Abstract
Background
It remains uncertain whether patients with atrial fibrillation (AF) requiring long‐term oral anticoagulation (OAC) and with stable coronary artery disease (CAD) should receive antiplatelet therapy (APT) in addition to OAC.
Hypothesis
APT in addition to OAC would be more effective than OAC alone in preventing ischaemic events in such patients.
Methods
In the international REduction of Atherothrombosis for Continued Health (REACH) Registry including 68 236 outpatients with or at risk for atherothrombosis, we identified 2347 patients with stable CAD and AF receiving vitamin K antagonists (VKA). Using propensity score matching, patients treated with VKA (n = 1481) were compared with those receiving VKA + APT at inclusion (n = 866). The primary outcome was major adverse cardiovascular events (MACE) at 4 years (cardiovascular death, myocardial infarction, or stroke). Secondary outcomes were all‐cause death and bleeding leading to hospitalization and transfusion.
Results
Patients receiving VKA only were older (74 vs 72 years, P < 0.01), had less diabetes (37% vs 42%, P = 0.02), and less frequent history of percutaneous coronary intervention (28.7% vs 43.9%, P < 0.01). The mean CHA2DS2‐VaSc score was 4.9 in the VKA group vs 4.7 in the VKA + APT group (P < 0.01). After propensity score matching, the rate of MACE was similar between groups: hazard ratio = 1.01 (0.77‐1.33) (P = 0.94), whereas bleeding tended to be more frequent in the VKA + APT group: odds ratio = 1.87 (0.99‐3.50) (P = 0.051).
Conclusions
In this observational analysis, the use of APT in addition to OAC in patients with stable CAD and AF was not associated with lower risk of ischemic events but possibly with higher bleeding rates. Randomized trials are necessary to determine the optimal long‐term antithrombotic strategy.
Keywords: Bleeding, Major Adverse Cardiovascular Events, Coronary Artery Disease, Atrial Fibrillation, Oral Anticoagulant, Antithrombotic, Antiplatelet Therapy
1. INTRODUCTION
Stable coronary artery disease (CAD) is frequent, with an incidence exceeding 10% in elderly patients above 65 years of age.1 In these patients, indications for long‐term oral anticoagulation (OAC) are frequent and mostly represented by atrial fibrillation (AF), the incidence of which also strongly increases with age.2 In recent large registries, patients with AF represented 10% or more of all patients with stable CAD.3, 4, 5, 6, 7 Conversely, CAD is present in 25% to 35% of the patients with AF.8, 9, 10 Patients with CAD and AF are at high‐risk of both ischemic and bleeding events, and their management is a frequent concern in clinical practice.3, 4, 5, 6, 7 For example, in the Suivi d'une cohorte de patients COROnariens stables en région NORd‐pas‐de‐Calais (CORONOR) registry, the rate of major adverse cardiovascular events (MACE) (eg, composite of cardiovascular death, MI, and stroke) was 3 times higher that in stable CAD patients without AF, at approximately 6.5% per year, and bleeding (Bleeding Academic Research Consortium (BARC) ≥3) was also 4.7 times higher at approximately 2.5% per year.3
Antithrombotic treatment of stable CAD outpatients is currently lifelong single antiplatelet therapy (APT).1 However, in patients with stable CAD and AF, the optimal antithrombotic regimen is still unknown. Given their high risk, identifying the optimal long‐term antithrombotic regimen in these patients is critical.11, 12, 13 These patients have in theory an indication to continue both a single APT and OAC, using either a vitamin K antagonist (VKA) or a direct oral anticoagulant, but European Society of Cardiology guidelines recommend the use of OAC alone in stable patients (ie, more than 12 months after percutaneous coronary intervention [PCI] or acute coronary syndromes).10, 14 Monotherapy using OAC is therefore the recommended lifelong treatment in this particular situation. However, there are little data to document the ischemic and bleeding risks of each strategy, using either OAC alone or combining OAC with APT.10, 15
Using the REduction of Atherothrombosis for Continued Health (REACH) registry, a large international, multiregional, observational cohort of stable patients with or at risk of atherothrombosis, we explored the profile and propensity‐adjusted outcomes of patients with stable CAD and AF receiving a VKA alone or with APT.
2. METHODS
2.1. Population of the REACH Registry
This study was conducted using data from the REACH registry. The REACH design and methods have been previously published in detail.16, 17, 18, 19 The study design was approved by local ethics board, and participants provided their written consent to participate. Briefly, the REACH registry is a large, prospective, international cohort of stable outpatients ages ≥45 years with either established atherothrombotic disease (CAD, peripheral artery disease and/or cerebrovascular disease [secondary prevention cohort]) or ≥3 risks factors of developing atherothrombosis (primary prevention cohort). Patients were enrolled between 2003 and 2004. Among all patients enrolled (n = 68 236), a total of 65 531 patients (96%) had follow‐up data available for primary outcomes.
The present analysis was restricted to patients with both stable CAD and AF at inclusion (n = 4767). We then further focused on patients under VKA at inclusion and with APT status available (n = 2347). Patients receiving VKA alone (n = 1481) were compared with those on a combination of VKA and APT (n = 866). The flowchart for the present analysis is displayed in Figure 1.
Figure 1.
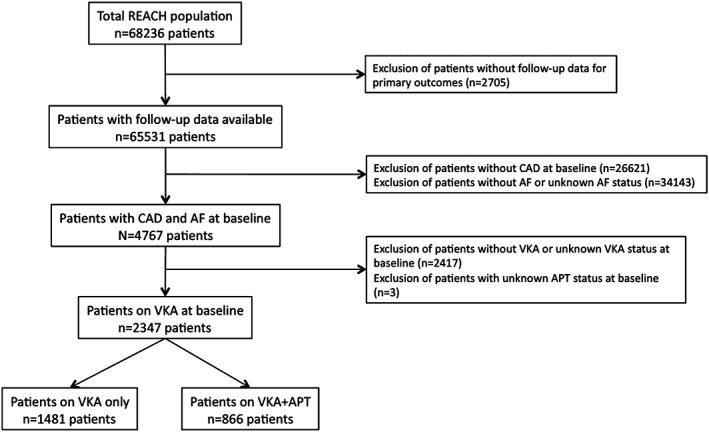
Flowchart of the study. Abbreviations: AF, atrial fibrillation; APT, antiplatelet therapy; CAD, coronary artery disease; REACH, Reduction of Atherothrombosis for Continued Health; VKA, vitamin K antagonist.
2.2. Definition of AF and OAC
Patients were categorized at enrollment based on the presence or absence of AF.4, 5 If physicians could not confirm whether patients had history of AF, patients were classified as “unknown” and were subsequently not considered for the present analysis. OAC was represented by VKA in all cases. Direct oral anticoagulants were not available at the time of the study initiation and during the 4‐year follow‐up.
2.3. Outcomes
Outcomes and their definitions in the REACH registry have been previously described.16, 17, 20 The primary outcome in the present study was major adverse cardiovascular events (MACE): a composite of cardiovascular death, myocardial infarction (MI), or stroke. Secondary outcomes included all‐cause death, cardiovascular death, MI, stroke and bleeding (defined by bleeding leading to hospitalization and also requiring transfusion, as previously published).20, 21 Outcomes were recorded by participating physicians and were not adjudicated, although stroke required documentation by a neurologist or hospital medical records.
2.4. Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Categorical variables are presented as absolute numbers and percentages.
Baseline characteristics and factors associated with APT use on top of VKA in the overall population of the present study were compared by using the χ2 test for categorical variables and the Student unpaired t test for continuous variables, as appropriate.
For the primary outcome (MACE), all‐cause death and each component of the primary outcome (cardiovascular death, MI, and stroke), cumulative event rates were estimated using the Kaplan‐Meier method and compared between groups by the log‐rank test. Cox proportional hazard model were conducted to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). The proportional hazards assumption was tested visually using Kaplan‐Meier curves. Because the dates of bleeding were not recorded in the database, this particular outcome was analyzed using logistic regression.
To account for differences in key characteristics according to APT use at baseline (Table 1), we calculated a propensity score, including as covariates age, sex, hypertension, diabetes mellitus, current smoker status, obesity, dyslipidemia, heart failure, history of PCI, history of MI, history of transient ischemic attack (TIA) or stroke, history of peripheral artery disease, β‐blocker use, statin use, and insulin use. Then, using propensity scoring, patients with APT use were matched 1‐to‐1 with patients without APT, using a SAS macro22 implementing a matching algorithm based on 8‐to‐1 digit matching (ratio of 1:1 without replacement), to obtain groups with similar baseline characteristics. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). Statistical significance was assumed at a P value <0.05. All tests were 2‐sided.
Table 1.
Baseline (demographic and clinical) characteristics of the population and of the 2 groups VKA only and VKA + APT
| Total Population, n = 2347 | VKA Only, n = 1481, 63.1% | VKA + APT, n = 866, 36.9% | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, mean ± SD | 73.17 ± 8.79 | 73.86 ± 8.59 | 71.98 ± 9.01 | <0.01 |
| Men, no. (%) | 1672 (71.2%) | 1023 (69.1%) | 649 (74.9%) | <.01 |
| Ethnic origin, no. (%) | ||||
| Caucasian | 1851 (84.44%) | 1170 (86.67%) | 681 (80.88%) | <0.01 |
| Hispanic | 50 (2.28%) | 34 (2.52%) | 16 (1.9%) | |
| East Asian | 115 (5.25%) | 37 (2.74%) | 78 (9.26%) | |
| South Asian | 6 (0.27%) | 2 (0.15%) | 4 (0.48%) | |
| Other Asian | 47 (2.14%) | 26 (1.93%) | 21 (2.49%) | |
| African American | 47 (2.14%) | 28 (2.07%) | 19 (2.26%) | |
| Other | 75 (3.42%) | 52 (3.85%) | 23 (2.73%) | |
| Cardiovascular risk factors, no. (%) | ||||
| Hypertension | 1927 (82.1%) | 1233 (83.3%) | 694 (80.1%) | 0.05 |
| Hyperlipidemia | 1655 (70.6%) | 1014 (68.5%) | 641 (74.1%) | <0.01 |
| Diabetes mellitus | 907 (38.9%) | 546 (37.2%) | 361 (41.9%) | 0.02 |
| Obesity | 708 (30.2%) | 451 (30.5%) | 257 (29.7%) | 0.69 |
| Current smoking | 164 (7.2%) | 92 (6.5%) | 72 (8.5%) | 0.07 |
| Heart failure, no. (%) | 1126 (48.7%) | 705 (48.3%) | 421 (49.5%) | 0.59 |
| Previous history of atherosclerotic disease, no. (%) | ||||
| History of PCI | 797 (34.3%) | 422 (28.7%) | 375 (43.9%) | <0.01 |
| History of CABG | 947 (40.6%) | 565 (38.4%) | 382 (44.4%) | <0.01 |
| History of MI | 1266 (54.8%) | 766 (52.5%) | 500 (58.6%) | <0.01 |
| History of MI within last year prior to inclusion, no. (%) | 257 (11.1%) | 130 (8.9%) | 127 (14.9%) | <0.01 |
| History of unstable angina | 448 (19.4%) | 249 (17.1%) | 199 (23.6%) | <0.01 |
| History of TIA | 377 (16.5%) | 229 (15.8%) | 148 (17.7%) | 0.24 |
| History of stroke | 433 (18.7%) | 278 (19%) | 155 (18.3%) | 0.66 |
| History of TIA or stroke | 659 (28.6%) | 422 (29%) | 237 (28.1%) | 0.65 |
| History of peripheral artery disease | 305 (13%) | 184 (12.4%) | 121 (14%) | 0.28 |
| Baseline medication use, no. (%) | ||||
| Aspirin | 735 (31.3%) | 0 (0%) | 735 (85.1%) | NA |
| Other APT | 223 (9.6%) | 0 (0%) | 223 (26%) | NA |
| Any APT | 866 (36.9%) | 0 (0%) | 866 (100%) | NA |
| Dual APT (aspirin + other APT) | 92 (3.9%) | 0 (0%) | 92 (10.7%) | NA |
| ≥1 Antihypertensive | 2169 (92.5%) | 1370 (92.6%) | 799 (92.3%) | 0.78 |
| β‐blocker | 1452 (62%) | 873 (59.1%) | 579 (67%) | <0.01 |
| Statin | 1626 (69.3%) | 987 (66.7%) | 639 (73.8%) | <.01 |
| ≥1 Diabetic medications | 777 (33.1%) | 457 (30.9%) | 320 (37%) | <.01 |
| Insulin | 276 (11.8%) | 158 (10.7%) | 118 (13.6%) | .03 |
| CHADS2 score, mean ± SD | 2.74 ± 1.38 | 2.77 ± 1.38 | 2.69 ± 1.38 | .20 |
| CHADS2VASc score, mean ± SD | 4.85 ± 1.62 | 4.92 ± 1.61 | 4.72 ± 1.62 | <.01 |
Abbreviations: APT, antiplatelet therapy; CABG, coronary artery bypass graft; MI, myocardial infarction; NA, not applicable; PCI, percutaneous coronary intervention; SD, standard deviation; TIA, transient ischemic attack; VKA, vitamin K antagonist.
3. RESULTS
3.1. Population, factors associated with APT use on top of VKA
The baseline characteristics of the 2347 patients with stable CAD and AF are summarized in Table 1. Their mean age was 73.2 years, and 38.9% of the patients had a history of diabetes. The rates of history of PCI and history of coronary artery bypass grafting (CABG) were 34.3% and 40.6%, respectively. A history of heart failure was present in 48.7% of the cases. The mean CHADS2 and CHA2DS2‐VaSc scores were 2.74 and 4.85, respectively. In this cohort, APT was used in addition to VKA in 866 patients (36.9%) at inclusion, including 92 (3.9%) patients on dual APT. Conversely, approximately two‐thirds of the patients (63.1%) received VKA alone.
There were important differences in baseline characteristics between patients according to the use of APT (Table 1). Patients in the VKA‐only group were older (73.9 vs 72 years) and less frequently men (69.1% vs 74.9%). They had more hypertension (83.3% vs 80.1%) and less diabetes (37.2% vs 41.9%). History of PCI was less frequent in the VKA‐only group (28.7% vs 43.9%). History of CABG was less frequent as well in the VKA‐only group (38.4% vs 44.4%). The rate of history of TIA or stroke was similar between both groups. The mean CHA2DS2‐VaSc score was 4.92 in the VKA only group vs 4.72 in the VKA + APT group (P < 0.01), whereas the CHADS2 score did not differ between groups.
After 1‐to‐1 propensity score matching, 2 subgroups of equal size were constituted (n = 755 in each group), which were well matched in terms of baseline characteristics (see Supporting Table 1 in the online version of this article).
3.2. Outcomes
Clinical follow‐up was obtained up to 4 years. In the whole study population, the unadjusted HR for the composite primary efficacy outcome (cardiovascular death, MI, or stroke) of patients with any APT use vs patients without APT use on top of VKA was 0.95 (95% CI: 0.69‐1.31), 25.5% vs 25%, P = 0.81). Figure 2 shows Kaplan‐Meier event curves for the primary efficacy outcome according to APT use in the overall study population. Similar results were observed in the adjusted analysis restricted to the propensity‐matched population (HR = 0.85 [95% CI: 0.64‐1.14], P = 0.27) (Figure 3). Of note, the rates of each component of the primary efficacy outcome as well as that of all‐cause mortality were similar between both groups (Figure 3).
Figure 2.
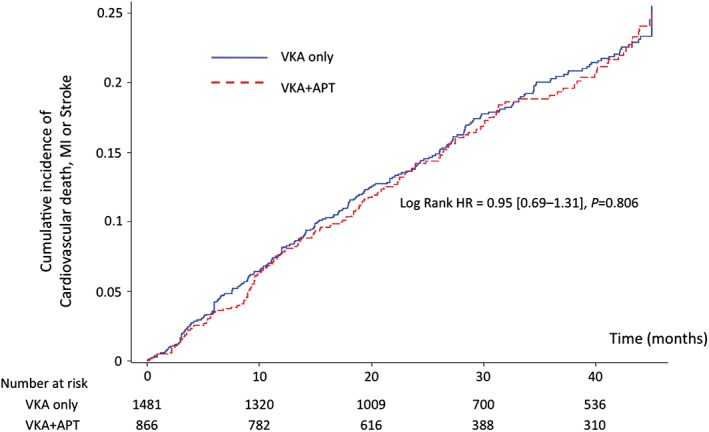
Cumulative incidence of cardiovascular death, myocardial infarction, or stroke in the overall population (unadjusted). The blue line represents the VKA‐only group, and the dotted red line represents the VKA + APT group. Abbreviations: APT, antiplatelet therapy; HR, hazard ratio; MI, myocardial infarction; VKA, vitamin K antagonist.
Figure 3.
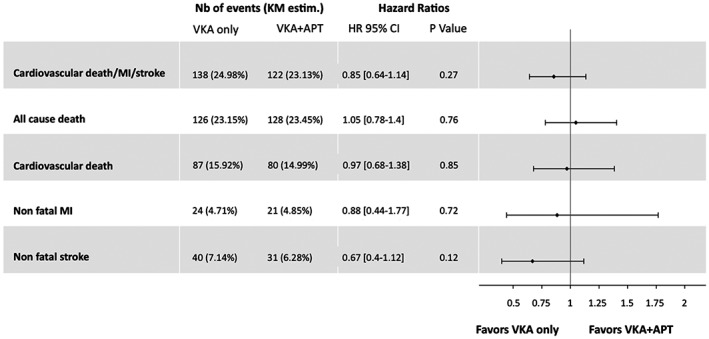
Adjusted comparison of the risk of ischemic outcomes between the VKA‐only and the VKA + APT groups, matched by propensity score (n = 755 patients in each group). Included as covariates in the model were age, sex, hypertension, diabetes mellitus, current smoker status, obesity, dyslipidemia, heart failure, history of percutaneous coronary intervention, history of MI, history of transient ischemic attack or stroke, and history of peripheral artery disease, β‐blocker use, statin use, and insulin use. Kaplan‐Meier estimates are derived from Kaplan‐Meier curves at end of follow‐up. Abbreviations: APT, antiplatelet therapy; CI, confidence interval; HR, hazard ratio; KM, Kaplan‐Meier, MI, myocardial infarction; Nb, number; VKA, vitamin K antagonist.
By contrast, there was a numerically, but not significantly, higher risk of bleeding in patients with APT use in the whole study population (unadjusted) (3.93% vs 2.57%, odds ratio [OR]: 1.55 [95% CI: 0.97‐2.48], P = 0.067). Likewise, there was a numerically higher risk of bleeding with combination APT and OAC in adjusted analyses restricted to the propensity‐matched population (OR: 1.87 [95% CI: 0.99‐3.50], P = 0.051).
Similar results were also observed in the overall population after adjustment on the propensity score, and these results were consistent, with no significant interaction across the major subgroups defined as a function of sex, age, diabetes, history of PCI, history of MI, CHADS2 score >2, and patients treated with aspirin alone (vs those under dual APT) on top of VKA (data not shown).
4. DISCUSSION
Patients with stable CAD and concomitant AF requiring long‐term OAC are frequent and present a therapeutic challenge in daily practice. In the present analysis of the REACH registry, the frequency of AF at inclusion was 12.3% among stable CAD patients, a proportion consistent with previous registries of stable CAD.3, 5, 6, 23
Long‐term aspirin is the cornerstone of stable CAD treatment and has been shown to reduce the risk of major cardiovascular events by approximately 20% to 25%.24 Therefore, the addition of aspirin to a long‐term OAC may in theory further improve outcomes in those patients who require a long‐term OAC for AF. However, this combination is likely to increase the risk of bleeding, which may counterbalance any benefit observed against ischemic events. For now, monotherapy using OAC is the recommended life‐long treatment in patients with stable CAD (after 12 months of stability) and AF. However, this is a largely "data‐free zone."10, 11 Most of the literature stems from cohorts or trials primarily dedicated to AF and focusing on the risk of bleeding related to combined antithrombotic therapy association.15, 25, 26, 27, 28, 29, 30, 31, 32 In these studies, approximately one‐third of AF patients are treated with the combination of OAC and APT, and these patients experience a higher risk of bleeding compared with patients on OAC only, as high as a 30% increase in major bleeding risk.11, 26, 27, 28, 29, 31 It should, however, be highlighted that the indication for APT in these post hoc analyses was not collected, as well as the degree of stability of the underlying CAD. Importantly, CAD was present in only 40% to 60% of the patients, even in the subgroups of patients on OAC and APT at inclusion.11, 30
Recently, Hamon et al reported similar conclusions in a subanalysis (n = 4149 patients) of the CORONOR registry focusing on the risk of bleeding related to VKA prescription in outpatients with stable CAD.3 In that study, 461 patients were under a VKA at inclusion (whatever the indication, analysis not limited to AF patients). The strongest predictor of bleeding (as defined by BARC ≥3 bleeding) was prescription of a VKA (HR: 4.69 [95% CI: 2.68.44], P < 0.0001, compared with aspirin alone) and the combination of APT and VKA resulted in a substantial further increase in bleeding risk (HR: 7.3 [95% CI: 3.91–13.64], P < 0.0001). However, results directly comparing a VKA alone and a VKA + APT regarding the risk of bleeding were not available in this post hoc analysis of the CORONOR registry. Authors also reported that patients on a VKA alone (n = 119) had a similar risk of cardiovascular death, MI, and stroke at 2 years than patients on a VKA and any additional APT (n = 342) after adjustment for confounders. However, that study was underpowered for analyzing ischemic events.
To the best of our knowledge, the present analysis is the first to describe, within the same cohort of outpatients with stable CAD and AF, the outcomes of OAC alone vs OAC and APT in terms of both ischemic and bleeding risk. Our results suggest that the addition of any APT to VKA in such patients is not associated with a lower risk of ischemic events (cardiovascular death, MI, or stroke) or all‐cause mortality. There was, however, a strong suggestion that patients receiving a combination of a VKA and APT had a higher risk of bleeding than patients on a VKA only. The risk of bleeding at 4 years was 90% higher in patients on a VKA and APT at inclusion after propensity score matching (P = 0.051).
4.1. Limitations
Our analysis has important limitations. As with all observational studies, there are differences between patients receiving a VKA with vs without APT. Although propensity score matching adjusts for the major known confounders, its power was limited, and unmeasured confounders cannot be ruled out. Therefore, the findings obtained with this approach are hypothesis generating rather than definitive.
The study was relatively modest in size and therefore has limited power to discriminate outcomes between groups. In that respect, lack of definitive evidence of a benefit of a combination of APT and VKA does not rule out real and clinically meaningful effects. However, considering the observed event rate of a MACE of 25% in the group under a VKA alone at the end of follow‐up in our study, 860 patients in each group provide 80% power (using an unilateral test) to detect a 20% risk reduction in a MACE (a risk reduction observed with aspirin in CAD in the literature24) in the group receiving APT and OAC compared to OAC alone, with an α risk of 0.05.
In addition, the comparison was based on APT use at baseline and does not account for subsequent changes in treatment.
The present analysis only pertains to VKA, as direct oral anticoagulants were not yet used at the time of data acquisition. As shown in Table 2, recent large international registries, including unselected AF patients, highlighted that direct OAC are getting more frequently used in real practice.8, 33, 34, 35 These agents have generally been shown to reduce the risk of bleeding when compared with VKA, and may offer a safer method for combining OAC and APT. Alternatively, there may be benefits of a low‐dose direct OAC alone for coronary events in stable patients, an issue that is currently being explored in the COMPASS trial (Cardiovascular OutcoMes for People Using Anticoagulation StrategieS; ClinicalTrials.gov Identifier NCT01776424). If this were the case, combining OAC with antiplatelet agents might be unnecessary to provide optimal protection against coronary events in patients receiving a long‐term OAC. Finally, our results are not applicable to the patients receiving OAC for other indications than AF.
Table 2.
Main registries focusing on antithrombotic management in atrial fibrillation since direct oral anticoagulant apparition
| Registry | Year of Publication | No. of Patients | Period of Inclusion | Design | Inclusion Criteria | Rate of OAC Use | Rate of Direct OAC Use | Rate of APT Use in Combination to Any OAC in Patients Under OAC |
|---|---|---|---|---|---|---|---|---|
| EORP‐AF | 2014 | 3119 | February 2012–March 2013 | Prospective registry, consecutive patients, multicenter (9 countries) | Inpatients and outpatients with AF, mean CHA2DS2VASc = 3.24 | 80% | 8.4% | 20.1% (16.4% + SAPT and 3.7% + DAPT) |
| PREFER in AF | 2014 | 7243 | January 2012–January 2013 | Prospective registry, consecutive patients, multicenter (7 countries) | History of AF within the preceding 12 months, mean CHA2DS2VASc = 3.37 | 82.3% | 6.1% | 10.9% (9.1% + SAPT and 1.6% + DAPT) |
| GARFIELD‐AF | 2017 | 39 670 | 2010–2015, 4 cohorts, C1: March 2010–October 2011, C2: August 2011–June 2013 , C3: April 2013–October 2014, C4: March 2014–July 2015 | Prospective registry, multicenter (35 countries) | Recently diagnosed nonvalvular AF (within 6 weeks), CHA2DS2VASc ≥1, median CHA2DS2VASc = 3 | 64.7% | 22.4% | 14.1% |
| GLORIA‐AF | 2017 | 15 641 | November 2011–December 2014 | Prospective registry, consecutive patients, multicenter (46 countries) | Newly diagnosed AF, CHA2DS2VASc ≥1, median CHA2DS2VASc = 3 | 79.9% | 47.6% | Not reported |
Abbreviations: AF, atrial fibrillation; APT, antiplatelet therapy; CAD, coronary artery disease; DAPT, dual antiplatelet therapy; EORP, EurObservational Research Programme–Atrial Fibrillation; GARFIELD‐AF, Global Anticoagulant Registry in the FIELD‐Atrial Fibrillation; GLORIA‐AF, Global Registry on Long‐Term Oral Anti‐thrombotic Treatment in Patients With Atrial Fibrillation; OAC, oral anticoagulation; PREFER in AF, Prevention of Thromboembolic Events—European Registry in Atrial Fibrillation; SAPT, single antiplatelet therapy.
5. CONCLUSION
The overlap between AF and stable CAD is not rare and exceeds 10% of cases. Currently, two‐thirds of these patients receive OAC alone, and one‐third a combination of OAC and APT. In the present cohort, the use of APT in addition to OAC in patients with stable CAD and AF was not associated with lower rates of ischemic outcomes (cardiovascular death, MI, or stroke) or all‐cause mortality compared with patients on OAC only. By contrast, there was a strong signal for an increased risk of bleeding. Our results are supporting, and may add some degree of evidence for, the most current guidelines, mainly based on expert opinion to date. Dedicated randomized trials are needed to guide physician decision making in this relatively frequent setting.
Conflicts of interest
Dr. Gilles Lemesle has received fees from Astra Zeneca, Bayer, Biopharma, Bristol‐Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, MSD, Lilly, Pfizer, sanofi‐aventis, Servier, and The Medicines Company as speaker and advisory board. Dr. Gregory Ducrocq has received speaker or consulting fees from Astra Zeneca, Biotronik, Bristol‐Myers Squibb, Daiichi Sankyo, and Lilly. Mr. Yedid Elbez has no conflicts of interest. Dr. Eric Van Belle has no conflicts of interest. Dr. Shinya Goto discloses the following relationships: advisory board: Medscape Cardiology; data monitoring committees: TIMI Study Group in Harvard University, Population Health Research Institute in McMaster University, Armethron; honoraria: the American Heart Association (Associate Editor, Circulation), Thrombosis Research Institute at the University College of London (clinical trial steering committees), TIMI Study Group in Harvard University (clinical trial steering committees), Thrombosis and Haemostasis (Section Editor), Archives of Medicine (Associate Editor), Journal of Cardiology Cases (Associate Editor), other: paid lecturer for sanofi‐aventis; research funding: sanofi‐aventis, Pfeizer, the Japanese Society of Promotion of Science, and Riken. Dr. Christopher P. Cannon discloses the following relationships: research grants from Amgen, Arisaph, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Janssen, Merck, and Takeda; and consulting fees from Alnylam, Amgen, Arisaph, Astra Zeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb, GlaxoSmithKline, Kowa, Merck, Takeda, Lipimedix, Pfizer, Regeneron, and sanofi‐aventis. Dr. Christophe Bauters has received travel grants from MSD‐Schering, Boehringer‐Ingelheim, and Servier. Dr. Deepak L. Bhatt discloses the following relationships: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; data monitoring committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Medtronic, Pfizer, Roche, sanofi‐aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Coinvestigator: Biotronik, Boston Scientific, St. Jude Medical; Trustee: American College of Cardiology; Unfunded Research: FlowCo, PLx Pharma, Takeda. Dr. Ph.Gabriel Steg discloses the following relationships: research grants from Merck, sanofi‐aventis, and Servier; speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myers‐Squibb, CSL‐Behring, GlaxoSmithKline, Janssen, Lilly, Merck, Novartis, Pfizer, Regeneron, sanofi‐aventis, Servier, The Medicines Company.
A full list of the REACH registry investigators can be found in Bhatt DL, Steg PG, Ohman EM, et al. JAMA. 2006;295:180–189.
Supporting information
Supplemental Table 1: Propensity‐matched subgroups. Baseline (demographic and clinical) characteristics in both groups: VKA only users versus VKA + APT users after matching on their propensity score (n = 755 patients in each group).
Lemesle G, Ducrocq G, Elbez Y, et al. Vitamin K antagonists with or without long‐term antiplatelet therapy in outpatients with stable coronary artery disease and atrial fibrillation: Association with ischemic and bleeding events. Clin Cardiol. 2017;40:932–939. 10.1002/clc.22750
Funding information The REACH registry was supported up to 2013 by sanofi‐aventis, Bristol‐Myers Squibb, and the Waksman Foundation, and is endorsed by the World Heart Federation.
REFERENCES
- 1. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation . 2012;126:e354–e471. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 3. Hamon M, Lemesle G, Tricot O, et al. Incidence, source, determinants, and prognostic impact of major bleeding in outpatients with stable coronary artery disease. J Am Coll Cardiol. 2014;64:1430–1436. [DOI] [PubMed] [Google Scholar]
- 4. Ruff CT, Bhatt DL, Steg PG, et al. Long‐term cardiovascular outcomes in patients with atrial fibrillation and atherothrombosis in the REACH Registry. Int J Cardiol. 2014;170:413–418. [DOI] [PubMed] [Google Scholar]
- 5. Goto S, Bhatt DL, Rother J, et al. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156:855–863, 863.e2. [DOI] [PubMed] [Google Scholar]
- 6. Fauchier L, Greenlaw N, Ferrari R, et al. Use of anticoagulants and antiplatelet agents in stable outpatients with coronary artery disease and atrial fibrillation. International CLARIFY Registry. PLoS One. 2015;10:e0125164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steg PG, Greenlaw N, Tardif JC, et al. Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective CLARIFY registry. Eur Heart J. 2012;33:2831–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lip GY, Laroche C, Dan GA, et al. A prospective survey in European Society of Cardiology member countries of atrial fibrillation management: baseline results of EURObservational Research Programme Atrial Fibrillation (EORP‐AF) Pilot General Registry. Europace. 2014;16:308–319. [DOI] [PubMed] [Google Scholar]
- 9. Bernard A, Fauchier L, Pellegrin C, et al. Anticoagulation in patients with atrial fibrillation undergoing coronary stent implantation. Thromb Haemost. 2013;110:560–568. [DOI] [PubMed] [Google Scholar]
- 10. Lip GY, Windecker S, Huber K, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia‐Pacific Heart Rhythm Society (APHRS). Eur Heart J . 2014;35:3155–3179. [DOI] [PubMed] [Google Scholar]
- 11. Schurtz G, Bauters C, Ducrocq G, et al. Impact of the adjunction of aspirin on top of oral anticoagulants in stable coronary artery disease outpatients with an indication for anticoagulation. Panminerva Med. 2016;58:271–285. [PubMed] [Google Scholar]
- 12. Depta JP, Bhatt DL. Atherothrombosis and atrial fibrillation: Important and often overlapping clinical syndromes. Thromb Haemost. 2010;104:657–663. [DOI] [PubMed] [Google Scholar]
- 13. Steg PG, Bhatt DL. Viewpoint: a proposal for a simple algorithm for managing oral anticoagulation and antiplatelet therapy in patients with non‐valvular atrial fibrillation and coronary stents. Eur Heart J Acute Cardiovasc Care. 2017;6:93–97. [DOI] [PubMed] [Google Scholar]
- 14. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 15. Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433–1441. [DOI] [PubMed] [Google Scholar]
- 16. Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 17. Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 18. Alberts MJ, Bhatt DL, Mas JL, et al. Three‐year follow‐up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J. 2009;30:2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steg PG, Bhatt DL, Wilson PW, et al. One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. [DOI] [PubMed] [Google Scholar]
- 20. Ducrocq G, Wallace JS, Baron G, et al. Risk score to predict serious bleeding in stable outpatients with or at risk of atherothrombosis. Eur Heart J. 2010;31:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alberts MJ, Bhatt DL, Smith SC Jr, et al. Risk factors and outcomes for patients with vascular disease and serious bleeding events. Heart. 2011;97:1507–1512. [DOI] [PubMed] [Google Scholar]
- 22. Parsons LS. Performing a 1:n case–control match on propensity score. Paper presented at: Twenty‐Ninth Annual SAS Users Group International (SUGI) Conference; May 9–12, 2004; Montreal, Canada. [Google Scholar]
- 23. Aguilar E, Garcia‐Diaz AM, Sanchez Munoz‐Torrero JF, et al. Clinical outcome of stable outpatients with coronary, cerebrovascular or peripheral artery disease, and atrial fibrillation. Thromb Res. 2012;130:390–395. [DOI] [PubMed] [Google Scholar]
- 24. Antithrombotic Trialists' Collaboration . Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ . 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander JH, Lopes RD, Thomas L, et al. Apixaban vs. warfarin with concomitant aspirin in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J . 2014;35:224–232. [DOI] [PubMed] [Google Scholar]
- 26. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 27. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 28. Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long‐Term Anticoagulation Therapy (RE‐LY) trial. Circulation. 2013;127:634–640. [DOI] [PubMed] [Google Scholar]
- 29. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 30. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) Registry. Circulation. 2013;128:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu H, Ruff CT, Giugliano RP, et al. Concomitant use of single antiplatelet therapy with edoxaban or warfarin in patients with atrial fibrillation: analysis from the ENGAGE AF‐TIMI48 Trial. J Am Heart Assoc . 2016;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lamberts M, Gislason GH, Lip GY, et al. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation. 2014;129:1577–1585. [DOI] [PubMed] [Google Scholar]
- 33. Camm AJ, Accetta G, Ambrosio G, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart. 2017;103:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Caterina R, Ammentorp B, Darius H, et al. Frequent and possibly inappropriate use of combination therapy with an oral anticoagulant and antiplatelet agents in patients with atrial fibrillation in Europe. Heart. 2014;100:1625–1635. [DOI] [PubMed] [Google Scholar]
- 35. Huisman MV, Rothman KJ, Paquette M, et al. The changing landscape for stroke prevention in AF: findings from the GLORIA‐AF Registry Phase 2. J Am Coll Cardiol. 2017;69:777–785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Propensity‐matched subgroups. Baseline (demographic and clinical) characteristics in both groups: VKA only users versus VKA + APT users after matching on their propensity score (n = 755 patients in each group).


