Abstract
BACKGROUND:
Chemicals that enter the body, especially benzene, will undergo a detoxification process. Unfortunately, at the detoxification process, sometimes benzene can produce free radicals. Free radical oxidation of lipids produces MDA compounds (malondialdehyde). To overcome these free radicals, the body will adapt to produce Glutathione (GSH) enzymes.
AIM:
The purpose of this study was to analyse the relationship between benzene concentration, MDA levels and glutathione enzymes in Shoe-Maker Home Industry workers exposed to benzene for more than 10 years.
METHODS:
Measurement of benzene concentration using a gas chromatography-flame ionisation detector (GC-FID). MDA levels used a modified spectrophotometric and GSH method of thiobarbituric acid (TBA) test.
RESULT:
The results showed that the majority of respondents had benzene concentrations still below the TLV value, mean of MDA levels were 6.94 mg/ml, while GSH was 4.54 mg/ml. Benzene concentration did not have a significant correlation with MDA and glutathione levels, whereas MDA levels had a strong correlation with glutathione levels (p = 0.000; r = -0.947).
CONCLUSION:
Workers should always use PPE and always eat foods that contain lots of glutathione enzymes such as spinach or broccoli to reduce the impact of free radicals from benzene inhalation.
Keywords: Benzene, MDA, Glutation, Inhalation, Shoe Worker
Introduction
Benzene is a liquid that is colourless and has a sweet smell, evaporates very rapidly in the air, and is difficult to dissolve in water [1], [2]. Benzene is also a raw material for making plastics, resins, synthetic fibres, dyes, detergents, medicines, pesticides, and components of crude oil, gasoline, and cigarette smoke [3], [4]. Pathways to benzene exposure can be through the skin, respiratory tract, mouth and then to the digestive tract [5].
A person who is exposed to high levels of benzene can experience several signs and symptoms, including drowsiness, dizziness, rapid or irregular heartbeat, headaches, tremors, confusion, unconsciousness, until death [6]. Excessive benzene in the body can become free radicals that can reduce blood cell production [7].
Free radicals are compounds or atoms that have one or more unpaired electrons [8]. Free radicals oxidise some of the body macromolecules such as proteins, nucleic acids and lipids [9]. Free radical oxidation of proteins, nucleic acids, and lipids each produces carbonyl compounds, MDA (malondialdehyde) and deoxyguanosine P [10].
To overcome free radicals, the body needs antioxidants. Antioxidants are obtained from outside the body (food) or produced by the body itself (endogenous) [11]. Examples of endogenous antioxidants are superoxide dismutase, glutathione (GSH), catalase and glutathione peroxidase. One antioxidant that is often measured to see the impact of an increase in free radicals in the body is GSH [12], [13]. The Glutathione molecular formula is C10H17N3O6S, with a molecular weight of 307.3235 g/mol. As an antioxidant for the body, glutathione is a tripeptide consisting of amino acids; glutamate, cysteine, and glycine [14]. The content of glutathione is found in most of the body’s cells, but the most are in the liver. The thiol (SH) group of cysteine functions as a proton donor and is responsible for the biological activity of glutathione. Suggested food sources because they contain glutathione as antioxidants namely asparagus, spinach, broccoli, garlic, kale, onions, watercress, cabbage, Brussels sprouts, some herbs like turmeric, cinnamon, watermelon, avocado, grapes, peaches, oranges, walnuts, granola, turkey and chicken meat, cottage cheese and yoghurt [13].
Several studies have shown that benzene exposure can increase free radicals and reduce the body’s antioxidant status, especially GSH enzymes. Research in Jakarta and Iraq shows that gas station workers exposed to benzene are more susceptible to DNA damage due to free radicals. Research on the relationship of benzene, MDA and glutathione concentrations in shoe Home Industry workers still does not exist in Indonesia.
Therefore, this study aims to analyse the relationship of benzene concentration, MDA levels and glutathione enzymes in Home Industry workers exposed to benzene.
Material and Methods
The type of research used is cross-sectional. Subjects are workers in the Tambak Osowilangun shoe industry in Surabaya. The inclusion criteria in this study were male and female workers who had worked in the shoe industry in Tambak Osowilangun for > 10 years and were willing to be used as research respondents. The study sample was 25 people.
The variables calculated were benzene levels, MDA levels, GSH levels, and measurements of benzene concentration at five points in the industry. The research subject was chosen after the person was willing to participate in the study by first describing the benefits and inconvenience of participating in the study. Willingness to participate in the study was made in writing through informed consent, and this study had received prior ethical approval by the Public Health Faculty Ethics Committee, Airlangga University with ethics number 516 KEP-K.
Measurement of length of work, average work every day, and work time in a week are obtained through in-depth interviews with respondents. Then, the measurement of benzene concentration in the work environment using a measurement method of NIOSH 1501 with an activated carbon (charcoal) pipe which uses a gas chromatography-flame ionisation detector (GC-FID) technique using NIOSH 1501 standard.
MDA measurements were carried out using a modified spectrophotometry method of thiobarbituric acid (TBA) test. A total of 400 µl of the sample was reacted with 200 μl of trichloroacetic acid (TCA) 20% for deproteination. Then the cortex and centrifuge at a speed of 5000 rpm for 10 minutes. The supernatant formed was taken and 400 μl TBA 0.67% was added. Then the sample was vortexed and incubated in a water heater at 96°C, 10 minutes then lift and cool at room temperature. Then read the absorption at a wavelength of 530 nm. The sample is taken immediately after the employee’s work shift is finished [14]. The normal average level for MDA is 2.61 µmol/L [15].
GSH measurement with a sample of blood taken from the left arm cubital vein as much as 2 mL of research subjects. The blood is then centrifuged at 2000 rpm for 3 minutes to get a plasma. Then the plasma is stored in a refrigerator -20°C, before GSH examination. GSH levels were measured by mixing 50 plasma with 1.78 mL phosphate buffer 0.1 M pH 8- and 0.2-mL TCA 5%. The mixture was then centrifuged at 1500 g for 5 minutes, a temperature of 40°C. The supernatant was then added with 0.01 mL DNTB and left for 1 hour. The mixture is then examined using spectrophotometry at a wavelength of 412 nm to determine plasma GSH levels [16]. GSH normal level is 3.8-5.5 µmol/L [17].
Statistical analysis using Pearson and Spearman’s Rank correlation test with an alpha of 0.05. The closer to 1, the stronger the correlation between variables and vice versa.
Results
Characteristics of Shoe Maker Worker
The majority of respondents have a high school senior education background (45.80%) and male sex (56%) and do not have smoking habits (60%). The majority of respondents, as many as 56% still have benzene concentrations still under TLV standard (0.01 mg/ml). In Figure 1, shows that the average MDA level in the respondent’s body is 6.94 mg/ml.
Figure 1.
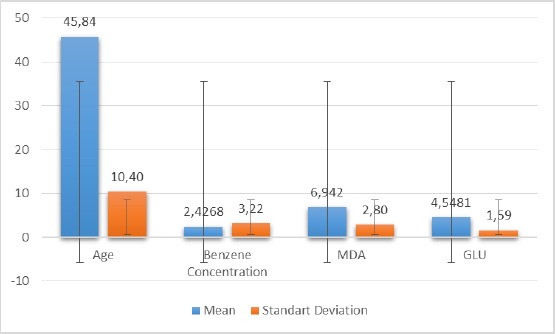
Average distribution and standard deviation of variables of age, benzene concentration, MDA and glutathione levels
The highest MDA level in the respondent’s body was 12.73 mg/ml, while the low level was 2.93 mg/ml. Figure 1 also shows that the average level of glutathione in the body of the respondent was 4.54 mg/ml. The highest level of glutathione in the body of the respondent was 8.28 mg/ml, while the lowest level was 1.86 mg/ml.
Correlation between Benzene, MDA and Glutathione Concentration in Shoe Home Industry Worker
Correlation between Benzene concentration and MDA
Figure 2 shows that there is no trend curve between the relationship between benzene concentration and MDA level. This is by the statistical test which states that there is no correlation between benzene concentration and MDA level (p = 1,000; r = 0.000). Correlation between Benzene Concentration with Glutation Level
Figure 2.
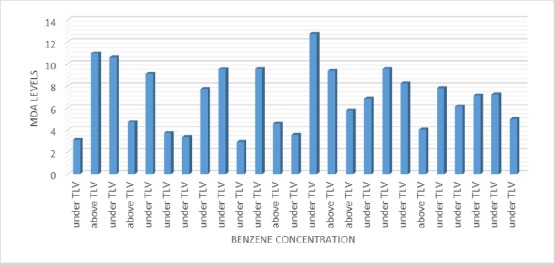
Graph of Relationship between Benzene Concentration and MDA Level
Figure 3 shows that there is no tendency curve between the relationship of benzene concentration and glutathione level. This is by the statistical test which states that there is no relationship between benzene concentration and glutathione level (p = 1,000; r = 0.000).
Figure 3.
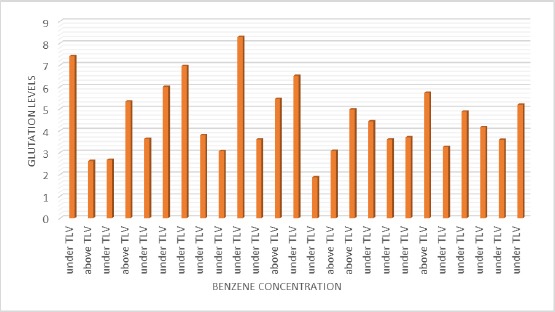
Graph of the Relationship between Benzene Concentration and Glutathione Level
Relationship of MDA Levels with Glutathione
Figure 4 shows that there is an inverse linear relationship between levels of MDA and Glutathione which means that the greater the MDA level, the lower the level of glutathione in the body. This is consistent with the statistical test which states that there is a strong relationship between MDA levels and glutathione levels (p = 0.000; r = -0.947).
Figure 4.
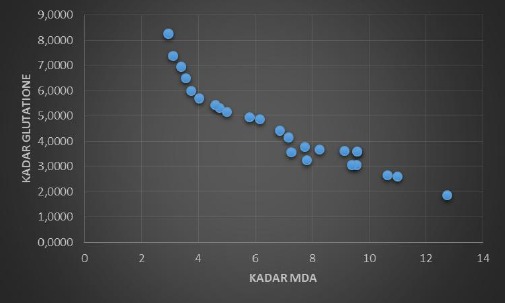
Graph of Relationship between MDA Levels and Glutathione Levels
Discussion
The majority of respondents have male gender and have a high school education background (high school). Benzene concentration in the majority of respondents has a value under the Threshold Limit Value (TLV). The Threshold value for the concentration of benzene according to the Regulation of the Minister of Manpower and Transmigration of the Republic of Indonesia Number 13 of 2011 concerning the Physics and Chemical Factor Threshold Value at the Workplace is 0.5 ppm (1.6 mg/m3) [18].
Malondialdehyde (MDA) is a compound that can describe the activity of free radicals in cells so that it is used as one of the indications of oxidative stress caused by free radicals [19]. Another study reinforces this statement by stating that the mediator Malondialdehyde (MDA) is a final product of fat peroxidation which is used as a biological biomarker of fat peroxidation and can describe the degree of oxidative stress [20]. The average MDA level in the respondent’s body was 6.94 mg/ml, and this value showed a higher value than previous studies in Indonesia (0.731 nmol/mL). Statistical tests also showed no significant relationship between benzene and MDA levels in shoe workers. This contrasts with similar research [10]. But the thing that distinguishes this research from the previous one is the location/place of research. The previous research location was located at a gas station which was known to have higher levels of benzene than the shoe industry factory which was only exposed to benzene in the glueing process. Other factors that influence can be too small a large sample and less exposure to benzene.
Glutathione (lgamma-glutamyl-cysteinyl-glycine) is a tripeptide consisting of glutamic acid, cysteine, and glycine. The compound has a sulfhydryl/thiol group (-SH) found in the amino acid cysteine. The sulfhydryl group causes GSH to act as a powerful electron donor (nucleophile) in counteracting free radicals. GSH can decompose H2O2 to H2O with the help of glutathione peroxidase enzymes [12], [21]. These compounds are metabolised by the liver to produce free radicals such as superoxide anions (O2-), hydroxyl radicals (OH-) and semicunionan radicals. GSH works to counteract these free radicals to prevent or reduce cell damage [22]. The longer you work at the benzene exposure site, the more exposure to benzene, toluene and xylene compounds will accumulate and are more likely to reduce the body’s antioxidants [11].
Figure 5.
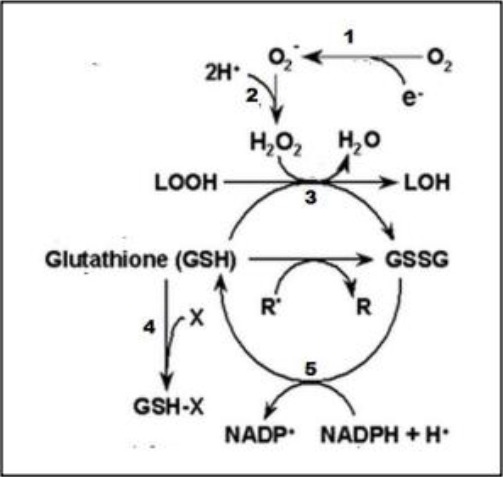
Role of GSH to Prevent Free Radicals 16 GSH helps the enzymes glutathione peroxidase and superoxide dismutase to break free radicals (LOOH, O2 -, R ’, X); 1. NADPH Oxidase; 2. Superoxide Dismutase; 3. Glutathione Peroxidase; 4. Glutathione S-Transferase; 5. Glutathione Reductase
GSH has a role as an antioxidant by reducing free radicals directly or as a cofactor of antioxidant enzymes such as glutathione peroxidase (Figure 1) and glutathione transhydrogenase [23]. The main function of GSH is to detoxify drugs, xenobiotics or pesticides catalysed by GSH-S-transferase enzymes. GSH also plays a role in maintaining thiol groups (-SH) in essential proteins, by reducing disulfide bonds in proteins, which are catalysed by the enzyme thioltransferase [12], [23]. However, the results of the statistical test analysis stated that there was no significant relationship between benzene concentration and glutathione. This has similarities with previous studies [16]. The thing that can be a significant factor of this correlation is the possibility that there are many other antioxidants (glutathione peroxidase, SOD and catalase) which play a role in reducing free radicals [8], [12].
MDA and glutathione levels have a strong significant correlation and have a reciprocal relationship which means that the higher the MDA level, the lower the level of glutathione in the worker’s body. This has similarities with another study which states that there is an increase in MDA levels and a decrease in Glutathione levels in cement workers [24]. Even studies in Jordan and Poland in automobile workers showed an increase in MDA levels by 48% and a decrease in GSH levels by 16-25% [25], [26]. High MDA levels indicate higher free radicals as well. This benzene radical can suppress detoxification enzymes, one of which is GSH. The most dangerous forms of free radicals benzene are superoxide (O2) anion, hydroxyl radical (OH) and hypochlorite acid (HOCl) and hydrogen peroxide (H2O2). Also, benzene free radicals can damage blood cells, can cause lipid peroxidation which can cause liver fibrosis [27]. What can be done to reduce benzene exposure in workers is to use PPE regularly such as masks, especially during the glueing process. Another approach is taken by consuming foods rich in detoxification enzymes, especially GSH which is usually found in the majority of vegetables such as asparagus, spinach, broccoli, garlic, kale, and onions [13].
In conclusion, the majority of respondents have male gender, high school education background, benzene concentration is still below the TLV value, the average MDA level is 6.94 mg/ml, and the average glutathione level is 4.54 mg/ml. Benzene concentration did not have a significant correlation with MDA and glutathione levels; on the contrary, MDA levels had a strong correlation with glutathione levels. The influencing factor was the benzene threshold which was still too small, so it still did not show high levels of GSH and MDA. Conversely, MDA and GSH have a strong correlation because the more benzene that becomes free radicals (MDA), the free radicals will directly interfere with the work of biotransformation enzymes (detoxification), one of which is GSH. Workers should always use PPE and always eat foods that contain lots of glutathione enzymes such as vegetables (spinach or broccoli) to reduce the impact of free radicals from benzene inhalation.
Acknowledgements
The authors would like thank the rector of Airlangga University. The authors would like to acknowledge workers at Shoe Industry Romokalisari Surabaya, East Java Indonesia and thanks to Wulan Meidikayanti and Fathimatul Tualeka for editing this article.
Footnotes
Funding: This article was supported by Activity Budget Plans 2017, Faculty of Public Health, Airlangga University
Competing Interests: The authors have declared that no competing interests exist
References
- 1.ATSDR. Toxicological Profile for Benzene. Atlanta, Georgia: ATSDR; 2007. [Google Scholar]
- 2.Tualeka AR, Jalaludin J, Salesman F, Wahyu A, Tukiran T, Setiawan S, Wibrata DA, Hasyim HN. Risk Analysis Characterization of Benzene and Demographic Factors toward Immunoglobulin A. Open Access Maced J Med Sci. 2018;6(12):2381. doi: 10.3889/oamjms.2018.488. https://doi.org/10.3889/oamjms.2018.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nirmawati S, Tualeka AR, Adi AC. Effect of Food Containing High Fe (Iron) Intake to Urinary Trans, Trans-muconic Acid (Tt-ma) Levels on Workers Exposed to Benzene. Indian Journal of Public Health Research &Development. 2018;9(1):53–57. https://doi.org/10.5958/0976-5506.2018.00010.4. [Google Scholar]
- 4.Smith MT. Advances in Understanding Benzene Health Effects and Susceptibility. California: Division of Environmental Health Sciences, School of Public Health, University of California, Berkeley; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putri YRP. Benzene in Urban Mara. Thesis, Department of Chemistry, Faculty of Mathematics and Natural Sciences, University of Indonesia, Depok. 2011 [In Indonesian] [Google Scholar]
- 6.Central Disease Center (CDC) Facts about Benzene. 2013. [Retrieved October 11, 2018]. https://emergency.cdc.gov/agent/benzene/basics/facts.asp .
- 7.Kamal A, Malik RN. Hematological evidence of occupational exposure to chemicals and other factors among auto-repair workers in Rawalpindi, Pakistan. Osong Public Health Res Perspect. 2012;3(4):229–238. doi: 10.1016/j.phrp.2012.10.003. https://doi.org/10.1016/j.phrp.2012.10.003 PMid:24159519 PMCid:PMC3747659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birben E, Sahiner UM, Erzurum S, Sackesen C, Kalayci O. Oxidative stress and antioxidant defense. WAO J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. https://doi.org/10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunwar A, Priyadarsini KI. Free radicals, oxidative stress, and importance of antioxidants in human health. J Med Allied Sci. 2011;1(2):53–60. [Google Scholar]
- 10.Ariyani R. Essay. Jakarta: Indonesia University; 2009. Study of detection DNA-adduct 8- hidroksi-2'-deoksiguanosin as a biomarker of cancer risk on worker at some Gas Station Jakarta. [Google Scholar]
- 11.Odewabi AO, Ogundahunsi OA, Oyalowo M. Effect of exposure to petroleum fumes on plasma antioxidant defense system in petrol attendants. J Pharmacol Toxicol. 2014;5(2):83–88. https://doi.org/10.19026/bjpt.5.5461. [Google Scholar]
- 12.Lushchak VI. Glutathione homeostasis and functions:potential targets for medical interventions. J Amino Acids. 2012;2012:1–26. doi: 10.1155/2012/736837. https://doi.org/10.1155/2012/736837 PMid:22500213 PMCid:PMC3303626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cadenas E, Jones PD. Bioavailability of Glutathione. In: Cadenas E, Packer L, editors. Handbook of Antioxidants. 2nd. ed. New York: Marcel Dekker, Inc; 2002. pp. 549–64. [Google Scholar]
- 14.Zainuri M, Wanandi SI. Specific Activity of Manganese Superoxide Dismutase (MnSOD) and Catalase in Rat Liver Induced Systemic Hypoxia:Its Relationship with Oxidative Damage. J Health Res Dev Media. 2012;22(2):87–2. [Google Scholar]
- 15.Singh Z, Karthigesu IP, Singh P, &Rupinder KAUR. Use of malondialdehyde as a biomarker for assessing oxidative stress in different disease pathologies:a review. Iranian J Pub Health. 2015;43(3):7–16. [PMC free article] [PubMed] [Google Scholar]
- 16.Safyudin S, &Subandrate S. Glutathione level (GSH) of the blood of SPBU employees in Palembang City. J Med Health:Scientific Publication, Faculty of Medicine, Sriwijaya University. 2015;2(3):277–81. [Google Scholar]
- 17.Tim Guilford Holistic Primary Care. What Every Doctor Should Know About Glutathione. [[October 24, 2018]]. Accessed on https://www.holisticprimarycare.net/topics/topics-o-z/vitamins-a-supplements/1421-what-every-doctor-should-know-about-glutathione.html .
- 18.The Republic of Indonesia. Regulation of the Minister of Manpower and Transmigration vNo. 13 of 2011 concerning the Threshold Value of Physical and Chemical Factors in the Workplace. Jakarta: State Secretariat; 2011. [In Indonesian] [Google Scholar]
- 19.Asni E, Harahap IP, Prijanti AR, Wanandi SI, Jusman SWA, Sadikin M. Effect of hypoxia on malondialdehyde levels, reduced glutathione and catalase activity of rat kidneys. Indonesian Medicine Magazine. 2009;59(12):595–600. [Google Scholar]
- 20.Rahardjani KB. Relationship between Malondialdehyde (MDA) and Outcome of Neonatorum Sepsis. Sari Pediatri. 2016;12(2):82–7. https://doi.org/10.14238/sp12.2.2010.82-7. [Google Scholar]
- 21.Al-Fartosy AJM, Awad NA, Shanan SK. Biochemical correlation between some heavy metals, malondialdehyde and total antioxidant capacity in blood of gasoline station workers. Int Res J Environment Sci. 2014;3(9):56–60. [Google Scholar]
- 22.Li C, Zhou HM. The role of manganese superoxide dismutase in inflammation defense. Enzyme Res. 2011;2011:1–6. doi: 10.4061/2011/387176. https://doi.org/10.4061/2011/308730 PMid:22145076 PMCid:PMC3226318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu G, Fang YZ, Yang Z, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–2. doi: 10.1093/jn/134.3.489. https://doi.org/10.1093/jn/134.3.489 PMid:14988435. [DOI] [PubMed] [Google Scholar]
- 24.Orman A, Kahraman A, Çakar H, Ellidokuz H, Serteser M. Plasma malondialdehyde and erythrocyte glutathione levels in workers with cement dust-exposure silicosis. Toxicology. 2005;207(1):15–20. doi: 10.1016/j.tox.2004.07.021. https://doi.org/10.1016/j.tox.2004.07.021 PMid:15590118. [DOI] [PubMed] [Google Scholar]
- 25.Kasperczyk A, Słowińska-Łożyńska L, Dobrakowski M, Zalejska-Fiolka J, Kasperczyk S. The effect of lead-induced oxidative stress on blood viscosity and rheological properties of erythrocytes in lead-exposed humans. Clin Hemorheol Microcirc. 2014;56(3):187–5. doi: 10.3233/CH-131678. PMid:23370159. [DOI] [PubMed] [Google Scholar]
- 26.Shraideh Z, Badran D, Hunaiti A, &Battah A. Association between occupational lead exposure and plasma levels of selected oxidative stress-related parameters in Jordanian automobile workers. Inter J Occup Med Environ Health. 2018 Jul 4;31(4):517–525. doi: 10.13075/ijomeh.1896.01243. https://doi.org/10.13075/ijomeh.1896.01243. [DOI] [PubMed] [Google Scholar]
- 27.Ellah AB, Rushdi M, Okada K, Yasuda J. Oxidative stress and bovine liver diseases:Role of glutathione. Japanese Journal of Veterinary Research. 2007;54(4):163–73. [PubMed] [Google Scholar]


