Abstract
Background
Heart failure is a significant cause of morbidity and mortality, yet patient risk stratification may be difficult. Prevention or treatment of atrial fibrillation (AF) may be an important strategy in these patients that could positively affect their outcome. It has been demonstrated that in patients with systolic dysfunction, prolonged QRS duration (QRSd), an easily measured electrocardiographic parameter, is associated with AF.
Hypothesis
Prolonged QRSd is associated with an increase in prevalence of AF in patients with heart failure with preserved ejection fraction(HFPEF).
Methods
Between February 2006 and February 2009, 718 patients were discharged with a diagnosis of HF from the Dartmouth‐Hitchcock Medical Center. Of these, 206 had EF ≥50% by echocardiography performed within 72 hours of admission. After exclusions, 82 patients remained, of which 25 had AF and 57 had sinus rhythm. Characteristics of the AF and sinus‐rhythm patients were compared in this pilot study.
Results
After adjustment for age, prior diagnosis of HF, and left atrial area, there was a nonsignificant trend (odds ratio: 2.2, 95% CI of 0.3‐17.2) for a QRSd >120 ms to be associated with AF.
Conclusions
Similar to results in patients with systolic dysfunction, patients with preserved EF may have an association between a prolonged QRSd and AF.
Keywords: Atrial Fibrillation, Heart Failure, Diastolic Heart Failure, Heart Failure With Preserved Ejection Fraction, QRS Duration
1. INTRODUCTION
Patients hospitalized for heart failure with reduced or preserved ejection fraction (HFrEF or HFpEF, respectively) have been shown to have similarly high rates of rehospitalization and mortality.1 Electrocardiographic (ECG) parameters are readily available and inexpensive, and may thus be useful indicators of future morbidity and mortality in HF patients. For instance, it has been demonstrated that QRS duration (QRSd) is an independent risk factor for mortality in patients with either HFrEF or HFpEF.1, 2, 3 In patients with HFpEF, QRSd has also been shown to predict a composite of adverse outcomes.4, 5
Atrial fibrillation (AF) is another ECG finding that has important prognostic information in HF, increasing mortality6, 7 or a combined endpoint of mortality/rehospitalization,8 regardless of EF. QRSd and AF may interrelate with regard to the development of AF, and also to prognosis once AF develops. In a study of 25,268 patients with left ventricular (LV) systolic dysfunction, 42% had a QRSd >120 ms, and QRSd was independently associated with AF.9 In addition, irrespective of HF status, QRSd has been shown to be an independent predictor of morbidity and mortality among patients with AF.10
To our knowledge, there are no studies where the primary objective was to examine ECG findings in patients with HFpEF, with and without AF. The purpose of this study was to examine whether QRSd is associated with AF prevalence in patients with HFpEF.
2. METHODS
We performed a retrospective data collection and analysis. Between February 2006 and February 2009, 718 patients were discharged with the diagnosis of HF from the Dartmouth‐Hitchcock Medical Center. Of these, 206 patients had EF ≥50% by echocardiography performed within 72 hours of admission.
After excluding patients with paced rhythm, atrial flutter, and severe valvular disease, and patients who did not meet Framingham criteria for HF, 82 patients remained and became our study cohort. Of these, 25 had AF on the admission ECG and 57 had sinus rhythm (SR). Clinical, echocardiographic, and ECG data were collected. QRSd, QRS axis, heart rate (HR), and corrected QT interval (QTc) were obtained from the automated measurement algorithm of the General Electric MUSE version 7 ECG system (GE Healthcare, Wauwatosa, WI).
2.1. Statistical analysis
Characteristics of the AF and SR patients were compared using the χ2 test, Student t test, and Wilcoxon rank‐sum test where appropriate.
The primary variable, QRSd, was assessed as a dichotomous variable (QRSd > or <120 ms) for its association with AF, using multivariate logistic analysis. Age, prior diagnosis of HF, and left atrial (LA) area have been shown to be correlated with AF11, 12 and were adjusted for in our analysis. Additionally, we included HR, as it was significantly different in the baseline analysis and may mechanistically be associated with AF and QRSd (aberrant conduction). Creatinine, although statistically different between the SR and AF groups (lower in the AF group), was not adjusted for, as a low normal creatinine has not previously been recognized as a risk factor for AF.
This was an exploratory analysis of a well‐defined cohort of patients with HFpEF. It was underpowered to prove the hypothesis that QRSd was a risk factor for AF, as this would have required a combined total of 3,592 patients with and without AF (assuming 90% power and 2‐tailed α of 0.05).
3. RESULTS
In this population of HFpEF patients, 30.5% had AF. Those with AF were notably older (mean age, 79 vs 69 years; P = 0.001; Table 1) and more likely to have a history of HF (80% vs 35%, P < 0.001; Table 1). Unexpectedly, AF patients also had lower creatinine on average (1.13 vs 1.91 µmol/L, P = 0.023; Table 1). Echocardiographically, AF patients had larger LA areas (30.0 vs 21.2 cm2, P < 0.001; Table 1), but there was no significant difference in E/e′ or deceleration time.
Table 1.
Baseline clinical and echocardiographic characteristics
| Characteristics | Patients With HFpEF and SR | Patients With HFpEF and AF | P Valuea |
|---|---|---|---|
| N | 57 (69.5) | 25 (30.5) | — |
| Age, y | 68.9 ± 12.7 | 78.5 ± 9.5 | 0.001 |
| Male sex | 24 (42.1) | 11 (44.0) | 0.873 |
| History of HF | 20 (35.1) | 20 (80.0) | <0.001 |
| History of HTN | 46 (80.7) | 18 (72.0) | 0.381 |
| History of CAD | 31 (54.4) | 16 (64.0) | 0.418 |
| sCr, µmol/L | 1.9 ± 1.7 | 1.1 ± 0.4 | 0.023 |
| GFR, mL/min | 51.4 ± 31.6 | 61.1 ± 23.1 | 0.172 |
| Hgb, mmol/L | 11.6 ± 2.3 | 11.9 ± 1.7 | 0.556 |
| History of DM | 32 (56.1) | 11 (44.0) | 0.311 |
| BMI, kg/m2 | 36.6 ± 22.5 | 30.4 ± 10.6 | 0.344 |
| E/e′ | 13.6 ± 6.0 | 14.4 ± 6.5 | 0.594 |
| LA area, cm2 | 21.2 ± 5.3 | 30.0 ± 8.7 | <0.001 |
| DT, ms | 213.0 ± 61.8 | 195.0 ± 59.7 | 0.225 |
| Pro‐BNP, pg/mL | 4951 ± 7576 | 6019 ± 5789 | 0.598 |
| SBP, mm Hg | 135.3 ± 24.8 | 124.5 ± 22.0 | 0.065 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; BNP, brain natriuretic peptide; CAD, coronary artery disease; DM, diabetes mellitus; DT, deceleration time; GFR, glomerular filtration rate; HF, heart failure; HFpEF, heat failure with preserved ejection fraction; Hgb, hemoglobin; HTN, hypertension; LA, left atrial; SBP, systolic blood pressure; sCr, serum creatinine; SD, standard deviation; SR, sinus rhythm.
Data are presented as n (%) or mean ± SD.
Unadjusted.
Before adjustment (Figure 1, Table 2), there was no significant difference between the SR and AF groups in mean QRSd (SR 103.0 vs AF 99.1 msec, P = 0.48; Table 2) or in the proportion of patients with a QRSd >120 ms (SR 15.8% vs AF 20.0%, P = 0.64; Table 2). QTc was similar in the 2 groups (SR 442.8 ms vs AF 447.3 ms, P = 0.58). HR was greater in the AF group, and this approached statistical significance (SR 77.7 bpm vs AF 86.6 bpm, P = 0.053). A box plot of QRS duration for patients in both groups demonstrated overlap of the middle 50% and 95% ranges (P = 0.59; Figure 1).
Figure 1.
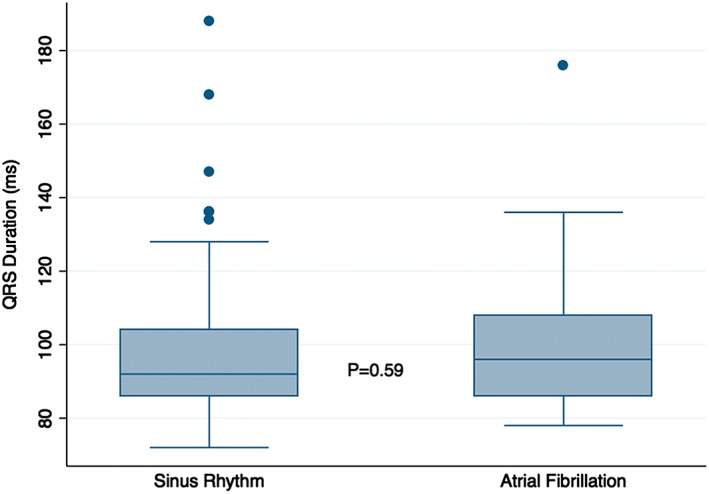
Box plot of QRSd, SR vs AF, with 50% and 95% ranges shown. Abbreviations: AF, atrial fibrillation; QRSd, QRS duration; SR, sinus rhythm.
Table 2.
Electrocardiographic characteristics
| Parameter | SR | AF | P Value, t Test |
|---|---|---|---|
| Mean QRSd, ms | 99.1 ± 22.9 | 103.0 ± 23.3 | 0.476 |
| % Patients with QRS >120 ms | 15.8 | 20.0 | 0.641 |
| QTc, ms | 442.8 ± 32.8 | 447.3 ± 35.3 | 0.583 |
| HR, bpm | 77.7 ± 16.1 | 86.6 ± 24.2 | 0.053 |
Abbreviations: AF, atrial fibrillation; HR, heart rate; QRSd, QRS duration; QTc, corrected QT interval; SR, sinus rhythm.
After adjustment for age (odds ratio [OR]: 1.2), history of HF (OR: 31.5), LA area (OR: 1.6), and HR (OR 1.2), there was a nonsignificant trend towards the association of a QRSd >120 ms with AF (OR: 2.2, 95% CI: 0.3‐17.2, Table 3).
Table 3.
Multivariate analysis with discrete QRSd
| Parameter | OR | 95% CI | P Value |
|---|---|---|---|
| QRSd >120 ms | 2.2 | 0.3‐17.2 | 0.463 |
| Age | 1.2 | 1.1‐1.3 | 0.006 |
| History of HF | 31.5 | 1.8‐565.4 | 0.019 |
| LA area | 1.6 | 1.2‐2.1 | 0.001 |
| HR | 1.1 | 1.0‐1.2 | 0.002 |
Abbreviations: CI, confidence interval; HF, heart failure; HR, heart rate; LA, left atrial; OR, odds ratio; QRSd, QRS duration.
4. DISCUSSION
HF is a significant cause of morbidity and mortality, and accurate risk assessment is essential for improving patient outcomes.13 AF is associated with an increased risk of mortality in HF patients,6, 7 and prevention or early detection of AF may be an effective way to reduce morbidity and mortality in these patients. However, “silent” AF poses a challenge with regard to early identification of patients for intervention.14, 15, 16
As our ability to detect asymptomatic AF is suboptimal in the absence of an implanted pacemaker, defibrillator, or loop recorder, QRSd might be a useful parameter to select patients for more aggressive screening. In a large study of patients with LV systolic dysfunction, QRSd was independently associated with AF.9 Mechanistically, a prolonged QRSd could facilitate the onset of AF by causing ventricular dyssynchrony, which in turn can cause adverse ventricular and atrial remodeling due to a reduction in the peak rate of rise in the LV pressure, reduced diastolic filling time, and increased mitral regurgitation.17, 18 The purpose of our pilot study was to determine if QRSd is associated with AF prevalence in HFpEF.
In this study, age, prior history of HF, LA area, and HR were independent predictors of AF in HFpEF. The first 3 parameters have predicted AF in previous studies in different patient populations.11, 12 Though more patients with AF had a QRSd >120 ms (20.0%, vs 15.8% of those in SR), this trend did not reach statistical significance. Interestingly, the 17% prevalence of QRS prolongation >120 ms in our cohort is similar to the 18% prevalence in larger cohorts of HFpEF,2, 5 and it is a higher prevalence than in the general population.2 This suggests that the pathophysiology of HFpEF (potentially via LV hypertrophy, ischemia, or other causes of fibrosis) contributes to QRS prolongation by causing fibrosis in the conduction system and/or myocardium.19 QRSd as a surrogate for myocardial fibrosis might explain in part the graded risk for cardiovascular mortality for every 10‐ms increase in QRSd, as observed in a cohort of 46,933 consecutive patients.20
We are aware of no other studies with a primary goal of assessing the relationship between QRSd and AF in HFpEF. In a study of 25,171 patients in the Swedish Heart Failure Registry, ≥4,783 patients had an EF of ≥50%, and 18% had a QRSd of ≥120 ms. In this study, AF prevalence was less frequent in those with a wide QRS—42% with a QRSd of ≥120 ms, vs 47% with a QRSd of <120 ms (P < 0.001). In this analysis of AF, patients with preserved and reduced EFs were combined and not separated by HF type.2 In the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial of 3,426 patients, the proportion of patients with a QRSd of ≥120 ms was again 18%. In this study, AF prevalence was more frequent in those with a QRSd of ≥120 ms (45%) vs those with a QRSd of <120 ms (33%; P < 0.0001).5
There are a number of differences between our patient population and the patients enrolled in TOPCAT, besides sample size. We identified patients at the time of hospitalization, whereas TOPCAT enrolled outpatients—although 72% of the TOPCAT patients had been hospitalized in the prior year. The EF cutoff was ≥50% in our study and ≥45% in TOPCAT, and TOPCAT excluded patients with AF and a mean ventricular rate >90 bpm.
4.1. Study limitations
Our analysis is a retrospective, observational pilot study without the ability to establish a causal relationship. The lack of longitudinal data is also a limitation. The study was not powered to allow a definitive determination of an association between QRSd and AF in the HFPEF population. The limited sample size does also not allow for subset analysis or stratification by QRSd to study a threshold effect (e.g., >120 ms vs >140 ms).
We used retrospective application of the Framingham criteria for HF to validate the initial index diagnosis of HF. This relies on the accuracy of documentation of subjective and objective data at the time of admission. We controlled for known, measurable confounders, but there may be other confounders in this population for which we were unable to control.
5. CONCLUSION
In this cohort of patients with HFpEF, we observed a nonsignificant trend for QRS prolongation (>120 ms) to be associated with AF. This finding is consistent with a large registry of patients with reduced EF and a smaller trial of HFpEF.5, 9 A large prospective trial is warranted to better understand whether ECG parameters including QRSd can predict the development of AF in HFPEF patients.
Conflicts of interest
The authors declare no potential conflicts of interest.
Gigliotti JN, Sidhu MS, Robert AM, et al. The association of QRS duration with atrial fibrillation in a heart failure with preserved ejection fraction population: a pilot study. Clin Cardiol. 2017;40:861–864. 10.1002/clc.22736
REFERENCES
- 1. Tsuchihashi‐Makaya M, Hamaguchi S, Kinugawa S, et al. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction: report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE‐CARD) [published correction appears in Circ J. 2009;73:2365]. Circ J . 2009;73:1893–1900. [DOI] [PubMed] [Google Scholar]
- 2. Lund LH, Jurga J, Edner M, et al. Prevalence, correlates, and prognostic significance of QRS prolongation in heart failure with reduced and preserved ejection fraction. Eur Heart J. 2013;34:529–539. [DOI] [PubMed] [Google Scholar]
- 3. Wang NC, Maggioni AP, Konstam MA, et al; EVEREST Investigators . Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299:2656–2666. [DOI] [PubMed] [Google Scholar]
- 4. Hummel SL, Skorcz S, Koelling TM. Prolonged electrocardiogram QRS duration independently predicts long‐term mortality in patients hospitalized for heart failure with preserved systolic function. J Card Fail. 2009;15:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joseph J, Claggett BC, Anand IS, et al. QRS duration is a predictor of adverse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:477–486. [DOI] [PubMed] [Google Scholar]
- 6. Dries DL, Exner DV, Gersh BJ, et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic function: a retrospective analysis of the SOLVD trials. J Am Coll Cardiol. 1998;32:695–703. [DOI] [PubMed] [Google Scholar]
- 7. Parkash R, Maisel WH, Toca FM, et al. Atrial fibrillation in heart failure: high mortality risk even if ventricular function is preserved. Am Heart J. 2005;150:701–706. [DOI] [PubMed] [Google Scholar]
- 8. Zareba KM, Shenkman HJ, Bisognano JD. Comparison of acute electrocardiographic presentation in patients with diastolic vs systolic heart failure. Congest Heart Fail. 2009;15:165–169. [DOI] [PubMed] [Google Scholar]
- 9. El‐Chami MF, Brancato C, Langberg J, et al. QRS duration is associated with atrial fibrillation in patients with left ventricular dysfunction. Clin Cardiol. 2010;33:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whitbeck MG, Charnigo RJ, Shah J, et al; AFFIRM Investigators . QRS duration predicts death and hospitalization among patients with atrial fibrillation irrespective of heart failure: evidence from the AFFIRM study. Europace . 2014;16:803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assn. 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenberg MA, Manning WJ. Diastolic dysfunction and risk of atrial fibrillation: a mechanistic appraisal. Circulation. 2012;126:2353–2362. [DOI] [PubMed] [Google Scholar]
- 13. Rogers JG. Defining and refining heart failure risk stratification to optimize patient selection for cardiac transplantation. Circ Heart Fail. 2013;6:2–3. [DOI] [PubMed] [Google Scholar]
- 14. Hickey KT, Reiffel J, Sciacca RR, et al. The utility of ambulatory electrocardiographic monitoring for detecting silent arrhythmias and clarifying symptom mechanism in an urban elderly population with heart failure and hypertension: clinical implications. J Atr Fibrillation. 2010;1:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high‐risk patients: the screening study for undiagnosed atrial fibrillation (STUDY‐AF). Clin Cardiol. 2015;38:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guenancia C, Binquet C, Laurent G, et al. Incidence and predictors of new‐onset atrial fibrillation in septic shock patients in a medical ICU: data from 7‐day Holter ECG monitoring. PLoS One. 2015;10:e0127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amiya E, Tanabe K, Ikari Y, et al. Prolonged QRS duration and severity of mitral regurgitation are unfavorable prognostic markers of heart failure in patients with nonischemic dilated cardiomyopathy. Circ J. 2006;70:57–62. [DOI] [PubMed] [Google Scholar]
- 18. Sade LE, Atar I, Özin B, et al. Determinants of new‐onset atrial fibrillation in patients receiving CRT: mechanistic insights from speckle tracking imaging. JACC Cardiovasc Imaging. 2016;9:99–111. [DOI] [PubMed] [Google Scholar]
- 19. Shantsila E, Shantsila A, Blann AD, et al. Left ventricular fibrosis in atrial fibrillation. Am J Cardiol. 2013;111:996–1001. [DOI] [PubMed] [Google Scholar]
- 20. Desai AD, Yaw TS, Yamazaki T, et al. Prognostic significance of quantitative QRS duration. Am J Med. 2006;119:600–606. [DOI] [PubMed] [Google Scholar]


