Abstract
BACKGROUND:
Hyaluronic acid (HA) based hydrogels for esthetic applications found widespread use. HA should be crosslinked for this application to achieve the correct viscoelastic properties and avoid fast degradation by the hyaluronidase enzyme naturally present in the skin: these properties are controlled by the amount of crosslinker and the fraction that is effectively crosslinked (i.e. that binds two HA chains).
AIM:
Crosslinking by polyethylene glycol diglycidyl ether (PEGDE) has been more recently introduced and showed attractive features in terms of viscoelastic properties and reduced biodegradation. Aim of this paper is to define a method for the determination of the crosslinking properties of these recently introduced fillers, method that is lacking at the moment.
MATERIAL AND METHOD:
The percentage of crosslinker and the fraction that is effectively crosslinked were determined by proton Nuclear Magnetic Resonance (1H NMR) and by 13C NMR, respectively. The filler were preliminarily washed with acetonitrile to remove residual PEG and then digested by hyaluronidase to obtain a sample that can be analysed by NMR.
RESULTS:
The crosslinking parameters were determined in four samples of NEAUVIA PEG-crosslinked dermal fillers (produced by MatexLab S.p.A., Italy). The percentage of crosslinker was between 2.8% and 6.2% of HA, whereas the effective crosslinker ratios were between 0.07 and 0.16 (ratio between the moles of effectively crosslinked PEG and total moles of PEG). Moreover, a digestion procedure alternative to enzymatic digestion, based on acidic hydrolysis, was successfully tested for the determination of crosslinker percentage.
CONCLUSIONS:
The proposed method successfully determined the two crosslinking parameters in PEG-crosslinked dermal fillers. The estimated percentage of crosslinker is similar to previously reported data for other crosslinkers, whereas the effective crosslinker ratio is lower for PEG crosslinked hydrogels.
Keywords: Hydrogels, Polyethylene glycol, Soft tissue augmentation
Introduction
Hyaluronic acid (HA) based hydrogels attracted much attention since the first years of the new millennium [1], [2]. Interest in this material and its chemical modifications stems from its high biocompatibility and flexibility, encompassing several very interesting and promising fundamental study and clinical applications. Tissue engineering [3], [4], drug delivery [5], cell scaffolding [4], [6], wound healing [7], dermal filler [8] are all extremely active field of fundamental research, product development and clinical activity. As far as dermal fillers are involved, the introduction of HA-based filler was a big change in paradigm in this field, moving from permanent or semipermanent to biodegradable fillers like the ones based on hyaluronic acid [8], [9]. The latter property prompted for the chemical modification of HA to reduce the biodegradation rate and give the possibility to modulate viscoelastic properties to achieve compatibility with different tissues [10]. The most successful chemical modification was achieved by crosslinking agents, i.e. chemical species connecting two sections of the HA chain in a bridge-like fashion. Several crosslinking species were introduced [11]: 1,4-Butanediol diglycidyl ether (BDDE), 1, 2, 7, 8-diepoxyoctane (DEO), divinyl sulfone (DVS), hexamethylenediamine (HMDA) and polyethylene glycol diglycidyl ether (PEGDE [12]). Crosslinked HA hydrogel is an adaptable material whose properties may be tailored for the intended purpose. Chemical and biochemical characterisation of hydrogels were accordingly performed with several different aims, ranging from safety assessment, quality assurance to the understanding of hydrogel properties (rheology, degradation, fitness for purpose). In particular, the crosslinking parameters play a major role in determining the rheological and swelling properties of the hydrogel [9], [10], [11], features that are of the utmost importance for clinical applications [10]. Two parameters are typically investigated: the degree of modification and the effective crosslinker ratio [13]. The first figure states the percentage of disaccharide units bound to a crosslinker molecule: as an example, a 50% modification means that half of the HA is modified by the crosslinker. The effective crosslinker ratio is esteem of the fraction of crosslinker that connects two disaccharide units, i.e. that acts as a crosslinker, bridge-like a binder.
Hyaluronic acid hydrogel crosslinked with polyethene glycol (PEG) has been recently introduced [12], [14], [15]. Several features of these materials have already been investigated, namely the properties directly involved in the safe use as dermal fillers (degradation by hyaluronidase [16] and biosafety [17]).
Nevertheless, the possibility to investigate crosslinking feature in these materials have not yet been investigated. Here we introduce a method for estimating the crosslinking parameters for PEG crosslinked hyaluronic acid-based hydrogels. Using as a starting point published general methodologies, several issues specific to this material were efficiently tackled, leading to the definition of a general approach for the characterisation of crosslinked hydrogels: critical steps were pinpointed, and an alternative procedure for sample treatment is proposed.
Instrumentation
Nuclear magnetic resonance (NMR) spectra were acquired on a Brucker 400 MHz Advance NMR spectrometer equipped with a 5 mm PABBO probe. The zg30 sequence from the TopSpin library was used to acquire 1H spectra, whereas 13C spectra were acquired by the zgig30 sequence. 1H spectra were acquired accumulating 64 scans with a recycle delay (D1) time of 5 seconds, whereas 13C spectra acquisition required a D1 of 10 seconds and the accumulation of 7000 scans. These delays ensured full relaxation of the signals achieving quantitative signals.
A VWR pHenomenal MU6100L pHmeter was used for pH measurements. A refrigerated incubator (model FOC225i) from VELP Scientifica was used for sample incubation at 37°C.
Reagents and solutions
Polyethylene glycol diglycidyl ether (PEGDE, mean MW 500 g/mol) was purchased from Fluka. The general formula of the product is shown below (see PEGDE characterization for a discussion on the structure of this species).
Hyaluronidase from sheep tested was purchased from Sigma Aldrich (type V, lyophilized powder, > 1500 U/mg). Sodium hyaluronate from Bacillus Subtilis was used for the synthesis of the crosslinked hydrogel.
Acetonitrile (> 99.5%, Sigma Aldrich), concentrated sodium hydroxide (32%, Carlo Erba), concentrated hydrochloric acid (37%, Sigma Aldrich), deuterium oxide (D2O, 99.97%D, Eurisotop), sodium azide (99%, Aldrich), sodium dihydrogenphosphate monohydrate (> 99.0%, Fluka), disodiumhydrogen-phosphate dihydrate (99.5%, Merck) and sodium chloride (> 99%, Carlo Erba) were used as received from the producers.
A phosphate buffer saline (PBS) solution was prepared dissolving 34.4 mg of NaH2PO4, 44.4 mg of Na2HPO4, 400 mg of NaCl and 20 mg of NaN3 in 50 mL of ultrapure water (final pH adjusted to 7.4).
Finally, crosslinked hydrogel samples were obtained from MatexLab S.p.A. (Brindisi, Italy) and consisted of PEG crosslinked hyaluronic acid hydrogels with HA concentrations ranging from 22 to 28 mg/L.
Hydrogel sample preparation
Five, 1 mL syringes of hydrogel samples were transferred into a 10 mL round-bottomed flask and washed three times with a 95:5 acetonitrile: water mixture. The resulting solid was dried under vacuum (diaphragm pump, KNF LABOPORT) at 60°C in a water bath for 30 minutes to remove any trace of the solvent. This procedure was used to remove the unreacted, hydrolysed PEGDE from the hydrogel.
The classic procedure (enzymatic digestion13) involves enzymatic digestion: the dried hydrogel was rehydrated in 5 mL of PBS to which 100 µL of the enzyme solution was added. The hydrogel was then incubated at 37°C for 18 hours. The simplified procedure (acidic digestion) involves the digestion for 1 hour at 60°C in 2 mL of 0.2 M HCl in D2O: the solution was buffered to pH 7 by neutralisation with 32% NaOH and the addition of 10 mM phosphate buffer.
1H NMR spectra were collected directly on the abovementioned solutions obtained after digestion, whereas solutions for 13C NMR were concentrated under vacuum to around 1 mL to increase hydrogel concentration and reduce acquisition time.
Results
PEGDE characterisation
Commercially available polyethylene glycol diglycidyl ether shows an average molar mass of 500 g/mol. It is a mixture of several compounds of the general structure depicted in the Reagent and solution section with n (number of –CH2–CH2–O– groups) ranging from 2 to 15 as shown by preliminary mass spectrometry measurements. This is the first feature specific to PEG crosslinked hydrogels: opposing to BDDE, DEO, DVS and HMDA, the crosslinker is not a single molecule but a mixture of oligomers with different chain length. Measuring the typical crosslinking parameters, i.e. degree of modification and effective crosslinker ratio, requires a preliminary investigation on the crosslinker to establish average parameters. In particular, as NMR spectroscopy was used for the hydrogel characterisation, the composition of the PEGDE crosslinker should be known in term of average hydrogen and carbon atoms. In other words, the term “n” in the formula reported in the Reagent and solution section should be experimentally determined and represents the average number of repetitive –CH2–CH2–O– groups. The 13C NMR spectrum of PEGDE dissolved in D2O obtained with a 20 s recycle time yielded a value of n of 7.63, which is close to the theoretical value of 8.4 expected for the commercially reported average molecular weight of 500 g/mol. This figure (n = 7.63) was used for all of the following calculations.
Hydrogel washing
The removal of unreacted polyethylene glycol, i.e. the fraction that did not react with hyaluronic acid, is of the utmost importance if reliable results on linked PEG is to be achieved. If any unreacted PEG is left in the sample, the following NMR analysis would not be able to distinguish between bound and unbounded PEG, leading to unreliable results. Unbounded crosslinker was typically removed by washing with water and saline aqueous solutions [18]: this procedure could not be followed for PEG crosslinked hydrogels as their swelling rate is much higher than other crosslinkers’, or at least than BDDE crosslinked hydrogels. Washing with water is completely inefficient in removing unreacted PEG as the only result is the swelling of the hydrogel. Two alternatives were accordingly tested: ethanol and acetonitrile. Both proved efficient, leading to an efficient washing accompanied by the removal of water from the hydrogel. A further complication is due to the possible interference in the NMR spectrum of the residual solvent (acetonitrile or ethanol) after drying (see Experimental): acetonitrile washing was preferred as its 1H signal (2.06 ppm in D2O) overlaps HA methyl signal (1.98 ppm) if acetonitrile is present in significant amounts, whereas traces do not cause any issue. On the other hand, ethanol –CH2– signal (3.66 ppm) falls in the region of both HA and PEG proton signals.
Calculation of crosslinking parameters
The calculation of crosslinking parameters by NMR for 1, 4-butanediol diglycidyl ether (BDDE) crosslinked HA has been recently introduced [13], [18]. The BDDE crosslinker enables a relatively easy determination of these figures as it is a single molecule and shows 1H and 13C signals well separated from HA signals [18]. Conversely, crosslinked PEG 1H signals overlap completely hyaluronic acid signals when a 400 MHz NMR spectrometer is used (see Figure 1). 13C signals of bound PEG, used to calculate the effective crosslinker ratio, do not overlap HA carbon signals but still fall in the region where the HA signals are (see Figure 3).
Figure 1.
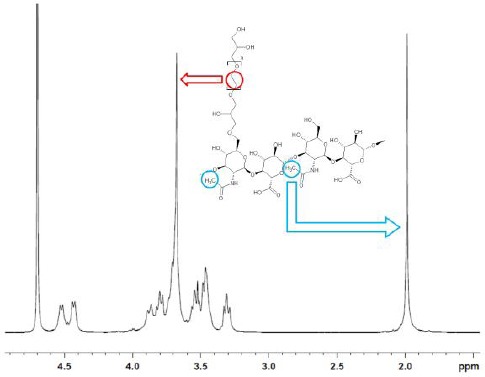
1H NMR spectrum of PEGDE crosslinked hydrogel disaccharide unit after enzymatic digestion (sample #1). Signals due to the protons in the repetitive unit –C2H4O– in PEG (red) and to the methyl group on the HA chain (blue) are evidenced. The signals from the remaining 10 PEG protons overlap the HA ones (3.2-4.0 ppm) completely
Figure 3.
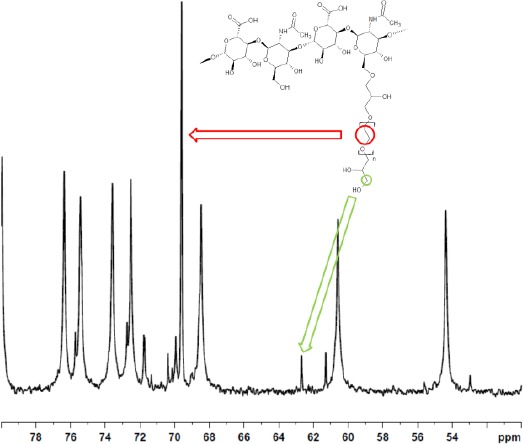
13C spectrum of PEGDE crosslinked hydrogel after enzymatic digestion limited to the 50-80 ppm range (sample #1)
The degree of modification, i.e. the moles of crosslinker bound per disaccharide unit (usually expressed as a percentage), was calculated as follows. The peaks with a chemical shift in the range 3.1-4.0 include both the hyaluronic acid protons and the PEG protons: the latter ones can be assigned to the peak at ~3.6 ppm, but cannot be integrated separately (see Figure 1). The integral of the region 3.1-4.0 ppm (Iδ3.1−4.0) is subtracted by 10 protons, which are the protons of hyaluronic acid in this range of chemical shifts (the chemical shift of the other two protons, the anomeric protons, is centered at 4.5 ppm). The subtracted integral ![]() is accordingly the integral of the PEG protons: based on the previously reported analysis (see PEGDE characterisation), 40.52 protons on average are present on the PEG residue. The degree of modification is consequently calculated as follows:
is accordingly the integral of the PEG protons: based on the previously reported analysis (see PEGDE characterisation), 40.52 protons on average are present on the PEG residue. The degree of modification is consequently calculated as follows:
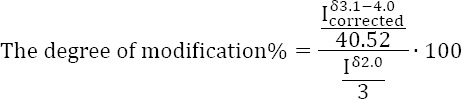
where Iδ2.0 Is the integral of the peak at 2.0 ppm of the three protons of the methyl group on the N-acetylglucosamine residue. This value is an average degree of modification as crosslinked PEG shows a distribution of molar masses as already mentioned.
The effective crosslinker ratio, i.e. the fraction of PEG molecules that cross-link between two HA chains, was calculated using 13C NMR data as follows. The signal at 69.58 ppm are attributed to the 15.26, on average, carbon atoms in the repetitive -C2H4O- unit of PEG, whereas the peak at 62.60 ppm was attributed to the carbon atom in the terminal -CH2OH group of the monolinked PEG molecule (see18 for the attribution of these signals and Figure 2 for the actual 13C NMR spectrum). The effective crosslinker ratio is determined according to the following equation:
Figure 2.
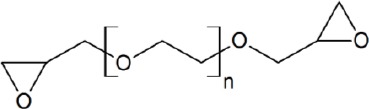
General formula polyethylene glycol diglycidyl ether (PEGDE)
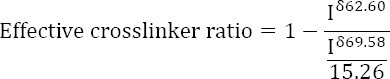
Accordingly, the effective crosslinker ratio is 1 when all of the PEG is crosslinked among two HA chains, and there is no -CH2OH group (Iδ62.60 = 0).
A grade of modification and effective crosslinker ratio for four samples of hydrogel crosslinked with PEG are reported in Table 1.
Table 1.
Grade of modification and effective crosslinker ratio for four PEG crosslinked HA hydrogels
| Sample | HA grade of modification | Effective crosslinker ratio |
|---|---|---|
| Neauvia Stimulate Lot HA1161101 |
6.2% | 0.07 |
| Neauvia Intense Lot HA2170204 |
5.6% | 0.14 |
| Neauvia Intense Flux Lot HA2170303 |
2.8% | 0.16 |
| Neauvia Rheology Lot HA2170207 |
5.0% | 0.09 |
Acidic digestion
Acidic digestion of the hydrogel was tested as an alternative, simplified procedure for hydrogel preparation before NMR measurements of crosslinking parameters. Acidic digestion is a known method to cleavage hyaluronic acid, leading to the hydrolysis of the glycosidic bond without inducing any further chemical modification [19]: accordingly, this treatment is expected to lead to a reduction in the length of HA chains without altering the HA structure and, possibly, the bound crosslinker. The reliability of this treatment was checked by applying the enzymatic and acidic digestion to six aliquots of the same sample and checking the results for comparability: three aliquots were treated by the enzymatic digestion and three by 0.2 M HCl. The 1H NMR spectra obtained by the two procedures are virtually indistinguishable, showing the same signal pattern. Signals were integrated and the crosslinking parameters calculated for the six sample aliquots: the results are reported in Table 2.
Table 2.
Comparison of the crosslinking properties obtained by enzymatic and acidic digestions. Data from the digestion of three different sample aliquots reported as average ± standard deviation for the calculation of the modification degree
| Modification degree % | ||
|---|---|---|
| Enzymatic | Acidic | |
| Lot#1 | 6.3 ± 0.63 | 5.8 ± 0.85 |
| Lot#2 | 3.4 ± 0.52 | 3.6 ± 0.70 |
| Lot#3 | 5.9 ± 0.78 | 6.4 ± 0.33 |
Data on the three samples clearly show that the acidic treatment leads to figures not significantly different from enzymatic digestion, as far as the calculation of the degree of modification is involved, leading to a simplification in the digestion procedure in terms of required time, manipulation and involved reagents.
In conclusion, the crosslinking properties of hydrogel based on hyaluronic acid crosslinked with polyethylene glycol were for the first time determined: the modification degree ranges from 2.8% to 6.2% and is similar to previously reported data (3.4%-8.2%, with one outlier at 32%, see [18]), whereas the effective crosslinker ratio seems on average lower than the reported ones (average in this work 0.11 against 0.27 in [18]). The latter difference is worth further investigations: at the present stage of investigation its significance should be assessed as the determination of the effective crosslinker ratio is affected by a relatively high error. Nevertheless, this difference may be one of the factors determining the contrast in the macroscopic behaviour of the two hydrogels like viscoelastic properties and swelling rate. The latter is an interesting property to be investigated: the evidence collected during this study point to a significant difference in swelling rate in vitro (speed of water inclusion into the hydrogel network) between different crosslinkers, with PEGDE showing a much higher rate than BDDE based formulations. In case this difference also occurred in vivo, it would clearly have a strong clinical significance. As a more general conclusion, this study puts forward a general method for the determination of crosslinking parameters in HA hydrogel with complex crosslinkers. Two critical steps were highlighted: hydrogel washing and data extraction from NMR spectra. The present study highlighted that, even for a complex crosslinker as PEGDE, the crosslinking parameters could be measured: generally, it is to be expected that this approach may be useful for any kind of crosslinker, under the unique, easily fulfilled condition, that it shows 1H or 13C NMR signals. Furthermore, a simplified sample treatment, i.e. acidic digestion, was successfully tested: although preliminary, results are indistinguishable from the ones obtained by enzymatic digestion.
A deeper understanding of the chemical factors controlling clinically relevant properties of the hydrogel, like viscoelastic behaviour and swelling, would be beneficial, finally leading to a rational approach to hydrogel design. In this regard, PEGDE shows unique features in that it is the longest crosslinker employed and it introduces unequal spacers among the HA chains as opposed to simpler molecules like BDDE, DVS and HMDA. An understanding of the role of these factors, i.e. crosslinker length and length distribution, on hydrogel properties should be accordingly achieved. More generally, establishing quantitative structure-activity relationship (QSAR) is surely a further goal for the research in this field. This achievement would allow a quantitative description of the effect of the formulation (crosslinker identity, HA/crosslinker ratio, reaction time, etc.) on hydrogel properties, which would translate into a deep understanding of the linkage between chemical structure and hydrogel characteristics. Nevertheless, a QSAR approach is at the moment a challenging task, mainly due to the lack of sufficient data.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2012;64(suppl):18–23. doi: 10.1016/s0169-409x(01)00239-3. https://doi.org/10.1016/j.addr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Huerta-Ángeles G, Nešporová K, Ambrožová G, Kubala L, Velebný V. An Effective Translation:The Development of Hyaluronan-Based Medical Products From the Physicochemical, and Preclinical Aspects. Front Bioeng Biotechnol. 2018;6 doi: 10.3389/fbioe.2018.00062. https://doi.org/10.3389/fbioe.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemshekhar M, Thushara RM, Chandranayaka S, Sherman LS, Kemparaju K, Girish KS. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int J Biol Macromol. 2016;86:917–928. doi: 10.1016/j.ijbiomac.2016.02.032. https://doi.org/10.1016/j.ijbiomac.2016.02.032 PMid:26893053. [DOI] [PubMed] [Google Scholar]
- 4.Drury JL, Mooney DJ. Hydrogels for tissue engineering:scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. https://doi.org/10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 5.Santos LF, Correia IJ, Silva AS, Mano JF. Biomaterials for drug delivery patches. Eur J Pharm Sci. 2018;118:49–66. doi: 10.1016/j.ejps.2018.03.020. https://doi.org/10.1016/j.ejps.2018.03.020 PMid:29572160. [DOI] [PubMed] [Google Scholar]
- 6.Diekjürgen D, Grainger DW. Polysaccharide matrices used in 3D in vitro cell culture systems. Biomaterials. 2017;141:96–115. doi: 10.1016/j.biomaterials.2017.06.020. https://doi.org/10.1016/j.biomaterials.2017.06.020 PMid:28672214. [DOI] [PubMed] [Google Scholar]
- 7.Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound Healing Dressings and Drug Delivery Systems:A Review. J Pharm Sci. 2008;97(8):2892–2923. doi: 10.1002/jps.21210. https://doi.org/10.1002/jps.21210 PMid:17963217. [DOI] [PubMed] [Google Scholar]
- 8.Jones DH. Semipermanent and Permanent Injectable Fillers. Dermatol Clin. 2009;27(4):433–444. doi: 10.1016/j.det.2009.08.003. https://doi.org/10.1016/j.det.2009.08.003 PMid:19850193. [DOI] [PubMed] [Google Scholar]
- 9.Tezel A, Fredrickson GH. The science of hyaluronic acid dermal fillers. J Cosmet Laser Ther. 2008;10(1):35–42. doi: 10.1080/14764170701774901. https://doi.org/10.1080/14764170701774901 PMid:18330796. [DOI] [PubMed] [Google Scholar]
- 10.Santoro S, Russo L, Argenzio V, Borzacchiello A. Rheological properties of cross-linked hyaluronic acid dermal fillers. J Appl Biomater Biomech. 2011;9(2):127–136. doi: 10.5301/JABB.2011.8566. https://doi.org/10.5301/JABB.2011.8566. [DOI] [PubMed] [Google Scholar]
- 11.Yeom J, Bhang SH, Kim B-S, et al. Effect of cross-linking reagents for hyaluronic acid hydrogel dermal fillers on tissue augmentation and regeneration. Bioconjug Chem. 2010;21(2):240–247. doi: 10.1021/bc9002647. https://doi.org/10.1021/bc9002647 PMid:20078098. [DOI] [PubMed] [Google Scholar]
- 12.Zerbinati N, D'Este E, Farina A, Rauso R, Cherubino M, Calligaro A. Morphological evidence following pegylated filler treatment in human skin. J Biol Regul Homeost Agents. 2017;31(2 Suppl. 2):79–85. PMid:28702967. [PubMed] [Google Scholar]
- 13.Kenne L, Gohil S, Nilsson EM, et al. Modification and cross-linking parameters in hyaluronic acid hydrogels - Definitions and analytical methods. Carbohydr Polym. 2013;91(1):410–418. doi: 10.1016/j.carbpol.2012.08.066. https://doi.org/10.1016/j.carbpol.2012.08.066 PMid:23044151. [DOI] [PubMed] [Google Scholar]
- 14.Zerbinati N, Rauso R, Gonzalez P, et al. In vitro evaluation of collagen production on human fibroblasts treated with hyaluronic acid peg cross-linked with micromolecules of calcium hydroxyapatite in low concentration. J Biol Regul Homeost Agents. 2017;31(Suppl 2):87–90. PMid:28702968. [PubMed] [Google Scholar]
- 15.Zerbinati N, Haddad RG, Bader A, et al. A new hyaluronic acid polymer in the augmentation &restoration of labia majora. J Biol Regul Homeost Agents. 2017;31(2 Suppl 2):153–161. PMid:28702976. [PubMed] [Google Scholar]
- 16.Zerbinati N, Lotti T, Monticelli D, et al. In vitro evaluation of the sensitivity of a hyaluronic acid PEG cross-linked to bovine testes hyaluronidase. Open Access Maced J Med Sci. 2018;6(1) doi: 10.3889/oamjms.2018.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zerbinati N, Lotti T, Monticelli D, et al. In vitro evaluation of the biosafety of hyaluronic acid PEG cross-linked with micromolecules of calcium hydroxyapatite in low concentration. Open Access Maced J Med Sci. 2018;6(1) doi: 10.3889/oamjms.2018.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wende FJ, Gohil S, Nord LI, Helander Kenne A, Sandström C. 1D NMR methods for determination of degree of cross-linking and BDDE substitution positions in HA hydrogels. Carbohydr Polym. 2017;157:1525–1530. doi: 10.1016/j.carbpol.2016.11.029. https://doi.org/10.1016/j.carbpol.2016.11.029 PMid:27987864. [DOI] [PubMed] [Google Scholar]
- 19.Tømmeraas K, Melander C. Kinetics of hyaluronan hydrolysis in acidic solution at various pH values. Biomacromolecules. 2008;9(6):1535–1540. doi: 10.1021/bm701341y. https://doi.org/10.1021/bm701341y PMid:18452332. [DOI] [PubMed] [Google Scholar]


