fold increase in the rate of expression in the CSCs with 0.0053±0.001. The expression rate of TET2 for non-CSCs was 0.0036±0.001. The levels were significant in the CSCs compared with non-CSCs (p≤0.02) (Figure 3B).
Similarly, TET3 had about 1.5-fold increase in the expression rate of the CSCs with 0.0038±0.0008. The rate of TET3 expression in the non-CSCs was 0.0026± 0.0009. The levels were not considerable in the CSCs, as compared with the non-CSCs (p≤0.59) (Figure 3C).
Discussion
In the current study, high expression of CD133 was found in the CSCs isolated from human colon cancer. CD133 is a known stem cell marker that is widely used to identify colon CSCs 1. Saigusa et al referred to an increase in the gene and protein expressions for CD133 in patients with rectal cancer and also a line between CD133 expression with poor prognosis and distant recurrence 12. Kemper et al also attested an association between CD133 expression with poor survival in patients suffering from CRC 13. On the other hand, Gazzaniga et al showed that CD133 expression in circulating tumor cells harvested from peripheral blood from metastatic CRC patients had no association with overall outcome in the patients 14. Despite having a contradictory single report, most of the studies reinforce the idea of the potential application of CD133 as a prognostic marker for colon CSCs 1. Therefore, a high rate of CD133 expression in the cells harvested from tumor samples subsequent to the second MACS indicates the collected cells obtained high levels of purity.
Expression of EpCAM is considered as a marker for detection of CRC 15. This marker is associated with proliferation 16 and metastasis 17 in cancer cell. In one report, however, a negative link between this marker with cancer cell proliferation has been documented 18. Due to most of the studies identified EpCAM as a prognostic marker for cancer progression, it can be assumed that high expression for EpCAM in the tumor cells may be a useful criterion for specific identification of the CSCs within the tumor milieu.
Overexpression of LGR5 was found in CSCs. LGR5 is a marker gene for detection of CSCs in colon cancer 19,20, indicating differentiation capacity and self-renewal for LGR5+ tumor cells 20. High activity for LGR5+ cells is at the crypt base 20 where CSCs are presumably located 21. High level for LGR5 in CRC is also positively associated with histological grade and invasiveness 8. Higher levels for mRNA and protein expressions of LGR5 in primary colon cancer compared with normal tissues in both animal and human 22 have also been reported to reflect shorter survival span in both models 22 so that selective LGR5 positive cell ablation limits primary colon tumor growth, but this does not lead to tumor regression. Instead, tumors are kept by proliferative LGR5 negative cells that continuously try to replenish the LGR5 positive CSC pool, resulting in rapid re-initiation of tumor growth upon treatment cessation 5.
BMI1 is an inducer of cancer cell migration and invasion 23. BMI1 is required for tumor growth maintenance in human CRC cells 24 in which its inhibition results in tumor growth arrest 25. BMI1 is also identified as a stem cell marker 23 and an important oncogene for promoting self-renewal of colon CSCs 23. Data from this work also showed significant expression of BMI1 in CSCs compared with non-CSCs. Lower expression for BMI1 was found than the expression for LGR5 in CSCs. To illustrate, BMI1 was initially identified through its ability for labeling quiescent intestinal stem cells 26, but LGR5 was identified in relation with rapid cycling cells 27. Therefore, it is conceivable to speculate the existence of more proliferative and active LGR5 and CD133 positive CSCs in human colon cancer than the existence of quiescent BMI1 positive cells.
Expression rates of TETs have also been surveyed in this work. Significant expressions of TET1 and TET2 in CSCs were observed. TET3, however, showed no significant changes between the two types of cells examined in the present study. Data about expression rate of TETs in CSCs is so limited, and most of the knowledge in this regard has come from studies on Embryonic Stem Cells (ESCs) or on colon tissue tumor in general. TET1 downregulation has been shown to promote cancer invasion and metastasis 28. In ESCs, TET1 was found to play an essential role in their self-renewal 29. However, in a study performed by Hu et al, ESCs lacking all three TET genes seemed normal in pluripotency and self-renewal 30. Neri et al noticed that downregulation of TET1 in colon tumor in mice was not only associated with tumor malignancy and progression but also connected to tumor initiation and growth 28. It is still unclear why CSCs over express TET1 and TET2 in human subjects and further studies are required to investigate the reason.
Conclusion
High rates of mRNA expressions for LGR5, BMI1, TET1 and TET2 in the CD133 and EpCAM positive CSCs of this study may be regarded as a useful criterion for detecting these cells from their progeny. When the cells are well identified, therapeutic protocols will be applied more specifically for these cells, as it has been done so far for glioblastoma 31 and ovarian cancer using melatonin 32.
Footnotes
Funding
This study was supported in part by grant 91-02-3017650 from Tehran University of Medical Sciences. The authors disclose no conflicts of interest.
References
- 1. Wahab SR, Islam F, Gopalan V, Lam AK-y. The identifications and clinical implications of cancer stem cells in colorectal cancer. Clin Colorectal Cancer 2017;16(2):93–102. [DOI] [PubMed] [Google Scholar]
- 2. Sahlberg SH, Spiegelberg D, Glimelius B, Stenerlöw B, Nestor M. Evaluation of cancer stem cell markers CD133, CD44, CD24: association with AKT isoforms and radiation resistance in colon cancer cells. PloS One 2014;9(4):e94621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445(7123):106–110. [DOI] [PubMed] [Google Scholar]
- 4. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2006;445 (7123):111–115. [DOI] [PubMed] [Google Scholar]
- 5. de Sousa e Melo F, Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 2017;543(7647):676–680. [DOI] [PubMed] [Google Scholar]
- 6. Fathi A, Mosaad H, Hussein S, Roshdy M, Ismail EI. Prognostic significance of CD133 and ezrin expression in colorectal carcinoma. IUBMB Life 2017;69(5):328–340. [DOI] [PubMed] [Google Scholar]
- 7. Sureban SM, Qu D, Houchen CW. Regulation of mi-RNAs by agents targeting the tumor stem cell markers DCLK1, MSI1, LGR5, and BMI1. Curr Pharmacol Rep 2015;1(4):217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu XS, Xi HQ, Chen L. Lgr5 is a potential marker of colorectal carcinoma stem cells that correlates with patient survival. World J Surg Oncol 2012;10(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scourzic L, Mouly E, Bernard OA. TET proteins and the control of cytosine demethylation in cancer. Genome Med 2015;7(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kinney SRM, Pradhan S. Ten eleven translocation enzymes and 5-hydroxymethylation in mammalian development and cancer. In: Epigenetic Alterations in Oncogenesis: Springer; 2013. p. 57–79. [DOI] [PubMed] [Google Scholar]
- 11. Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, et al. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res 2010;70(2):719–729. [DOI] [PubMed] [Google Scholar]
- 12. Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, et al. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol 2009;16(12):3488–3498. [DOI] [PubMed] [Google Scholar]
- 13. Kemper K, Versloot M, Cameron K, Colak S, de Sousa e Melo F, de Jong JH, et al. Mutations in the Ras-Raf Axis underlie the prognostic value of CD133 in colorectal cancer. Clin Cancer Res 2012;18(11):3132–3141. [DOI] [PubMed] [Google Scholar]
- 14. Gazzaniga P, Gradilone A, Petracca A, Nicolazzo C, Raimondi C, Iacovelli R, et al. Molecular markers in circulating tumour cells from metastatic colorectal cancer patients. J Cell Mol Med 2010;14(8):2073–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu F, Zhu J, Mao Y, Li X, Hu B, Zhang D. Associations between the epithelial-mesenchymal transition phenotypes of circulating tumor cells and the clinicopathological features of patients with colorectal cancer. Dis Markers 2017;2017: 9474532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou F, Qi Y, Xu H, Wang Q, Gao X, Guo H. Expression of EpCAM and Wnt/beta-catenin in human colon cancer. Genet Mol Res 2015;14(2):4485–4494. [DOI] [PubMed] [Google Scholar]
- 17. Hanusova V, Skalova L, Kralova V, Matouskovs P. The effect of flubendazole on adhesion and migration in SW-480 and SW620 colon cancer cells. Anticancer Agents Med Chem 2018;18(6):837–846. [DOI] [PubMed] [Google Scholar]
- 18. Lugli A, Iezzi G, Hostettler I, Muraro MG, Mele V, Tornillo L, et al. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer 2010;103(3):382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Z, Dai W, Jiang L, Cheng Y. Over-expression of LGR5 correlates with poor survival of colon cancer in mice as well as in patients. Neoplasma 2014;61(2):177–185. [DOI] [PubMed] [Google Scholar]
- 20. Oost KC, van Voorthuijsen L, Fumagalli A, Lindeboom RGH, Sprangers J, Omerzu M, et al. Specific labeling of stem cell activity in human colorectal organoids using an ASCL2-responsive minigene. Cell Rep 2018;22(6):1600–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takahashi H, Ishii H, Nishida N, Takemasa I, Mizushima T, Ikeda M, et al. Significance of Lgr5+ ve cancer stem cells in the colon and rectum. Annals Surg Oncol 2011; 18(4):1166–1174. [DOI] [PubMed] [Google Scholar]
- 22. Liu Z, Dai W, Jiang L, Cheng Y. Over-expression of LGR5 correlates with poor survival of colon cancer in mice as well as in patients. Neoplasma 2013;61(2):177–185. [DOI] [PubMed] [Google Scholar]
- 23. Fesler A, Liu H, Ju J. Modified miR-15a has therapeutic potential for improving treatment of advanced stage colorectal cancer through inhibition of BCL2, BMI1, YAP1 and DCLK1. Oncotarget 2018;9(2):2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Espersen MLM, Olsen J, Linnemann D, Høgdall E, Troelsen JT. Clinical implications of intestinal stem cell markers in colorectal cancer. Clin Colorectal Cancer 2015;14(2):63–71. [DOI] [PubMed] [Google Scholar]
- 25. Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med 2014;20(1): 29–36. [DOI] [PubMed] [Google Scholar]
- 26. López-Arribillaga E, Rodilla V, Pellegrinet L, Guiu J, Iglesias M, Roman AC, et al. Bmi1 regulates murine intestinal stem cell proliferation and self-renewal down-stream of Notch. Development 2015;142(1):41–50. [DOI] [PubMed] [Google Scholar]
- 27. Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen R, et al. Krt19+/Lgr5- cells are radioresistant cancer initiating stem cells in the colon and intestine. Cell Stem Cell 2015;16(6):627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neri F, Dettori D, Incarnato D, Krepelova A, Rapelli S, Maldotti M, et al. TET1 is a tumour suppressor that inhibits colon cancer growth by derepressing inhibitors of the WNT pathway. Oncogene 2015;34(32):4168–4176. [DOI] [PubMed] [Google Scholar]
- 29. Chen ZX, Riggs AD. DNA methylation and demethylation in mammals. J Biol Chem 2011;286(21):18347–18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu X, Zhang L, Mao SQ, Li Z, Chen J, Zhang RR, et al. Tet and TDG mediate DNA demethylation essential for mesenchymal-to-epithelial transition in somatic cell re-programming. Cell Stem Cell 2014;14(4):512–522. [DOI] [PubMed] [Google Scholar]
- 31. Zheng X, Pang B, Gu G, Gao T, Zhang R, Pang Q, et al. Melatonin inhibits glioblastoma stem-like cells through suppression of EZH2-notch1 signaling axis. Int J Biol Sci 2017;13(2):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akbarzadeh M, Movassaghpour AA, Ghanbari H, Kheirandish M, Fathi Maroufi N, Rahbarghazi R, et al. The potential therapeutic effect of melatonin on human ovarian cancer by inhibition of invasion and migration of cancer stem cells. Scientific Reports 2017;7(1):17062. [DOI] [PMC free article] [PubMed] [Google Scholar]