Abstract
Coronary artery aneurysm is defined as a coronary dilation that exceeds the diameter of adjacent segments or the diameter of the patient's largest normal coronary vessel by 1.5×. It is an uncommon disease that has been diagnosed with increasing frequency since the widespread appearance of coronary angiography. The published incidence varies from 1.5% to 5%, suggesting male dominance and a predilection for the right coronary artery. Although several causes have been described, atherosclerosis accounts for ≥50% of coronary aneurysms in adults. Reported complications include thrombosis and distal embolization, rupture, and vasospasm, causing ischemia, heart failure, or arrhythmias. The natural history and prognosis remain unknown, as definitive data are scarce. Controversies persist regarding the use of medical management (antithrombotic therapy) or interventional/surgical procedures. Only some case reports or small case series are available about this condition. The Coronary Artery Aneurysm Registry (CAAR; http://www.ClinicalTrials.gov NCT02563626) is a multicenter international ambispective registry that aims to provide insights on anatomic, epidemiologic, and clinical aspects of this substantially unknown entity. In addition, the registry will assess management strategies (conservative, interventional, or surgical) and their short‐ and long‐term results in a large cohort of patients.
Clinical Trial registration: ClinicalTrials.gov. Unique identifier: NCT02563626.
Keywords: Coronary Aneurysm, Registry, Prognosis, Epidemiology, CAAR
1. INTRODUCTION
Coronary artery aneurysm has been classically defined as a coronary dilation that exceeds the diameter of normal adjacent segments or the diameter of the patient's largest coronary vessel by 1.5×. Termed by Bourgon,1 it is an uncommon disease that has been diagnosed with increasing occurrence since the advent of coronary angiography.2, 3 The incidence has been reported to vary from 1.5% to 5%, with suggested male dominance and a predilection for the right coronary artery.2, 3 Although several causes have been shown, atherosclerosis accounts for ≥50% of coronary aneurysms in adults. Reported complications include thrombosis and distal embolization, vasospasm, and rupture, producing ischemia, heart failure, or arrhythmias. The natural history and long‐term outcomes remain unclear, as definitive data are lacking. In addition, controversies persist regarding the use of medical treatment (antithrombotic therapy) or interventional/surgical procedures.1, 2, 3, 4, 5
Thus, we propose a multicenter, international registry gathering patients with confirmed coronary aneurysm by means of an invasive coronary angiography. Because most of the available data on this disease derive from limited case series published several years ago, we consider that an update is needed, performing at the same time a thorough assessment on several points where there is no real current evidence (eg, incidence, etiology, clinical features, management, long‐term outcomes).
2. METHODS
2.1. Registry design
The Coronary Artery Aneurysm Registry (CAAR) is a multicenter, international, and anonymized (blind sample) observational registry with ambispective recruitment and prospective follow‐up (Figure 1). It will be coordinated by the team at the Clínico San Carlos Hospital (HCSC) in Madrid, Spain. HCSC will serve as the core center to solve angiographic doubts as well.6 (For details on the scientific committee and Angiographic Core Laboratory of the registry, see Supporting Information, Appendix, in the online version of this article.) This study has been endorsed by the interventional cardiology section of the Spanish Society of Cardiology.
Figure 1.
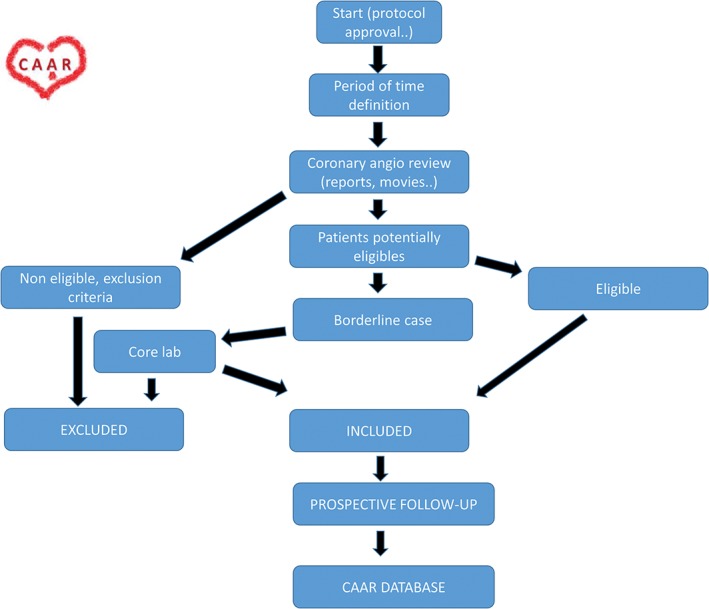
CAAR flow diagram. Abbreviations: CAAR, Coronary Artery Aneurysm Registry.
Using standardized case‐report forms, data will be recorded prospectively and consecutively by local investigators. This data will feed an anonymized electronic database, and a centralized analysis at HCSC will be carried out after queries solution. Inclusion in the registry will not imply any change in clinical management, which will be decided by the local attending physician.
The design of this registry has been approved by the HCSC clinical research ethics committee (C.P.‐C.I.15/458‐E), complies with the Declaration of Helsinki and, when required by local or national legislation, was submitted and approved by the local hospital committee. The http://www.ClinicalTrials.gov identifier is NCT02563626. This is an investigator‐initiated protocol that does not rely on internal/external funding or financial relationships requiring disclosure.
2.2. Definitions and inclusion/exclusion criteria
2.2.1. Definitions
Coronary aneurysm: A focal coronary dilation that exceeds the diameter of normal adjacent segments or the diameter of the patient's largest coronary vessel by 1.5×. Multiple aneurysms are accepted in the CAAR.
Coronary ectasia: Commonly defined as inappropriate dilation of the coronary arteries exceeding the largest diameter of an adjacent normal vessel more than 1.5‐fold. The term “ectasia” refers to diffuse dilation of a coronary artery, whereas focal dilation is called “coronary aneurysm.”7, 8 This condition has been suggested as a variant of coronary atherosclerosis.9 Patients with coronary ectasia (without clear aneurysm, as previously defined) are not eligible for this study (Figure 2).
Giant coronary aneurysm: A coronary dilation that exceeds the diameter of normal adjacent segments or the diameter of the patient's largest coronary vessel by 4×. This is an eligible condition for CAAR.
Figure 2.
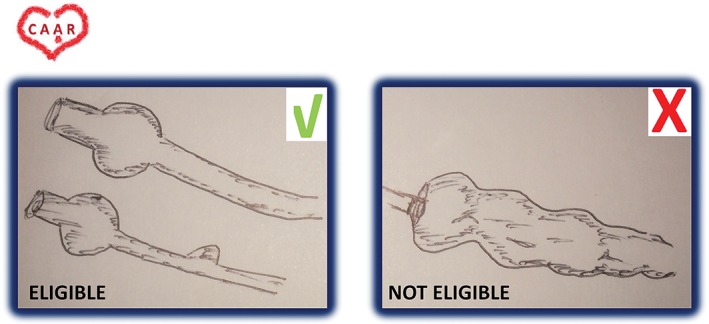
Example of eligible and noneligible CAAR candidates. Abbreviations: Coronary Artery Aneurysm Registry.
2.2.2. Inclusion/exclusion criteria
All patients (age ≥18 years) who, after carrying out an invasive angiography, have been diagnosed with coronary aneurysm, either single or multiple, are eligible. We established a lower age limit of 18 years to homogenize the registry (and its outcomes). This way, it is likely that we focus more on atherosclerotic aneurysms and ischemic coronary disease. In addition, most of the participating centers are adult cardiology departments.
Every center will consecutively review all of its coronary angiographies during a period of time that could be variable and chosen based on the availability of investigator data. The protocol will allow each center to choose its own time interval of study. For logistic reasons, some centers will include patients prospectively (some cannot review the movies retrospectively) and other centers will do it ambispectively (retrospectively and prospectively).
To include retrospectively recruited patients, the center should be able to review the complete angiographies, all in an uninterrupted period of time (all consecutive angiographies were reviewed, and all consecutive patients fulfilling criteria included). In all cases, the follow‐up will be prospective.
The retrospective recruitment is considered of paramount importance to obtain an adequate follow‐up length, because estimated incidence is low.
Each local study team, composed of experienced interventional cardiologists, will choose those consecutive patients clearly eligible for the CAAR. If the investigator team is uncertain about the inclusion of a certain patient, angiographic images will be submitted to the core center (HCSC) to determine that patient's final inclusion/exclusion.
The only exclusion criterion is patient refusal to participate in the registry.
2.3. Objectives of the CAAR
2.3.1. Anatomic objectives
To characterize the prevalence of coronary aneurysms among all comers undergoing invasive coronary angiography.
To describe anatomical, location, and aneurysm features.
To assess intracoronary imaging data, if available, by intravascular ultrasound or optical coherence tomography.
To describe the natural history and angiographic evolution when multiple angiograms are available (angiographic changes, new aneurysms, complications at the aneurysm level).
2.3.2. Clinical objectives
To describe the clinical presentation that further triggers the diagnostic catheterization.
To compile patients’ clinical features. In this registry, we will focus on the epidemiological profile of the patients to make an educated guess about etiology. Of course, without histology, the exact diagnosis is complex. One of the direct questions the protocol poses is childhood background and history of vasculitis/collagenopathies.
Long‐term outcomes (cardiovascular and all‐cause death, reinfarction, unstable angina, heart failure, bleeding, stroke).
2.3.3. Therapeutic objectives
To assess short‐ and long‐term results of management strategies (conservative, interventional, and surgical).
To determine outcomes regarding management.
Because several thousand coronary angiographies will be reviewed, a central core for all the procedures was not feasible, so we will provide… this core laboratory task only for the doubtful cases (borderline patients, or when the local team had doubts about patient inclusion or exclusion). The registry aims to… to include >1000 patients. The exact size calculation was not performed because the data about this disease are scarce.
The HCSC network will provide a matched cohort of patients with coronary artery disease (without aneurysms), to compare the outcomes.
The Table 1 displays the provisional current recruitment and a list of hospitals already included in the registry.
Table 1.
CAAR current recruitment (December 2016)
| Center | City | Country |
|---|---|---|
| H Clínico San Carlos | Madrid | Spain |
| H Severo Ochoa | Leganes | Spain |
| H Príncipe de Asturias, Alcalá | Alcalá | Spain |
| H Álvaro Cunqueiro | Vigo | Spain |
| Instituto de Cardiología y Cirugía Cardiovascular | La Habana | Cuba |
| Kerckhoff Klinik | Bad Nauheim | Germany |
| H Arnau Vilanova | Lerida | Spain |
| H Universitario de Lozano Blesa | Zaragoza | Spain |
| University Hospital, London Health Sciences Centre | London, Ontario | Canada |
| H La Sapienza | Roma | Italy |
| H Molinette 1 | Turin | Italy |
| H Molinette 2 | Turin | Italy |
| Hospital de Clínicas Dr. Manuel Quintela | Montevideo | Uruguay |
| H de Valdecilla | Santander | Spain |
| H Universitario de Albacete | Albacete | Spain |
| H Universitario de Valladolid | Valladolid | Spain |
| H Cabueñes | Gijon | Spain |
| H P Giaccone | Palermo | Italy |
| H Fatebenefratelli | Milan | Italy |
| H Valdecilla | Oviedo | Spain |
| H Central de la Defensa Gomez Ulla | Madrid | Spain |
| H Bolognini | Bolognini | Italy |
| H Rivoli | Turin | Italy |
| Utrecht Medisch Centrum (University Medical Center Utrecht) | Utrecht | Netherlands |
| H Juan Ramón Jimenez Huelva | Huelva | Spain |
| H Universitario de Canarias | Canarias | Spain |
| H Bellvitge | Barcelona | Spain |
| H San Giovanni Bosco | Turin | Italy |
| Hospital Brno | Brno | Czech Republic |
| Hospital Universitario de La Princesa | Madrid | Spain |
| Istituto Clinico Sant'Ambrogio | Milan | Italy |
| Montefiore Medical Center | New York City | United States |
| Mayo Clinic | Phoenix | United States |
| Provisional recruitment, n | 1569 |
Abbreviations: CAAR, Coronary Artery Aneurysm Registry; H, Hospital.
2.4. Statistical analysis
For statistical analysis, we will use SPSS version 20.0 (IBM Corp., Armonk, NY) and the Office 2010 software package (Microsoft, Redmond, WA). The data will be presented as mean ± SD or median and range, as applicable. The study will be descriptive and differences between groups will be analyzed using the most appropriate statistic test in each case, according to data spread and depending on whether it includes quantitative or qualitative variables. Comparisons between groups will be performed using the Pearson χ2 test for qualitative variables and the t test or Mann‐Whitney and Wilcoxon tests, as appropriate, for continuous variables. Long‐term event‐free survival curves for the different exploratory analyses and groups will be obtained using the Kaplan‐Meier method, and comparisons between groups shall be performed using the log‐rank test. Regarding the multivariate analysis, Cox logistic regression is to be used to analyze and select those variables independently associated with the development of long‐term clinical events. To avoid excess of variables included in the multivariate analysis, these will be reduced using a prespecified model including variables that obtained a value of P ≤ 0.10 in univariate study. Risk ratios (hazard ratio) and 95% confidence intervals will be calculated according to the backward stepwise logistic regression analysis (Wald). The level of statistical significance will be set at P < 0.05 (2‐tailed).
3. DISCUSSION
The CAAR will be the largest registry assessing coronary artery aneurysms so far. The international multicentric character of this investigation would pose additional and informative advantages.
With very limited information about this potentially severe and complex disease, the only data available are based on clinical cases, small series, or post‐hoc substudies from other research, usually noncontemporary, with different profiles and probably different therapeutics.10, 11, 12, 13, 14, 15 Thus, when diagnosing a coronary aneurysm (acutely or not), there is no clear therapeutic approach and prognostic data. In fact, it is remarkable that coronary artery disease practice guidelines do not refer to this condition and do not provide recommendations at all, even in acute settings. Considering this, the CAAR team, composed of clinician‐researchers from various centers and different continents, will try to answer some questions that are still unclear and also provide insight into clinical best practices.
Goals are to define the natural history of the disease (epidemiology, aneurysm growing patterns, etiologies),14 whether the clinical presentation (acute or not) should modify our management strategy,13 main medical treatment aspects (eg, antiplatelet therapy, how and for how long, need for anticoagulation), revascularization strategies (interventional or surgical approaches preferred, and when),16 and, for angioplasty procedures, if we should choose drug‐eluting stents or covered stents rather than others.17, 18, 19
3.1. Registry limitations
We expect some limitations in this registry. The true and accurate incidence calculation will be difficult since we will only obtain data from patients who underwent cardiac catheterization for certain reasons. Therefore, the extrapolation to the general population can be difficult. In addition, we invited several centers around the world (>60) to participate, and finally >30 accepted our invitation. With this distribution, we feel the prevalence will be closer to reality for Caucasians and Latinos/Hispanics, not so close for Blacks, and still unknown for Asians. Race is recorded in the registry. The etiology is a difficult issue. Without histology, the exact diagnosis is complex (Kawasaki's, connective tissue disorder, idiopathic), and the profile of our patients will indicate more atherosclerotic aneurysms and ischemic coronary disease. Other potential diagnoses will be carefully considered. Ideally, a core laboratory should review all angiographies assessed in this study. Nevertheless, the researchers of the registry reviewed >400 000 angiographies, so this is unfortunately out of our reach (logistically and economically). However, in theory, the definition is clear and the participating hospitals are experienced and able to adequately recognize this condition (easy diagnosis and a focal condition). Ectasia is a related condition; because this is sometimes more difficult to define, in our experience, we decided to focus mainly on aneurysm. For doubtful cases, we established a core laboratory.
4. CONCLUSION
The results of CAAR will contribute to a better understanding of coronary artery aneurysms. The registry will define the approximate incidence and depict a precise epidemiological profile for these patients. We aim to describe the management of this condition as well as the outcomes in relation to the chosen therapy, and so gain insight to improve patient's care in the future.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
APPENDIX S1.
Núñez Gil IJ, Nombela‐Franco L, Bagur R, et al. Rationale and design of a multicenter, international and collaborative Coronary Artery Aneurysm Registry (CAAR). Clin Cardiol. 2017;40:580–585. 10.1002/clc.22705
REFERENCES
- 1. Bourgon A (cited by Scott DH). Aneurysm of the coronary arteries. Am Heart J . 1948;36:403–421. [DOI] [PubMed] [Google Scholar]
- 2. Swaye PS, Fisher LD, Litwin P, et al. Aneurysmal coronary artery disease. Circulation. 1983;67:134–138. [DOI] [PubMed] [Google Scholar]
- 3. Syed M, Lesch M. Coronary artery aneurysm: a review. Prog Cardiovasc Dis. 1997;40:77–84. [DOI] [PubMed] [Google Scholar]
- 4. Dutary J, Zakhem B, De Lucas CB, et al. Treatment of a giant coronary artery aneurysm: intravascular ultrasound and optical coherence tomography findings. J Interv Cardiol. 2012;25:82–85. [DOI] [PubMed] [Google Scholar]
- 5. Maehara A, Mintz GS, Ahmed JM, et al. An intravascular ultrasound classification of angiographic coronary artery aneurysms. Am J Cardiol. 2001;88:365–370. [DOI] [PubMed] [Google Scholar]
- 6. Núñez‐Gil IJ, Bas M, Fernández‐Ortiz A, et al. Long term experience with a novel interventional cardiology network model: learned lessons. J Hosp Admin. 2016;5:87–94. [Google Scholar]
- 7. Li JJ, Li Z, Li J. Is there any link between inflammation and coronary artery ectasia? Med Hypotheses. 2007;69:678–683. [DOI] [PubMed] [Google Scholar]
- 8. Díaz‐Zamudio M, Bacilio‐Pérez U, Herrera‐Zarza MC, et al. Coronary artery aneurysms and ectasia: role of coronary CT angiography. Radiographics. 2009;29:1939–1954. [DOI] [PubMed] [Google Scholar]
- 9. Li JJ, Nie SP, Qian XW, et al. Chronic inflammatory status in patients with coronary artery ectasia. Cytokine. 2009;46:61–64. [DOI] [PubMed] [Google Scholar]
- 10. Velasco M, Zamorano JL, Almería C, et al. Multiple coronary aneurysms in a young man: a diagnostic approach via different techniques [article in Spanish]. Rev Esp Cardiol. 1999;52:55–58. [DOI] [PubMed] [Google Scholar]
- 11. Merchán A, López‐Mínguez JR, Alonso F, et al. Giant left main coronary aneurysm without associated coronary lesions [article in Spanish]. Rev Esp Cardiol. 2002;55:308–311. [DOI] [PubMed] [Google Scholar]
- 12. Warisawa T, Naganuma T, Nakamura S, et al. How should I treat multiple coronary aneurysms with severe stenoses? EuroIntervention. 2015;11:843–846. [DOI] [PubMed] [Google Scholar]
- 13. Chiu P, Lynch D, Jahanayar J, et al. Bilateral giant coronary artery aneurysms complicated by acute coronary syndrome and cardiogenic shock. Ann Thorac Surg. 2016;101:e95–e97. [DOI] [PubMed] [Google Scholar]
- 14. Befeler B, Aranda JM, Embi A, et al. Coronary artery aneurysms: study of their etiology, clinical course and effect on left ventricular function and prognosis. Am J Med. 1977;62:597–607. [DOI] [PubMed] [Google Scholar]
- 15. Letac B, Cazor JL, Cribier A, et al. Large multiple coronary artery aneurysm in adult patients: a report on three patients and a review of the literature. Am Heart J. 1980;99:694–700. [DOI] [PubMed] [Google Scholar]
- 16. Sadeghi MM, Jouzdani SR. Giant left anterior descending coronary artery aneurysm in an adult male patient with ST elevation myocardial infarction. J Surg Case Rep . 2016. doi: 10.1093/jscr/rjw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levisay JP, Roth RM, Schatz RA. Coronary artery aneurysm formation after drug‐eluting stent implantation. Cardiovasc Revasc Med. 2008;9:284–287. [DOI] [PubMed] [Google Scholar]
- 18. Alfonso F, Pérez‐Vizcayno MJ, Ruiz M, et al. Coronary aneurysms after drug‐eluting stent implantation: clinical, angiographic, and intravascular ultrasound findings. J Am Coll Cardiol. 2009;53:2053–2060. [DOI] [PubMed] [Google Scholar]
- 19. Aggarwal V, Mishkel G, Goswami N. Percutaneous exclusion of a rapidly enlarging left main coronary artery aneurysm using coils and an Amplatzer septal occluder. Catheter Cardiovasc Interv. 2016;88:209–214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1.


