Abstract
By screening for enhanced ethylene-response (eer) mutants in Arabidopsis, we isolated a novel recessive mutant, eer1, which displays increased ethylene sensitivity in the hypocotyl and stem. Dark-grown eer1 seedlings have short and thick hypocotyls even in the absence of added ethylene. This phenotype is suppressed, however, by the ethylene biosynthesis inhibitor 1-aminoethoxyvinyl-glycine. Following ethylene treatment, the dark-grown eer1 hypocotyl response is greatly exaggerated in comparison with the wild type, indicating that the eer1 phenotype is not simply due to ethylene overproduction. eer1 seedlings have significantly elevated levels of basic-chitinase expression, suggesting that eer1 may be highly sensitive to low levels of endogenous ethylene. Adult eer1 plants display exaggerated ethylene-dependent stem thickening, which is an ethylene response previously unreported in Arabidopsis. eer1 also has enhanced responsiveness to the ethylene agonists propylene and 2,5-norbornadiene. The eer1 phenotype is completely suppressed by the ethylene-insensitive mutation etr1-1, and is additive with the constitutive ethylene-response mutation ctr1-3. Our findings suggest that the wild-type EER1 product acts to oppose ethylene responses in the hypocotyl and stem.
Ethylene is a simple gaseous hydrocarbon that has profound effects on plant growth and development. Considered as one of the five classic plant hormones, ethylene controls many physiological processes ranging from seed germination to tissue senescence (Abeles et al., 1992; Johnson and Ecker, 1998). Our current understanding of the molecular mechanisms of ethylene signaling has largely resulted from the isolation of ethylene-response mutants in Arabidopsis (Kieber, 1997; Johnson and Ecker, 1998; Chang and Shockey, 1999). These mutants have been isolated on the basis of defects in the ethylene-mediated triple response of dark-grown seedlings, which consists of shortening and thickening of the hypocotyl and root and exaggeration of apical hook curvature and proliferation of root hairs (Bleecker et al., 1988). Mutants lacking the triple response in the presence of ethylene are known as ethylene-response (etr) (Bleecker et al., 1988), ethylene-insensitive (ein) (Guzmán and Ecker, 1990; Roman et al., 1995), or ACC-insensitive (ain) (Van Der Straeten et al., 1992). Those that display the triple response even in the absence of exogenous ethylene are either constitutive ethylene-response (ctr and ran) mutants (Kieber et al., 1993; Hirayama et al., 1999; Woeste and Kieber, 2000) or ethylene overproducer (eto) mutants (Guzmán et al., 1990). Genetic epistasis analyses and isolation of the corresponding genes have provided molecular insight into the ethylene signal transduction pathway in Arabidopsis.
Ethylene action is initiated by a family of five ethylene receptors, which have significant similarity to His protein kinase receptors of two-component regulatory systems (Bleecker, 1999). ETR1 and ERS1 possess all of the required motifs for His kinase activity (Chang et al., 1993; Hua et al., 1995), and ETR1 was shown to have His autokinase activity in vitro (Gamble et al., 1998). Both ETR1 and ERS1 bind ethylene when expressed in yeast cells (Schaller and Bleecker, 1995; Rodriguez et al., 1999; Hall et al., 2000). Ethylene binding requires a copper cofactor as part of the functional receptor (Rodriguez et al., 1999), and a copper transporter protein, RAN1, is thought to provide copper ions to the receptors (Hirayama et al., 1999; Woeste and Kieber, 2000). The receptor genes ETR1, ETR2, and EIN4 were identified as dominant gain-of-function mutations that confer ethylene insensitivity (Bleecker et al., 1988; Hua et al., 1998; Sakai et al., 1998). Introduction of the identical missense mutations into ERS1 and ERS2 results in similar ethylene insensitivity (Hua et al., 1995, 1998). The loss of function of three or more of the receptors results in a constitutive ethylene-response phenotype, indicating that the receptors act as negative regulators of the ethylene-signaling pathway (presumably maintaining activation of the negative regulator CTR1 in the absence of ethylene) (Hua and Meyerowitz, 1998).
CTR1, a negative regulator of ethylene responses, was identified based on loss-of-function mutations that result in pleiotropic constitutive ethylene responses independent of ethylene (Kieber et al., 1993). CTR1 has sequence similarity to the Raf family of MAPKKKs, suggesting the involvement of a MAP kinase cascade in ethylene signaling (Kieber et al., 1993). Epistasis analysis demonstrated that CTR1 acts downstream of all identified ethylene receptors (Kieber et al., 1993; Roman et al., 1995; Hua et al., 1995, 1998; Sakai et al., 1998). The regulatory domain of CTR1 physically interacts with the presumed cytoplasmic domains of ETR1 and ERS1, implicating a direct role for the receptors in regulating CTR1 activity (Clark et al., 1998).
Additional components in the pathway acting downstream of CTR1 were identified through ethylene-insensitive mutations (Roman et al., 1995). The EIN2 protein has an N-terminal integral membrane domain, similar to the Nramp family of metal-ion transporters, and a C-terminal domain of unknown function (Alonso et al., 1999). Loss-of-function mutations in ein2 result in ethylene insensitivity (Guzmán et al., 1990; Roman et al., 1995), however the function of EIN2 at the biochemical level has yet to be determined. Several positive regulators of a transcriptional cascade have also been uncovered. EIN3 and the related EIN3-like proteins are novel transcriptional activators of primary ethylene-response genes (Chao et al., 1997; Solano et al., 1998). A target of EIN3 is the ERF1 gene, which encodes a protein that directly binds to the ethylene response element found in the promoters of many ethylene-inducible genes, including several pathogen-response genes such as basic chitinase (Solano et al., 1998).
The isolation of ethylene-response mutants in Arabidopsis has led to substantial insight regarding the components and mechanisms of ethylene signal transduction. The response pathway contains an intricate series of positive and negative regulators of ethylene responses, beginning with ethylene binding and leading to gene regulation. To further build upon this framework, the identification and analysis of additional components is required. Most of the ethylene mutant screens performed in the past have used saturating levels of ethylene or no added ethylene. The mutants obtained, consequently, have been generally limited to one of three classes: ethylene insensitive, constitutive ethylene response, or ethylene overproducer. Alternative mutant screens will probably be necessary to uncover additional components, including those that are redundant or tissue specific. With this in mind, we sought to isolate a new class of ethylene response mutants, which we designated enhanced ethylene response (eer). To identify such mutants, we screened for the induction of triple response phenotypes using sub-threshold levels of ethylene. This approach resulted in the isolation of a novel ethylene-dependent mutant, eer1, described in this paper. Analysis of the eer1 mutant suggests that the EER1 product acts to oppose ethylene responses primarily in the hypocotyl and stem.
RESULTS
Isolation of the eer1 Mutant
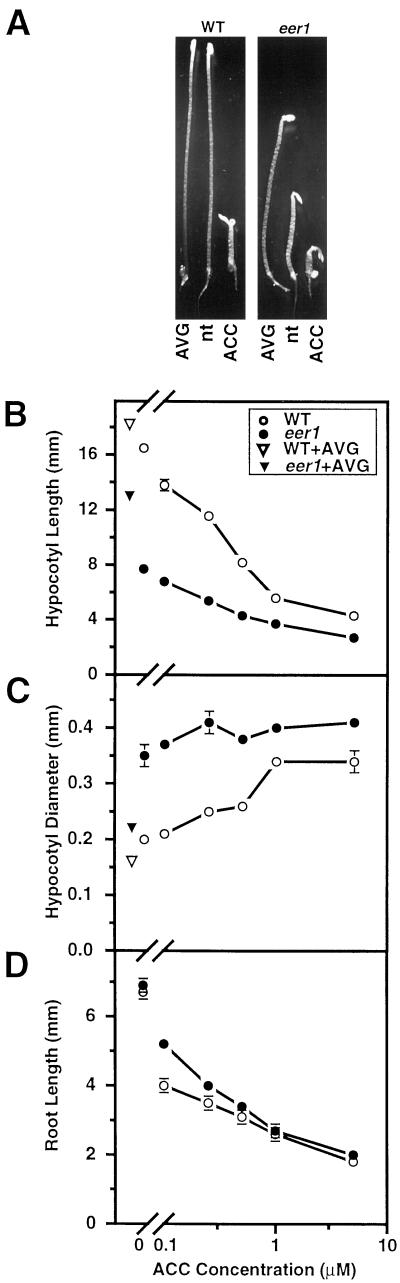
An enhanced ethylene response (eer1) mutant was isolated by screening T-DNA- and ethyl methanesulfonate (EMS)-mutagenized Arabidopsis for dark grown seedlings that exhibited facets of the ethylene-mediated triple response in the presence of sub-threshold levels of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC). ACC is the immediate precursor to ethylene and is converted to ethylene via ACC oxidase (Yang and Hoffman, 1984). As shown in Figure 1A, characteristics of the seedling triple response include inhibition of hypocotyl and root elongation, radial swelling of the hypocotyl and root, increased curvature of the apical hook, and proliferation of root hairs (Bleecker et al., 1988). Putative eer mutants exhibited one or more of these phenotypes when grown on nutrient agar containing a low concentration of ACC (0.1 μm), which causes only a slight manifestation of the triple response in the wild type (Fig. 1). The eer1 mutant was identified on the basis of severe inhibition of hypocotyl elongation in conjunction with an increase in hypocotyl thickness at this concentration of ACC (Fig. 1).
Figure 1.
Dark-grown eer1 seedlings have an enhanced ethylene response when exposed to the ethylene precursor ACC. A, Dark-grown wild-type and eer1 seedlings treated with either 10 μm AVG, 0 μm ACC (nt, no treatment), or 10 μm ACC for 3.5 d. B, ACC dose response curves for hypocotyl length of 3.5-d-old dark-grown wild-type and eer1 seedlings. Control treatments included no ACC and 10 μm AVG. Mean ± se values were determined from 25 to 30 seedlings. C, ACC dose response curves for hypocotyl diameter of 3.5-d-old dark-grown wild-type and eer1 seedlings as in B. D, ACC dose response curves for root length of 3.5-d-old dark-grown wild-type and eer1 seedlings as in B.
The eer1 phenotype results from a recessive mutation at a single locus. The F1 progeny of eer1 back-crossed to the wild type showed wild-type ethylene responses. The F2 progeny from self-fertilized F1 plants segregated 3:1 (141 wild type to 48 eer1; χ2 = 0.0018, P > 0.95). Although eer1 was isolated from a T-DNA-mutagenized population, eer1 was not kanamycin resistant (data not shown), suggesting that the mutation was not due to a T-DNA insertion. No EMS-derived alleles of eer1 were identified.
Enhanced Ethylene Response in the eer1 Hypocotyl
In dark-grown wild-type seedlings, the hypocotyl response to ethylene involves a re-orientation of cell expansion, resulting in the inhibition of hypocotyl elongation with a concomitant increase in radial thickness. The hypocotyl of eer1 was significantly shorter and thicker than that of the wild type over a broad range of ACC concentrations (Fig. 1, B and C). This phenotype was restricted to the basal portion of the hypocotyl; the apical portion of the hypocotyl was not thicker, and there was no exaggerated hook formation. Roots of eer1 seedlings were somewhat less sensitive than the wild type to low concentrations of ACC (Fig. 1D).
It should be noted that the hypocotyl of eer1 was shorter and thicker than that of the wild type even in the absence of exogenously supplied ACC. This phenotype was substantially alleviated by treatment with 1-aminoethoxyvinyl-Gly (AVG), which inhibits ethylene biosynthesis by blocking ACC synthase activity (Yang and Hoffman, 1984), thus indicating that the eer1 mutant phenotype is ethylene-dependent rather than a product of constitutive ethylene signaling or a growth defect unrelated to ethylene (Fig. 1, A–C). The addition of 10 μm AVG to the growth medium caused a 70% increase in hypocotyl length for eer1 compared with a 15% increase for the wild type; concomitantly, there was a 37% reduction in hypocotyl thickness for eer1 compared with a 20% reduction for the wild type. The effect of AVG on roots could not be assessed because AVG treatment severely inhibited wild-type root growth. The AVG treatment did not fully revert eer1 to wild type, perhaps due to incomplete inhibition of ethylene biosynthesis. However, as described later, the eer1 phenotype was completely suppressed by the etr1-1 ethylene receptor mutation, which blocks ethylene perception.
Having established the ethylene dependence of the eer1 phenotype, we next assessed whether the phenotype could result from increased ethylene biosynthesis. Rates of ethylene biosynthesis were determined for dark-grown seedlings of the wild type and eer1. Ethylene was collected after a period of 12 h from 3-d-old seedlings enclosed in airtight vials. As shown in Table I, eer1 seedlings produced 2.8-fold more ethylene than the wild type (based on fresh weight).
Table I.
Ethylene production by dark-grown seedlings
| Strain | Ethylene Productiona |
|---|---|
| nL g−1 fresh wt h−1 | |
| Ws-2 | 0.10 ± 0.01 |
| eer1 | 0.28 ± 0.03 |
Mean ± se values were determined from five samples.
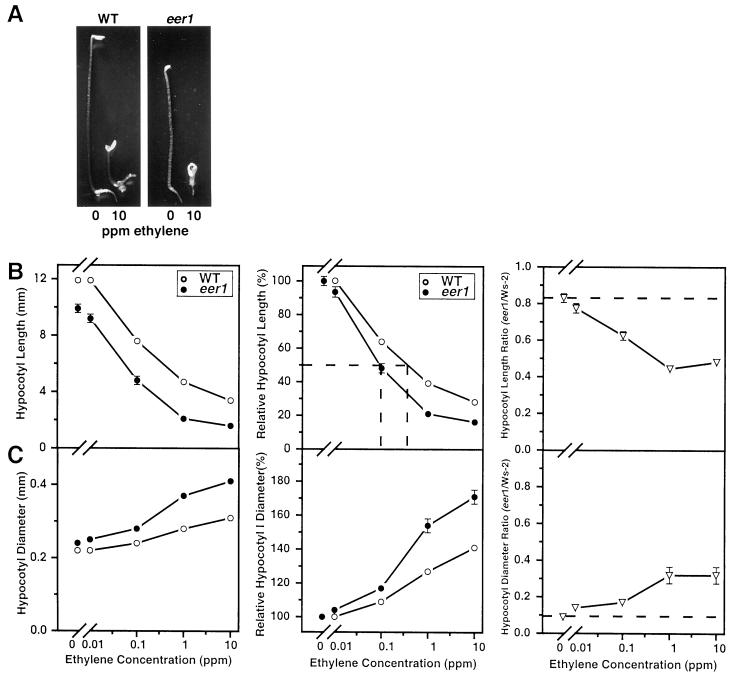
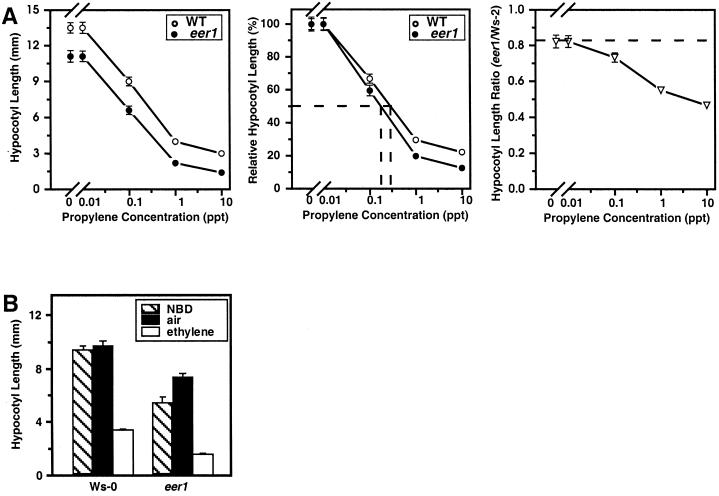
Despite the observed increase in ethylene synthesis by eer1, it was determined that ethylene overproduction cannot be the primary defect causing the eer1 phenotype. This conclusion is based on the response of eer1 to treatment with ethylene gas as shown in Figure 2. All of the ethylene treatments were performed in the presence of 10 μm AVG, which was added to inhibit ethylene biosynthesis. AVG treatment effectively removed the ethylene overproduction aspect of eer1, so that we could measure the ethylene response of eer1. As in Figure 1, the eer1 hypocotyl phenotype was substantially alleviated by AVG in the absence of added ethylene (Fig. 2). When treated, however, with a range of ethylene gas concentrations in the presence of AVG, the eer1 hypocotyl was consistently shorter and thicker than that of the wild type (Fig. 2, A–C). For example, the hypocotyl length of eer1 was inhibited 50% at an ethylene concentration of 0.1 μL L−1, whereas the wild type required greater than 0.3 μL L−1 ethylene to achieve the same level of inhibition (Fig. 2B). These results suggest an increased sensitivity to ethylene in the eer1 hypocotyl. If the eer1 phenotype resulted from only a general growth defect, then this differential could be expected to remain constant (Fig. 2, B and C).
Figure 2.
Ethylene treatment causes an exaggerated response in dark-grown eer1 seedlings. A, Dark-grown wild-type and eer1 3.5-d-old seedlings grown in air or 10 μL L−1 ethylene in the presence of 10 μm AVG. B, Ethylene dose response curves for hypocotyl length of 3.5-d-old dark-grown wild-type and eer1 seedlings grown in the presence of 10 μm AVG. Left, Actual hypocotyl length. Center, Relative inhibition of hypocotyl length (length/length at 0 μL L−1 ethylene) with the concentration of ethylene that gives 50% inhibition denoted by (- - -). Right, The ratio of eer1 hypocotyl length over wild-type hypocotyl length for each ethylene concentration with (- - -) denoting the predicted ratio if the eer1 mutant were not ethylene responsive. Mean ± se values were determined from 25 to 30 seedlings. C, Ethylene dose response curves for hypocotyl diameter of dark-grown wild-type and eer1 seedlings treated as in B. Left, Actual hypocotyl diameter. Middle, The relative increase in hypocotyl diameter (diameter/diameter at 0 μL L−1 ethylene). Right, The ratio of eer1 hypocotyl diameter over wild-type hypocotyl diameter for each ethylene concentration, with (- - -) denoting the predicted ratio if the eer1 mutant were not ethylene-responsive.
At 10 μL L−1 ethylene, the severity of the eer1 phenotype reached a level that has not been reported for the wild type (e.g. Bleecker et al., 1988). If the eer1 phenotype was the result of ethylene overproduction, then the ethylene dose response curves of the wild type and eer1 would be expected to converge at a compensation point at which the effects of exogenously added ethylene mimic the effects of endogenously produced ethylene. The apparent increase in sensitivity coupled with the severity of the eer1 phenotypic response indicate that ethylene overproduction is not the primary defect of eer1.
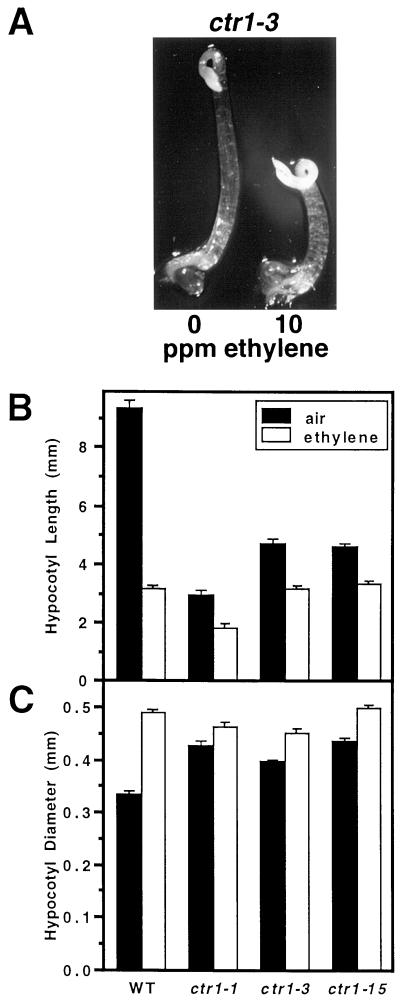
Because the extreme phenotype achieved by eer1 is not reproduced by ethylene-treated wild-type seedlings, we were interested to see if eer1 could be phenocopied by ethylene treatment of ctr1 mutants. ctr1 loss-of-function mutants display a constitutive ethylene-response phenotype less severe than the phenotype of ethylene-treated eer1. Although ethylene responsiveness of ctr1 mutants had not been previously reported, all three of the ctr1 mutant lines that we tested (including a newly isolated allele ctr1-15 [Larsen and Chang, unpublished data]) were capable of responding to ethylene both in terms of hypocotyl shortening and increased radial thickness. This result, shown in Figure 3, argues for the existence of an alternative ethylene signaling pathway in Arabidopsis that bypasses CTR1. Despite being ethylene-responsive, ctr1 (or wild-type) seedlings treated with ethylene did not produce the exaggerated phenotype of eer1, perhaps due to the presence of functional EER1. As described later, when eer1 is combined with ctr1, the double mutant has a more severe phenotype than ctr1 alone, supporting the idea that wild-type EER1 limits the hypocotyl response in both ethylene-treated wild type and ctr1.
Figure 3.
Dark-grown ctr1 seedlings respond to ethylene treatment. A, Dark-grown ctr1-3 seedlings grown in air or 10 μL L−1 ethylene for 3.5 d. B, Hypocotyl length of the wild type (Col-0) and three different ctr1 mutant alleles grown in the dark for 3.5 d either in the presence or absence of 10 μL L−1 ethylene gas. The ctr1-1 missense mutation results in an amino acid substitution of a highly conserved residue within the kinase domain. ctr1-3 carries a stop codon in the N-terminal regulatory domain. The ctr1-15 mutation has not yet been identified. Mean ± se values were determined from 25 to 30 seedlings. C, Hypocotyl diameter of the wild type (Col-0) and the three ctr1 mutant alleles treated as in B.
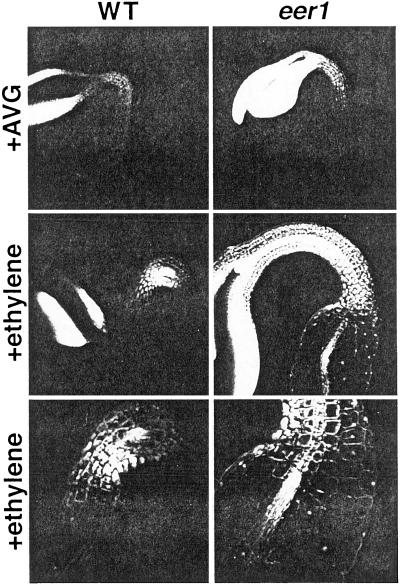
Exaggerated Cell Expansion in the eer1 Hypocotyl
Confocal microscopy revealed that the ethylene-dependent radial thickening of the eer1 hypocotyl is due to exaggerated cell expansion as shown in Figure 4. Dark-grown seedlings were exposed to either 10 μm AVG or a high level of exogenous ethylene (10 μL L−1). AVG treatment resulted in etiolated hypocotyls with no pronounced apical hook formation for both the wild type and eer1 (Fig. 4). Etiolation was correlated with cell elongation parallel to the vertical plane of the hypocotyl with cell shape in the wild type and eer1 being indistinguishable. In contrast, high concentrations of ethylene produced dramatic differences in the morphologies of the wild type and eer1 hypocotyl (Fig. 4). In the ethylene-treated wild type, there was pronounced apical hook formation along with a re-orientation of cell expansion that resulted in compact, less elongated cells compared with untreated hypocotyls. In ethylene-treated eer1 seedlings, there was no pronounced apical hook formation, perhaps due to constraints applied by the structure of the lower hypocotyl. In contrast, cell expansion in eer1 was severely exaggerated in the basal portion of the hypocotyl correlating with a dramatic increase in radial thickness. It is interesting that this phenotype was strictly localized as there was a sharp transition in cell size between the apical hook region and the basal portion of the hypocotyl (Fig. 4).
Figure 4.
The enhanced ethylene response of eer1 is restricted to the basal portion of the hypocotyl. Confocal microscopy of dark-grown wild-type and eer1 seedlings treated with either 10 μm AVG or 10 μL L−1 ethylene for 3.5 d. Top two sets, The cotyledons, apical hook, and apical portion of the hypocotyl (10× magnification). Bottom, The transition zone between the apical hook region and basal portion of the hypocotyl (20× magnification).
Response of eer1 to Ethylene Agonists
Since the eer1 phenotype was dependent upon the ethylene signal, we tested whether other compounds that are known to bind to the ethylene receptors could elicit a similar response in eer1 hypocotyls. Both propylene, which is known to elicit ethylene responses when applied at concentrations 100-fold higher than ethylene (Abeles et al., 1992), and 2,5-norbornadiene (NBD) (Sisler, 1991), which is a competitive inhibitor of ethylene action, were tested for their effects on eer1 hypocotyls.
Dark-grown wild-type and eer1 seedlings were exposed to a range of propylene concentrations (10 μL L−1–10 mL L−1) in the presence of 10 μm AVG to limit endogenous ethylene production. As with the ethylene dose response curve, eer1 hypocotyls were both more sensitive and more responsive to propylene (Fig. 5A). This enhanced response included an increase in sensitivity in comparison with the wild type, as demonstrated by the lower level of propylene required for 50% inhibition of hypocotyl length. In addition, eer1 hypocotyls demonstrated the same increase in level of response as seen with ethylene treatment, again indicating that the eer1 phenotype results from both an increase in sensitivity and in the amplitude of response.
Figure 5.
eer1 hypocotyls have an enhanced response to ethylene agonists. A, Propylene dose response curves for hypocotyl length of 3.5-d-old dark-grown wild-type and eer1 seedlings grown in the presence of 10 μm AVG. Left, Actual hypocotyl length. Middle, Relative inhibition of hypocotyl length (length/length at 0 μL L−1 ethylene) with the concentration of propylene causing 50% inhibition denoted by (- - -). Right, The ratio of eer1 hypocotyl length over wild-type hypocotyl length for each propylene concentration with (- - -) denoting the predicted ratio if the eer1 mutant were not propylene responsive. Mean ± se values were determined from 25 to 30 seedlings. B, Hypocotyl length was measured for dark-grown wild-type and eer1 seedlings exposed to either 229 μL L−1 NBD, air, or 1 μL L−1 ethylene for 3.5 d. Mean ± se values were determined from 25 to 30 seedlings.
The effect of NBD on eer1 was also tested. Dark-grown wild-type and eer1 seedlings were exposed to either 229 μL L−1 NBD, air, or 1 μL L−1 ethylene, each in the presence of 10 μm AVG to limit ethylene production. As shown in Figure 5B, instead of reverting eer1 to wild type, NBD actually elicited a partial ethylene response; eer1 seedlings displayed a 25% decrease in hypocotyl length, whereas no response was detected in the wild type. Since olefin compounds other than ethylene are capable of eliciting limited ethylene responses (Sisler, 1991; Abeles et al., 1992), binding of NBD to the ethylene receptors possibly triggers the signaling pathway but at a level that is imperceptible in the wild type. It is conceivable that eer1 is sensitized to even the low level of signaling that might be initiated by NBD.
Basic-Chitinase Expression in eer1
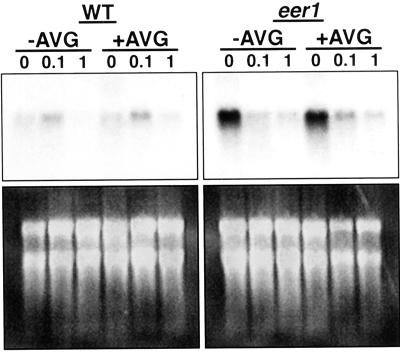
Basic-chitinase is an ethylene-regulated pathogenesis-related gene (Solano et al., 1998). Northern analysis was performed using basic-chitinase as an indicator of whether the eer1 mutation results in changes in ethylene-regulated gene expression. Total RNA was isolated from wild-type and eer1 dark-grown seedlings treated with air, 0.1, or 1 μL L−1 ethylene either in the presence or absence of 10 μm AVG. In both the wild type and eer1, AVG had little effect on the levels of basic-chitinase expression. As shown in Figure 6, the basic-chitinase transcript was slightly induced in wild-type seedlings at 0.1 μL L−1 ethylene and was reduced to the level of the air-treated control at 1 μL L−1 ethylene. In contrast, eer1 seedlings had high levels of basic-chitinase expression in the absence of exogenous ethylene, and expression was reduced to nearly undetectable levels with ethylene treatment. It is notable that the level of basic chitinase expression in untreated eer1 seedlings was substantially higher than ever detected in the wild type. This is consistent with the exaggerated morphological responses displayed by eer1 in the absence of ACC or ethylene. The altered pattern of basic-chitinase expression further suggests that the eer1 mutation results in both a shift in ethylene sensitivity and a dramatic increase in the amplitude of response.
Figure 6.
Ethylene-dependent regulation of basic-chitinase expression in dark-grown seedlings. Top, Autoradiograms of northern blots hybridized with a basic-chitinase gene probe. Dark-grown seedlings of the wild type and eer1 were treated with either 0, 0.1, or 1 μL L−1 ethylene, either in the presence or absence of 10 μm AVG for 3.5 d. Bottom, Ethidium bromide-stained rRNA bands in the corresponding gels prior to blotting.
Ethylene-Dependent Stem Thickening
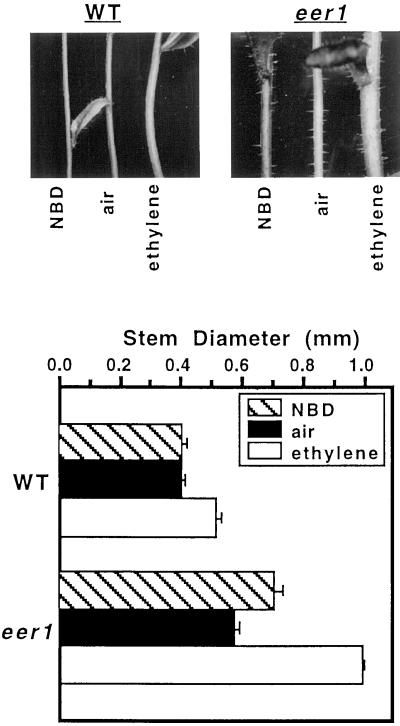
Adult eer1 plants showed no obvious mutant phenotypes with respect to leaf or rosette size, height, flower morphology, inflorescence development, or flowering time. The examination of senescence phenotypes was inconclusive (data not shown). In contrast, the stems of eer1 were noticeably thicker than those of the wild type. ctr1 mutants, which have constitutive ethylene responses throughout their life cycle, also have thicker stems (data not shown). These observations led us to examine whether stem thickening represents an ethylene response in Arabidopsis, and to determine whether stem thickening in eer1 is an enhanced ethylene response. Adult wild-type and eer1 plants (3 weeks old), which were just beginning to bolt, were exposed to either 229 μL L−1 NBD, air, or 1 μL L−1 ethylene for 8 d. The thickness of the stem was measured immediately following this treatment. As shown in Figure 7, wild-type stems were thicker with exposure to ethylene, suggesting that increased stem thickness is an ethylene-response phenotype in Arabidopsis. eer1 stems that developed in the presence of high levels of ethylene were nearly 2-fold thicker than eer1 stems in air (Fig. 7). As with the hypocotyl response, the extent of this phenotype was not achieved in the wild type treated with ethylene. Furthermore, we found that eer1 stems were responsive to NBD as seen for the eer1 hypocotyl; stems of NBD-treated eer1 plants were significantly thicker than the air-treated controls, whereas no response to NBD was detected in the wild type (Fig. 7). These findings indicated that the eer1 mutant possesses enhanced ethylene response in the adult stem and that the thicker stems in the absence of ethylene treatment were likely due to increased sensitivity to endogenous ethylene.
Figure 7.
Ethylene treatment causes radial swelling of Arabidopsis stems. A, Stems of 3-week-old wild-type and eer1 plants treated with either 229 μL L−1 NBD, air, or 1 μL L−1 ethylene for 8 d. B, Measurements of stem thickness immediately following the treatments described in A. Mean ± se values were determined from 10 plants.
Double Mutant Analysis
Double mutant analysis was performed with eer1 and three mutants: the dominant ethylene-insensitive mutant etr1-1, the recessive ethylene-insensitive mutant 2-1; and the recessive constitutive ethylene-response mutant ctr1-3. Dark-grown seedlings of selfed eer1/eer1 etr1-1/+ individuals segregated approximately 3:1 (79 etr1 to 23 eer1, χ2 = 0.21, P > 0.6) on 10 μm ACC. This high concentration of ACC induces a severe triple response phenotype in eer1 but does not elicit a response in etr1-1. As shown in Table II, the hypocotyl length of the eer1 etr1-1 double mutant was indistinguishable from that of etr1-1 alone. The same was found for the eer1/eer1 etr1-1/+ mutant (data not shown). Moreover, the eer1 phenotype was completely suppressed by the ein2-1 mutation in ACC-treated dark-grown seedlings of the eer1 ein2-1 double mutant (data not shown). The finding that both etr1-1 and ein2 completely masked the eer1 phenotype indicated that eer1 acts in (or upon) the ethylene-response pathway.
Table II.
Hypocotyl lengths of dark-grown ethylene-response mutantsa
| Treatmentb | Wild typec (Ws) | Wild typec (Col) | eer1c (Ws) | etr1-1c (Col) | eer1 etr1-1d | ctr1-3c (Col) | eer1 ctr1-3e |
|---|---|---|---|---|---|---|---|
| No ACC | 10.6 ± 0.6 | 9.7 ± 0.2 | 6.9 ± 0.1 | NDf | ND | 5.0 ± 0.2 | 2.1 ± 0.1 |
| 10 μm ACC | 2.8 ± 0.2 | 2.9 ± 0.3 | 1.4 ± 0.1 | 12.3 ± 0.4 | 13.3 ± 0.2 | ND | ND |
The unit of measure for hypocotyl length is mm.
Seedlings were grown for 4 d in the dark in either the presence or absence of ACC.
Mean ± se values were determined from 15 individuals.
Mean ± se values were determined from 102 individuals.
Mean ± se values were determined from 37 individuals.
ND, Not determined in this experiment.
The eer1 ctr1-3 double mutant displayed primarily additive phenotypes, suggesting that CTR1 and EER1 are both necessary to repress ethylene responses. Dark-grown seedlings of selfed eer1/eer1 ctr1-3/+ individuals segregated roughly 3:1 (108 eer1 to 37 eer1 ctr1, χ2 = 0.0023, P > 0.95) in the absence of ACC. As shown in Table II and Figure 8, the hypocotyl of the dark-grown eer1 ctr1-3 double mutant had a more severe phenotype than either untreated eer1 or ctr1, resembling eer1 treated with high levels of ethylene. In contrast, the root growth inhibition of ctr1-3 seedlings was partially alleviated in the eer1 ctr1-3 double mutant (Fig. 8), consistent with the earlier observation of decreased ethylene sensitivity in eer1 roots (Fig. 1). At the adult stage, the eer1 ctr1-3 double mutant exhibited phenotypes that were more severe than in either parent, including a reduction in rosette size that primarily resulted from reduced petiole length (data not shown). Stem thickening, which occurs independently in eer1 and ctr1-3, was greatly exaggerated in the double mutant in both the primary and secondary stems (data not shown).
Figure 8.
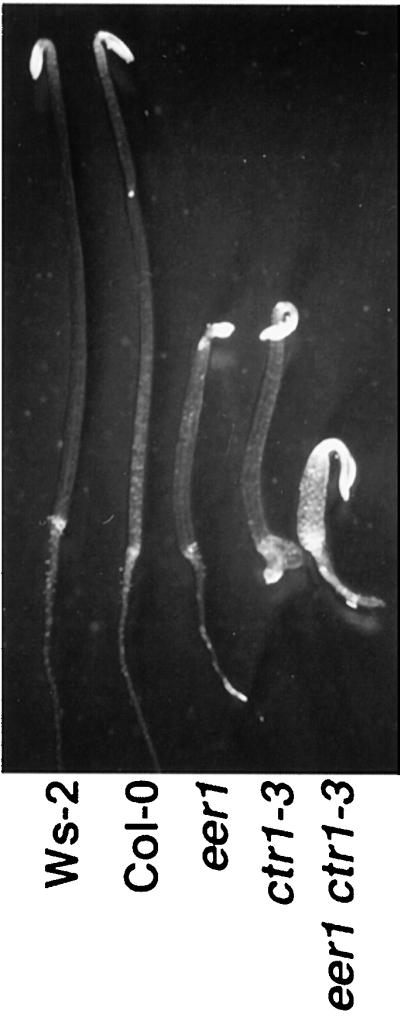
The eer1 ctr1-3 double mutant phenotype is additive. Dark-grown 3.5-d-old seedlings of the wild type (Ws-2 and Col-0), eer1, ctr1-3, and the double mutant eer1 ctr1-3 without ethylene treatment. Hypocotyl inhibition of the eer1 ctr1-3 double mutant was greater than for either eer1 or ctr1-3 alone, indicating that the two mutations are additive.
Map Location of the EER1 Locus
EER1 was mapped to a position on the top arm of chromosome 1 using a cross between eer1 (ecotype Wassilewskija) and wild-type Landsberg (data not shown). The eer1 phenotype showed linkage with two cleaved amplified polymorphic sequence (CAPS) markers, placing the EER1 locus approximately 4.7 cM south of CAPS marker m235 (position 34 cM) and 9.2 cM north of the CAPS marker UFO (unusual floral organs; position 49.6 cM). To our knowledge, no other mutants involved in ethylene signaling have been mapped to this location.
DISCUSSION
We have identified an Arabidopsis locus, EER1, which modulates the magnitude of ethylene responses specifically in the hypocotyl and stem. EER1 was identified on the basis of a recessive mutant that displays a short thick hypocotyl in response to either sub-threshold levels of ACC, ethylene, or the ethylene agonists propylene and NBD. The eer1 mutation causes a profound enhancement in both the sensitivity and amplitude of response to ethylene, such that the mutant is sensitive to even low levels of endogenously produced ethylene. In dark-grown eer1 seedlings, the mutant phenotype is restricted to the lower portion of the hypocotyl; there is no enhanced response in either the apical hook or root. In adult eer1 plants, there is enhanced ethylene-dependent thickening of the stem. Stem thickening has not been previously reported as an ethylene response in Arabidopsis. This thickening, or swelling, might result from ethylene-induced changes in cell wall structure or synthesis, producing effects similar to those observed in the hypocotyl. How ethylene triggers these cell wall changes is not fully understood (Abeles et al., 1992). EER1 action does not seem to be specific to cell expansion in that the eer1 mutant also displays enhanced expression of basic-chitinase, which is an ethylene-regulated gene involved primarily in pathogen responses.
There are several lines of evidence indicating that the eer1 mutant phenotype results from a defect in ethylene response. The alleviation of this phenotype by the ethylene biosynthesis inhibitor AVG shows that eer1 is ethylene-dependent. In the absence of AVG, ethylene levels in eer1 seedlings are approximately 3-fold higher than in wild type, however ethylene overproduction is unlikely to be the primary defect, because eer1 displays greater sensitivity to ethylene and has a more extreme ethylene-response phenotype than the maximal ethylene-response phenotype in the wild type. In addition, propylene and NBD, two ethylene agonists that bind to the ethylene receptors and trigger a signaling event, elicit the same enhanced responsiveness in eer1 as seen with ethylene treatment. These results are consistent with eer1 having a primary defect in ethylene response rather than in ethylene overproduction. The basis for the increase in ethylene production in eer1 remains unclear; presumably, it is a downstream effect of the eer1 mutation.
AVG did not fully restore the eer1 phenotype to wild type. This was perhaps due to incomplete inhibition of ethylene biosynthesis. Although the incomplete rescue of the eer1 phenotype by AVG raises the possibility that a portion of the eer1 phenotype is due to an ethylene-independent growth defect, the double mutant results with etr1-1 or ein2 argue against this. The finding that the eer1 phenotype is completely suppressed by either of the ethylene-insensitive mutations etr1-1 or ein2-1 (which block ethylene signaling) indicates that the entire eer1 phenotype is fully ethylene dependent. Therefore, the most plausible explanation for why the AVG treatment did not completely restore the eer1 hypocotyl length to wild type is that suppression of ethylene biosynthesis by AVG was incomplete, and eer1 responded to the low level of ethylene, which was still produced.
The eer1 mutant displays a greater sensitivity and amplitude of response than the wild type, as indicated by both the lower ethylene concentration required to achieve 50% inhibition of hypocotyl length and the exaggerated phenotypes in the hypocotyl and stem. The enhanced responsiveness to propylene and NBD can also be interpreted as a heightened response to ethylene signaling. By binding to the ethylene receptor, NBD might initiate a low level of ethylene signaling as seen for other olefins (Sisler and Yang, 1984; Bleecker et al., 1987; Sisler, 1991; E. Sisler, personal communication). Stimulation of ethylene responses by NBD binding has been previously described for deep water rice, in which treatment with high concentrations of NBD causes an ethylene-like response independent of ethylene (Bleecker et al., 1987). In wild-type Arabidopsis, the low level of signaling that is presumably triggered by NBD may be incapable of producing an observable phenotypic response. The responsiveness of eer1 to the predicted signaling initiated by NBD is consistent with eer1 having enhanced ethylene sensitivity and an increased amplitude of response.
A potentially similar result was obtained with respect to gene expression. Basic-chitinase, which is commonly used as a reporter gene for ethylene response, was highly expressed in untreated eer1 seedlings. In fact, the levels of basic-chitinase expression attained in untreated eer1 seedlings were not observed in wild-type seedlings even following ethylene treatment. Wild-type seedlings exposed to 0.1 μL L−1 ethylene displayed a mild induction of basic-chitinase expression, whereas identically treated eer1 seedlings already showed a dramatic reduction of expression. It is unclear why AVG did not significantly reduce basic-chitinase expression in eer1 seedlings, but it is possible that eer1 seedlings are highly sensitive to even the low levels of ethylene production that may still occur during AVG treatment. The effect of ethylene on basic-chitinase in seedlings is apparently complex. The results do parallel, however, the morphological manifestations of the mutant phenotype and reflect two aspects of the mutant phenotype: (a) the kinetics of the eer1 response are shifted toward higher sensitivity, e.g. low levels of ethylene cause the same degree of response in eer1 as obtained by high levels of ethylene in wild type and (b) the magnitude of the eer1 response, both in terms of morphology and gene expression, is much greater than ever observed for ethylene-treated wild type.
The masking of the eer1 mutant phenotype by etr1-1 and ein2-1 indicates that EER1 acts in or upon the ethylene-response pathway. The specific step at which EER1 acts cannot be established, because the usual interpretations of epistasis do not apply to double mutants in which enhanced sensitivity is involved. For instance, if the ethylene signal is required for the enhanced response to be exhibited, then blocking of the signal with the etr1-1 mutation will mask the phenotype of mutations acting downstream of etr1-1. Thus, one interpretation of the eer1 etr1-1 and eer1 ein2-1 double mutants is that ethylene signaling is required for the eer1 mutant phenotype, and EER1 acts at a point downstream of ETR1 or EIN2. An alternate interpretation is that EER1 acts at or upstream of the ethylene receptors, similar to RAN1. The ran1 mutant shows responsiveness to the ethylene antagonist trans-cyclooctene. ran1 mutants, however, do not display enhanced response to ethylene (Hirayama et al., 1999; Woeste and Kieber, 2000). Like eer1, ran1 is suppressed by etr1 and ein2 (Hirayama et al., 1999). The RAN1 protein is thought to provide the ethylene receptors with copper ions necessary for ethylene binding as well as for stability of the receptor complex (Hirayama et al., 1999; Woeste and Kieber, 2000). The latter idea is based on the observation that ran1 loss-of-function mutants display responsiveness to trans-cyclooctene (Hirayama et al., 1999). It is speculated that a copper deficiency in the ethylene-binding site of the receptors may destabilize the receptor complex, making it easier for less potent ethylene antagonists to trigger a signaling event. EER1 may similarly act at or upstream of the ethylene receptors and be involved in an aspect of regulating receptor function. Taking this view, the eer1 phenotypes might result from tissue-specific modification of the ethylene receptors, which does not occur when the etr1-1 or ein2-1 alleles are present (as in the double mutants).
The additive phenotypes of the eer1 ctr1-3 double mutant suggest that EER1 acts in addition to CTR1 to oppose ethylene responses. Wild-type EER1 is likely to counter the ethylene-independent response generated in the ctr1 loss-of-function mutant. This could explain why the eer1 ctr1-3 double mutant phenotype is both similar to that of ethylene-treated eer1 and more severe than either of the single mutant phenotypes (in the absence of ethylene). We can also take into account the finding that ctr1 loss-of-function mutations are capable of responding to ethylene. This result provides evidence that Arabidopsis seedlings possess an alternative ethylene-signaling pathway that bypasses the requirement for functional CTR1. The existence of such a pathway is also supported by the observation that ctr1 mutations do not fully suppress mutations in the ethylene receptor genes (Hua and Meyerowitz, 1998; Hua et al., 1998). The alternative pathway perhaps involves a functionally redundant CTR1 homolog. EER1 might oppose both the alternative ethylene-response pathway as well as the primary pathway.
The eer1 mutant has striking similarities to the previously described Arabidopsis mutant sabre. sabre was identified as having exaggerated radial swelling of cortical cells in the root (Aeschbacher et al., 1995). Although dose response analysis was not used to determine whether the sabre phenotype results from enhanced ethylene sensitivity, cell swelling in sabre is suppressed by etr1-1 and can be partially rescued by an ethylene biosynthesis inhibitor. As in eer1, the ethylene-dependent cell enlargement in sabre is exaggerated in comparison with the wild type, and reflects a re-orientation of cell expansion perpendicular to the axis of growth. Dark-grown sabre and eer1 also have similar hypocotyls and apical hooks. eer1 produces only a partial apical hook, which is perhaps somehow constrained by the extreme thickness of the lower part of the hypocotyl. This same exaggerated hypocotyl thickening appears to occur in sabre along with a partial apical hook. In terms of basic-chitinase expression, sabre shows no increase in basic-chitinase message levels in leaves. However, expression levels of basic-chitinase are unknown in the root cortex, which is the tissue having the most dramatic ethylene-related phenotype. Thus we cannot rule out the possibility that EER1 and SABRE are functionally analogous. SABRE has been cloned and codes for a hydrophilic protein of unknown function (Aeschbacher et al., 1995). It remains unclear how the SABRE protein opposes ethylene-regulated cell expansion.
It is worth noting that unlike the additive hypocotyl phenotype in the eer1 ctr1-3 double mutant, eer1 partially suppresses ctr1-dependent root growth inhibition and root hair formation. This is consistent with the insensitivity of eer1 roots to low doses of the ethylene precursor ACC and suggests that EER1 may have an opposite function in roots. Future isolation of the EER1 gene will hopefully allow us to better understand its function and dissect the regulation of ethylene signaling. The EER1 gene may also advance our understanding of how the ethylene hormone regulates processes such as cell expansion and gene expression.
MATERIALS AND METHODS
Plants and Growth Conditions
The Wassilewskija, Columbia, and Landsberg ecotypes of Arabidopsis were used in this study. T-DNA-mutagenized seeds of Wassilewskija (Ws-2) were obtained from the Arabidopsis Biological Resource Center (ABRC; Columbus, OH). In all experiments comparing eer1 with the wild type, strain Ws-2 from the ABRC was used as the wild type and is referred to as Ws in the text. EMS-mutagenized seeds of ecotype Columbia (Col-0) were obtained from Lehle Seeds (Round Rock, TX). The Landsberg (La-0) ecotype (ABRC) was used for genetic mapping.
For seedling growth, seeds were surface-sterilized then cold-stratified at 4°C for 4 d in the dark to synchronize germination. Seeds were then suspended in 0.15% (w/v) agarose and sown in rows on plant nutrient agar medium with Suc (Lincoln et al., 1992) [2 mm KNO3, 0.2 mm KH2PO4, 2 mm MgSO4, 0.25 mm (NH4)2SO4, 1 mm Ca(NO3)2, 1 μm MnSO4, 5 μm H3BO3, 0.05 μm CuSO4, 0.2 μm ZnSO4, 0.02 μm NaMoO4, 0.1 μm CaCl2, 0.001 μm CoCl2, 0.5% (w/v) Suc, and 0.8% (w/v) agar, pH 5.7]. The medium was supplemented with ACC (Sigma, St. Louis) or AVG (Sigma) as needed. The medium was contained in Petri dishes or vials as indicated. Seedlings were germinated for 3.5 d in the dark at 20°C. In experiments using ACC, the Petri dishes were oriented vertically for seedling growth.
All adult plants in this study were grown in soil under a 16-h-light/8-h-dark cycle at 20°C in plant growth chambers.
Mutant Screening
In all, approximately 6,500 T-DNA-mutagenized T4 seeds and approximately 30,000 EMS-mutagenized M2 ( approximately 3,125 M1) seeds were screened. Seeds were sown on nutrient agar supplemented with 0.1 μm ACC, which is a sub-threshold level for the triple response in the wild type. Seedlings were germinated in the dark at 20°C with the Petri dishes vertically oriented. After 3.5 d, the seedlings were scored for individuals exhibiting one or more of the triple response phenotypes. Putative mutants were rescued to nutrient medium lacking ACC and subsequently transferred to soil. The self-progeny of putative mutants were rescreened using the same concentration of ACC, and the confirmed mutants were tested using the same conditions on nutrient agar supplemented with 10 μm AVG instead of ACC.
Treatment with Ethylene, Propylene, or NBD
Surface-sterilized seeds were sown on 60 × 15-mm Petri dishes (Fisher Scientific, Pittsburgh) containing 6 mL of nutrient agar (as described above) supplemented with 10 μm AVG [(2-aminoethoxyvinyl Gly), Sigma Chemical Company]. Petri dishes were then enclosed in wide mouth Mason jars sealed with a perforated lid containing a rubber syringe cap. For ethylene treatment, ethylene gas (Air Products, Allentown, PA) was first diluted to 100 or 1,000 μL L−1 in a sealed flask, and the resulting ethylene concentration was determined by gas chromatography. Ethylene was then injected into the Mason jars to specific concentrations using a syringe. Treatment with propylene (Scott's Specialty Gas, Plumsteadsville, PA) was conducted in the same manner.
For NBD treatment, liquid NBD (Fluka, Milwaukee, WI) was first diluted to a concentration of 100 μL L−1 then added to the Mason jars to a final liquid concentration of 1 μL L−1, which is equivalent to 229 μL L−1 NBD gas. The growth conditions were as described for the ACC treatment.
Adult plants were grown in air until the point of bolting (approximately 3 weeks), then exposed to either ethylene or NBD in an airtight chamber (Plas Labs, Lansing, MI) or air for 8 d. The chamber was flushed with air every 2 d. Immediately after this treatment, stem thickness was measured under a dissecting microscope fitted with an eyepiece micrometer.
Measurement of Ethylene Production
Surface-sterilized seeds were placed in 5-mL glass scintillation vials containing 0.5 mL of nutrient agar. The vials (uncapped) were placed into a sterile covered beaker and incubated in the dark for 72 h at 20°C. The vials were then sealed in the dark with a rubber syringe cap, allowing ethylene to collect in the vial. After 12 h of further incubation in the dark, 1 mL of headspace was sampled from each vial and the ethylene content was measured by gas chromatography as above. The total fresh weight of the tissue was measured for each sample.
Confocal Microscopy
Germinating seedlings were exposed to either 10 μm AVG or 10 μL L−1 ethylene in the dark for 3.5 d as described above. The seedlings were fixed and stained according to Running et al. (1995) and were visualized by confocal microscopy (model MRC 1024; Bio-Rad Laboratories, Hercules, CA).
Northern Analysis
Germinating seedlings were treated with either 0.1 or 1.0 μL L−1 ethylene gas, and/or 10 μm AVG in the dark for 4 d as described above. Total RNA was extracted from whole seedlings using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA). Total RNA (5 μg) from each sample was separated by electrophoresis in a 1% (w/v) denaturing agarose gel, and the gel was blotted to nylon membrane (Hybond N+, Amersham, Arlington Heights, IL). A 32P-labeled basic-chitinase gene probe was generated using a random primed DNA labeling kit (Boehringer Mannheim, Indianapolis). Prehybridization, hybridization, and washes were carried out at 65°C following the manufacturer's instructions (Amersham), and the results were visualized by autoradiography.
Genetic Analysis
For backcrossing, eer1 (male) was crossed to wild-type Ws-2 (female), and the F1 and F2 progeny were scored on plant nutrient agar medium with Suc containing 0.1 μm ACC.
Double mutants were created by crossing eer1 (male) to etr1-1, ein2-1, and ctr1-3 plants (females). Self-fertilization of the resulting F1 produced the F2 progeny. F2 progeny of the cross with etr1-1 were screened on 10 μm ACC to isolate ethylene-insensitive individuals (which were thus etr1-1 homozygous or heterozygous). Among those, eer1 homozygotes were identified using a CAPS marker tightly linked to the EER1 locus. Double homozygotes were identified in the next generation after selfing. F2 progeny of the cross with ein2-1 were screened on 0.1 μm ACC and progeny demonstrating the eer1 phenotype were selected. F3 families from self-fertilized homozygous eer1 F2 lines were scored on 10 μm ACC for segregation of ethylene-insensitive individuals, which were the eer1 ein2-1 double mutants. F2 progeny of the cross with ctr1-3 were screened in the absence of ACC, and those individuals displaying the eer1 phenotype (homozygous for eer1), but not the ctr1-3 phenotype, were isolated. F3 families from F2 self-fertilized lines were scored in the absence of ACC to observe segregation of potential eer1 ctr1 double mutants.
For genetic mapping of the EER1 locus, eer1 (male) was crossed to wild-type La-0 (female). The mapping population consisted of 400 F2 individuals (F1 self-progeny), all of which were homozygous for eer1. The map location was determined by identifying simple sequence length polymorphism (Bell and Ecker, 1994) and CAPS (Konieczny and Ausubel, 1993) markers that cosegregated with the eer1 phenotype. Genetic distance was determined using the Mapmaker II program (Lander et al., 1987).
ACKNOWLEDGMENTS
The technical assistance of Jason Shockey, Josh Russell, and Jesse Cancel and the suggestions of Dr. Anthony Bleecker and Dr. Joseph Ecker are much appreciated. We also thank Dr. Robert Franks for invaluable assistance with confocal microscopy; Dr. Saeid Nourizadeh for providing the basic chitinase gene and ctr1-3 seeds; and Drs. Daniel Gallie, Paul Bottino, and Theophanes Solomos for use of equipment. We acknowledge the Arabidopsis Biological Resource Center (Columbus, OH) for providing seed stocks.
Footnotes
This work was supported by the National Research Initiative Competitive Grants Program/U.S. Department of Agriculture (postdoctoral grant no. 97–35304–4921 to P.B.L.); by the Department of Energy (grant no. 02–99ER20329 to C.C.); by the National Research Initiative Competitive Grants Program/U.S. Department of Agriculture (grant no. 98–35304–67–95 to C.C.); and by the Maryland Agricultural Experiment Station.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME., Jr . Ethylene in Plant Biology. Ed 2. New York: Academic Press; 1992. [Google Scholar]
- Aeschbacher RA, Hauser M-T, Feldmann KA, Benfey PN. The SABRE gene is required for normal cell expansion in Arabidopsis. Genes Dev. 1995;9:330–340. doi: 10.1101/gad.9.3.330. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Ecker JE. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Bleecker AB. Ethylene signaling: an evolutionary perspective. Trends Plant Sci. 1999;4:269–274. doi: 10.1016/s1360-1385(99)01427-2. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Rose-John S, Kende H. An evaluation of 2,5-norbornadiene as a reversible inhibitor of ethylene action in deepwater rice. Plant Physiol. 1987;84:395–398. doi: 10.1104/pp.84.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chang C, Shockey JA. The ethylene-response pathway: signal perception to gene regulation. Curr Opin Plant Biol. 1999;2:352–358. doi: 10.1016/s1369-5266(99)00004-7. [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Clark KL, Larsen PB, Wang X, Chang C. Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci USA. 1998;95:5401–5406. doi: 10.1073/pnas.95.9.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Coonfield ML, Schaller GE. Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc Natl Acad Sci USA. 1998;95:7825–7829. doi: 10.1073/pnas.95.13.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Findell JL, Schaller GE, Sisler EC, Bleecker AB. Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiol. 2000;123:1449–1457. doi: 10.1104/pp.123.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T, Kieber JJ, Hirayama N, Kogan M, Guzman P, Nourizadeh S, Alonso JM, Dailey WP, Dancis A, Ecker JR. RESPONSIVE-TO-ANTAGONIST1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell. 1999;97:383–393. doi: 10.1016/s0092-8674(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM. Ethylene insensitivity conferred by Arabidposis ERS gene. Science. 1995;269:1712–1714. doi: 10.1126/science.7569898. [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Ecker JR. The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet. 1998;32:227–254. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- Kieber JJ. The ethylene response pathway in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:277–296. doi: 10.1146/annurev.arplant.48.1.277. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM. A procedure for mapping Arabidopsis mutations using the co-dominant ecotype-specific PCR-based markers. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L. Mapmaker, an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Lincoln C, Turner J, Estelle M. Hormone-resistant mutants of Arabidopsis have an attenuated response to Agrobacterium strains. Plant Physiol. 1992;98:979–983. doi: 10.1104/pp.98.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB. A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science. 1999;283:996–998. doi: 10.1126/science.283.5404.996. [DOI] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Running MP, Clark SE, Meyerowitz EM. Confocal microscopy of the shoot apex. In: Galbraith DW, Burgue DP, Bohnert HJ, editors. Methods in Cell Biology: Plant Cell Biology. Vol. 49. San Diego: Academic Press; 1995. pp. 217–229. [DOI] [PubMed] [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:5812–5817. doi: 10.1073/pnas.95.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB. Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science. 1995;270:1809–1811. doi: 10.1126/science.270.5243.1809. [DOI] [PubMed] [Google Scholar]
- Sisler EC. Ethylene-binding components in plants. In: Mattoo AK, Suttle JC, editors. The Plant Hormone Ethylene. Boca Raton, FL: CRC Press; 1991. pp. 81–99. [Google Scholar]
- Sisler EC, Yang SF. Anti-ethylene effects of cis-2-butene and cyclic olefins. Phytochemistry. 1984;23:2765–2768. [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Straeten D, Djudzman A, Van Caeneghem W, Smalle J, Van Montagu M. Genetic and physiological analysis of a new locus in Arabidopsis that confers resistance to 1-aminocyclopropane-1-carboxylic acid and ethylene and specifically affects the ethylene signal transduction pathway. Plant Physiol. 1992;102:401–408. doi: 10.1104/pp.102.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeste KE, Kieber JJ. A strong loss-of-function mutation in RAN1 results in constitutive activation of the ethylene response pathway as well as a rosette-lethal phenotype. Plant Cell. 2000;12:443–455. doi: 10.1105/tpc.12.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]