Abstract
BACKGROUND:
Cancer is a major burden and threat to global society. A wide range of chemotherapeutic agents is extensively used to treat cancer at different stages. Inappropriate drug use may also lead to the raised cost of medical care, adverse drug effects, and patient mortality. Hence, in recent years, drug utilisation studies have become a potential tool to be used in the evaluation of different health care systems including cancer.
AIMS:
The objectives of the study were to identify the various types of cancer, the commonly prescribed drugs, rational use of anticancer drugs, and analyse the prescribing indicators in a tertiary care government hospital of India.
MATERIAL AND METHODS:
Newly diagnosed cancer and/or known case of carcinoma of either sex which required treatment/on treatment with chemotherapy aged > 18 yrs admitted in Radiotherapy Department from April 2016 to September 2016 were included in the study and analysed for prescribing indicators.
RESULTS:
The head & neck cancers were the prevalent cancers observed with more preponderance among males. Most of the patients were prescribed with a single anticancer drug. Cisplatin was the most commonly used cytotoxic drug followed by carboplatin, and antimetabolites. The most commonly used adjuvant drugs in our study were anti-emetics and anti-peptic ulcer drugs. Over 82% of anticancer agents were taken from the essential drug list and were prescribed in generic names, indicating rational use.
CONCLUSION:
Over 82% of anticancer agents were taken from the essential drug list and were prescribed in generic names, indicating rational use.
Keywords: WHO prescribing indicators, Anticancer drugs, Drug utilisation review, Pre-chemotherapy drugs
Introduction
Cancer is a major burden and threat to global society. It is one of the leading causes of death in both developed and developing countries [1]. A survey conducted by the World Health Organization (WHO) indicated that 8.2 million people succumbed from cancer in 2012, and it may rise to 19 million by 2025 [2]. In India, the estimated number of people living with this disease is around 2.25 million, and the estimated numbers of new cancers are about 1.1 million per year [3]. More than 0.6 million die because of cancer each year [3], and approximately 42% of cancers are tobacco related [4]. The main modalities used for its treatment include surgery, radiation, chemotherapy, immunotherapy, and hormones. The decision of therapy depends upon patient factors, tumour factors, and treatment factors [5]. A wide range of chemotherapeutic agents is extensively used to treat cancer at different stages. Chemotherapy refers to the usage of antineoplastic drugs to treat cancer as a standardised treatment regimen [6]. It is the only therapy which acts systematically to reduce the disease from the entire body [5]. These drugs usually act on rapidly dividing cells and are either cell cycle specific or non-specific [7].
The drug use for the treatment of diseases is a complicated process since optimal benefits of drug therapeutics in patient care may not be obtained because of under-use, overuse or misuse of the drugs. Inappropriate drug use may also lead to the raised cost of medical care, adverse drug effects, and patient mortality. Hence, in recent years, DUS has become a potential tool to be used in the evaluation of different health care systems including cancer [8]. The evaluation of drug utilisation of anticancer drugs is necessary as their irrational use has generated a significant health problem in the current medical practice. Drug utilisation has been defined by the WHO as the marketing, distribution, prescription, and use of drugs in a society with specific emphasis on the resulting medical and social consequences [9]. Drug utilisation research promotes the rational use of drugs in the population [9]. Monitoring of drug utilisation patterns helps to improve the therapeutic efficacy, provides feedback to the prescriber to assure rational use of medicines and decrease the adverse drug reactions. The ultimate goal of drug utilisation research is to assess whether drug therapy is rational or not. Therefore, the present study aimed to analyse and evaluate the trends and patterns of prescribing anticancer drugs.
The objectives of the study were to identify the various types of cancer, the commonly prescribed drugs, rational use of anticancer drugs, and analyse the prescribing indicators in a tertiary care government hospital of India.
Material and Methods
Study design
The study was an observational, prospective, non-interventional study
Study site
The study was conducted in the cancer radiotherapy department of Vijayanagara Institute of Medical Sciences, Ballari, one of the major government tertiary care hospitals for cancer treatment near the Hyderabad- Karnataka region. The study was performed after receiving the necessary ethical clearance from the Institutional Ethics Committee.
Study Duration
It was carried out for six months, period from April 2016 to September 2016
Inclusion criteria
Newly diagnosed cancer &/or known case of carcinoma of either sex which required treatment/on treatment with chemotherapy aged > 18yrs admitted in the Radiotherapy Department from April 2016 to September 2016 was included in the study. The written informed consent was taken before the start of the study. All the patients were observed for the complete duration of the study.
Exclusion criteria
Patients who were pregnant or having insufficient records and data were excluded from the study.
Sample size calculation
The sample size was designed based on the average number of inpatients admitted and registered in the earlier six months of the study period. A total number of 144 patients were recruited in the study from April 2016 to September 2016.
Data collection
Data were collected and entered in specially designed patient data entry forms. The prescription parameters needed for the study were recorded.
Data analysis
The data were entered in Microsoft Excel (Windows 7; Version 2007). The collected data of demographic and clinical variables were analysed using descriptive statistics such as frequencies and percentage, were represented in tables and figures. WHO core prescribing indicators was compiled at the end of the study to know the number of prescriptions with polypharmacy, per cent of prescriptions with injectables, per cent of drugs prescribed from Essential Drugs list.
Results
Age
The majority of patients (36.8%) were in the age group of 56-65 years followed by 18.05% in 46-55 years (26 patients), 17.36% > 65 years (25 patients), 14.58% in 36-45 years (21 patients), 9.02% in 26-35 years (13 patients) and 4.16% in 18-25 years (6 patients). The mean age of the participants was found to be 53.86 years.
Sex
Males constituted 54.86% (79 patients), and females constituted 45.13% (65 patients) of the total study population.
Distribution of cancer
Table 1, shows the system-wide distribution of cancer among study participants. The head and neck cancers were more predominant (46.7%), followed by gastrointestinal cancer (24.1%), reproductive system (14.51%), breast (11.29%) and respiratory cancers (3.22%).
Table 1.
System-wise distribution of cancers
| Organ System name | % pts |
|---|---|
| Gastrointestinal | 24.1 |
| Head and neck cancers | 46.52 |
| Breast cancers | 11.29 |
| Reproductive system | 14.51 |
| Respiratory | 3.22 |
The organ-wise distribution is shown in Figure 1.
Figure 1.
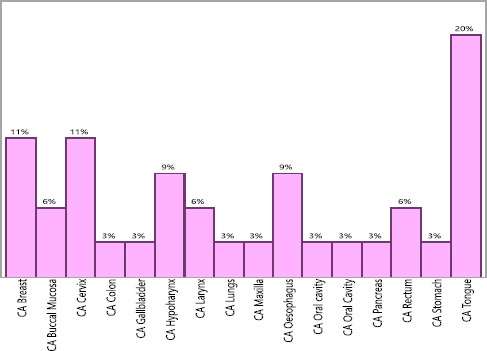
Organ-wise distribution of the cancers
Drug utilisation pattern: Out of 144 cancer patients analysed, 65.27% patients (94) was prescribed with single anticancer drug therapy while the remaining patients were given multiple anticancer drugs therapy.
The treatment regimens used in different organ system cancer is shown in Table 2.
Table 2.
The different treatment regimens used in cancer patients
| Organ System name | % pts | Treatment regimens used |
|---|---|---|
| Gastrointestinal | 24.1 | 1. Cisplatin/Carboplatin + Radiation therapy |
| 2.Oxaliplatin+Capacetabine | ||
| 3. 5-FU(5-Fluorouracil) + Radiation therapy | ||
| 4.5FU + Oxaliplatin(FOLFOX) | ||
| 5. DCF-docetaxel, cisplatin + 5-fluorouracil | ||
| 6. GEMOX- Gemcitabine + oxaliplatin | ||
| Head and neck cancers | 46.52 | 1.Cisplatin/Carboplatin (+ o r-) Radiation therapy |
| 2.Cisplatin+ Paclitaxel (+or-) Radiation Therapy | ||
| Breast cancers | 11.29 | 1.Doxorubicin+Cyclophosphamide+Radiation Therapy |
| 2. Paclitaxel | ||
| Reproductive system | 14.51 | 1. Cisplatin+ Radiation therapy |
| 2. Carboplatin+ Paclitaxel | ||
| Respiratory | 3.22 | Carboplatin+ Paclitaxel |
The anticancer drugs, the adjuvant drugs and other supportive drugs pattern is depicted in Figures 2, 3 and Tables 3, 4 and 5.
Figure 2.
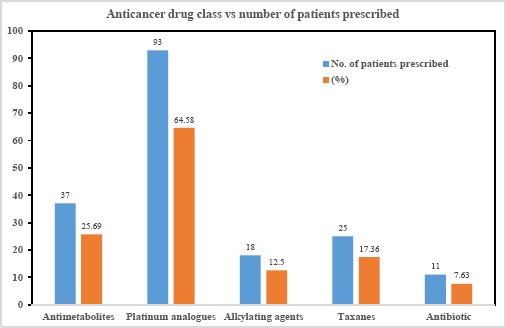
Anticancer class- wise usage of the drugs
Figure 3.

Drug class-wise usage of adjuvant & supportive drugs
Table 3.
Prescription pattern of each anticancer drug
| Name of Anticancer drug | Number of pts prescribed(n) | (%) |
|---|---|---|
| 5-Fluorouracil | 16 | 8.7 |
| Capecitabine | 13 | 7.07 |
| Carboplatin | 38 | 20.65 |
| Cisplatin | 45 | 24.46 |
| Cyclophosphamide | 18 | 9.78 |
| Docetaxel | 8 | 4.35 |
| Doxorubicin | 11 | 5.98 |
| Gemcitabine | 8 | 4.35 |
| Oxaliplatin | 10 | 5.43 |
| Paclitaxel | 17 | 9.24 |
| 184 | 100 |
Table 4.
Prescription pattern of each adjuvant drug
| Drug name | Number of pts given | (%) |
|---|---|---|
| Aprepitant | 4 | 0.586 |
| Dexamethasone | 131 | 19.2 |
| Furosemide | 63 | 9.23 |
| Potassium Chloride | 67 | 9.82 |
| Leucovorin | 3 | 0.439 |
| MESNA | 4 | 0.586 |
| Magnesium sulphate | 69 | 10.11 |
| Ondansetron | 143 | 20.96 |
| Pantaprozole | 60 | 8.79 |
| Rantac | 82 | 12.01 |
| Chlorpheniramine | 8 | 1.17 |
Table 5.
Prescription pattern of other supportive drugs
| Drug name | Number of pts given | (%) |
|---|---|---|
| Multivitamin injection | 41 | 6.01 |
| Sucracure | 3 | 0.439 |
| Zoledronic acid | 4 | 0.586 |
| Total- Adjuvant & other supportive drugs | 682 | 100 |
Discussion
Anticancer drugs utilisation
Totally 184 anticancer drugs were given to 144 study participants. The average number of these drugs in our study was 1.27 per patient. The present research showed that the most common class of cytotoxic agents prescribed was platinum compounds (64.58%).
Amongst the platinum analogues, the most commonly used drug was cisplatin that comprised 24.46%, followed by carboplatin and oxaliplatin which formed 20.65% and 5.43% respectively of all anticancer drugs. Cisplatin was used with radiotherapy in 33.3% of patients for the management of carcinoma of tongue, cervix, oesophagus and hypopharynx which were the most common types of cancers in the study population.
Carboplatin with radiotherapy was the second common standard regimen in cancer of the tongue and oral cavity in the present study. The antimetabolites were the next commonly prescribed anticancer drug class accounting for 25.69% of all anticancer drugs. 5-Fluorouracil was most common antimetabolite given (43.24%).
The other antimetabolites used were capecitabine (35.13%) and gemcitabine (21.6%). They were used in gastrointestinal cancers like carcinoma of rectum, colon and stomach. The use of cisplatin (18.5 %) and 5-FU (13 %) were also higher in a comparable study conducted by Dave et al., at PDU Govt. Medical College and Hospital, Gujarat [10]. Similar findings were seen in other related studies of Mary Rohini et al., 2015, Goyal et al., 2014 and Darshan et al., 2014 [11], [12], [13].
After antimetabolites, taxanes and alkylating agents were the 3rd and 4th commonest class of agents used. Amongst the taxanes which accounted for 17.36% anticancer drug use, the commonest taxane used was paclitaxel (68%), followed by docetaxel (32%). They were prescribed in carcinoma of the breast with secondaries, cervix, lung and stomach.
Adjuvant and supportive drugs utilisation pattern
Cancer chemotherapy includes anticancer medicines accompanied by adjuvant and supplementing therapeutic measures. These additional medications other than the anti-neoplastic drugs are used for reducing the adverse effects seen with the cancer chemotherapy. In total, 682 adjuvant drugs were given to 144 study participants. The average number of these drugs in our study were 4.73 per patient. The prescribing pattern of the different adjuvant and supportive drugs used is shown in Table 4 and 5. The antiemetics were the most commonly prescribed pre-chemotherapy drug accounted for 21.54% of total adjuvant drugs given, followed by the drugs used to reduce gastric acidity which constituted 20.8%. Inj Ondansetron was the most common used antiemetic dug which was administered intravenously in almost all patients, and Aprepitant was used in only in 4 patients of the study. On an average 90.9% of the patients were prescribed dexamethasone along with the chemotherapy. This adjuvant steroid, when used with chemotherapy, minimises the adverse effects like nausea and vomiting caused by chemotherapy medications. Also, it has found to improve appetite, decrease inflammation at the cancer site, and also decrease the elevated blood calcium levels (which is connected with some bone cancer cases) [14].
Cisplatin/carboplatin-based chemotherapy regimen causes hypomagnesemia and hypokalemia due to renal magnesium (Mg) and potassium (K) losses. Therefore potassium chloride and magnesium sulphate were given to almost all the patients receiving a cisplatin/carboplatin-based chemotherapy regimen. These patients also have a high risk of renal tubular dysfunction and a cumulative impairment in renal function, manifested by a decline in the glomerular filtration rate [15]. To prevent the development of nephrotoxicity, forced hydration in the form of saline infusion before, on the day of chemotherapy, and following cisplatin [16] and diuresis, by furosemide was given to the patients in our study. Although some researchers have already reported the effect of furosemide on reducing the renal toxicity, its effect on the prevention of nephrotoxicity is still controversial [17]. It has been reported that furosemide protects renal function, while it worsens renal histopathology [18]. However, it is discovered that prophylactic magnesium supplementation, in addition to curbing adverse effects that occur directly from magnesium deficiency, can minimise the severity of cisplatin-induced renal damage without interfering with the anticancer effect of the drug [19]. Magnesium performs an essential role in the preservation of intracellular K+ loss too. Therefore, in the present study, furosemide was always co-prescribed with MgSO4 to reduce the nephrotoxicity of cisplatin.
CPM was used as an antiallergic among eight patients. Chlorpheniramine maleate has found to be effective, patient convenient and very useful in preventing allergic reaction due to paclitaxel [20]. Mesna was used along with cyclophosphamide to prevent hemorrhagic cystitis. The other drugs prescribed included zoledronic acid in breast cancer with secondaries in bones. Administration of anticancer drugs is associated in some patients with severe acid reflux diseases which require the prescription of proton pump inhibitors, H2 antagonist and antacid prophylactically as well as therapeutically to the patients [21]. In our study, as gastric protectants, Ranitidine or Pantaprozole were given intravenously. Inj Ranitidine formed 12.01%, followed by Inj Pantaprozole which was 8.79% of the total prechemotherapy drugs in all the study groups. Inj Ranitidine was given in 82 patients and Inj Pantaprozole in 60 patients.
Table 6.
WHO prescribing indicators
| Prescribing Indicators | In patients |
|---|---|
| Number of drugs prescribed per patient | 6.01 |
| Percentage of drugs prescribed by Generic name | 76.7% |
| Number of anti-cancer drugs prescribed per patient | 1.27 |
| Number of adjuvant/supportive drugs prescribed per patient | 4.73 |
| Percentage of drugs prescribed from national essential drug list | 82.2% |
The prescribing indicator shows that the average number of cytotoxic drugs per prescription was 1.277, which was near to study conducted by B. Sajeev Kumar et al., (1.73) [22] and relatively lower than that of comparable studies conducted in Brazil (2.4), Jordan (2.3) and in other places in India (2.7) [23]. The average number of drugs per prescript was 6.01. The number of adjuvant/supportive drugs prescribed per patient was 4.73. The percentage of drugs prescribed by the Essential Drug List was 82%. The drugs were prescribed based on the hospital formulary and supplied on a nonprofit basis by the government. The percentage of drugs ordered by the generic name was 77%. Prescribing medicines by generic name must be strengthened since generic medicines are as efficient as brand ones, and they cost less which lowers the medical expenditure.
In conclusion, the head & neck cancers were the prevalent cancers observed with more preponderance among males. Most of the patients were prescribed with a single anticancer drug. Cisplatin was the most commonly used cytotoxic drug followed by carboplatin, and antimetabolites. The most commonly used adjuvant drugs in our study were anti-emetics and anti-peptic ulcer drugs. Over 82% of anticancer agents were taken from the essential drug list and were prescribed in generic names, indicating rational use. WHO promotes that more such drug utilisation studies are needed in every health care setting to assess and assure the rational drug use.
Acknowledgement
We thank Dr K. Laxminarayana N., Professor and H.O.D, Department of Pharmacology, Vijayanagara Institute of Medical Sciences, Ballari and Dr Meher Kumar, Head of Department, Department of Radiotherapy, Vijayanagara Institute of Medical Sciences, Ballari for their encouragement and the support for our study.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.WHO. The global burden of disease:2004 update. Geneva: World Health Orga¬nization; [updated 2008. [Google Scholar]
- 2.Cancer. World Health Organization:WHO. Available from: http://who.int/cancer/en .
- 3.American Cancer Society. Chemotherapy Principles. 2013. Available from: http://www.cancer.org/treatment/treatmentsandsideeffects/treatmenttypes/chemotherapy/chemotherapyprinciplesanindepthdiscussionofthetechniquesanditsroleintreatment/chemotherapy-principles-what-is-chemo .
- 4.Times of India. 48% cancers due to tobacco chewing. 2011. Available from: https://timesofindia. indiatimes.com/city/chandigarh/48-cancers-due-to-tobacco-chewing/articleshow/10649252. cms?
- 5.Haskell CM. Introduction Cancer treatment. 4th edn. Philadelphia, USA: WB Saunders; 1995. pp. 3–9. [Google Scholar]
- 6.Malhotra V, Perry MC. Classical chemotherapy:mechanisms, toxicities and the therapeutic window. Cancer Biol Ther. 2003;2:S2–4. https://doi.org/10.4161/cbt.199 PMid:14508075. [PubMed] [Google Scholar]
- 7.Rang HP, Dale MM, Ritter JM, Flower RJ. Rang and Dale's Pharmacology. Philadelphia: Elsevier Publisher; 2007. pp. 718–38. https://doi.org/10.1016/B978-0-443-06911-6.50056-6. [Google Scholar]
- 8.Sachdeva PD, Patel BG. Drug utilization studies- Scope and future perspectives. IJPBR. 2010;1:11–7. [Google Scholar]
- 9.World Health Organization. WHO:Introduction to drug utilization research/WHO International Working Group for Drug Statistics Methodology, WHO Collaborating Centre for Drug Statistics Methodology, WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services. Geneva: WHO; 2003. Available from: http://www.whocc.no/filearchive/publications/drug_utilization_research.pdf . [Google Scholar]
- 10.Dave DJ, Pillai A, Shah DV, Agarwal S, Goel A. An analysis of utilization pattern of anticancer drugs in diagnosed cases of carcinoma in a tertiary care teaching hospital. Int J Basic Appl Med Sci. 2014;4:251–59. [Google Scholar]
- 11.Pentareddy MR, Suresh AV, Shailendra D, Subbaratnam Y, Prasuna G, Naresh DT, Rajsekhar K. Prescription pattern of anticancer drugs in a tertiary care hospital. Journal of Evidence based Medicine and Healthcare. 2015;2(20):3001–9. https://doi.org/10.18410/jebmh/2015/435. [Google Scholar]
- 12.Yash N Goyal, Krunal C Solanki, Rusva A Mistry, Niasrg D Joshi, Anil P Singh, Maganlal V Gajera. Pattern of adverse drug reactions due to cancer chemotherapy in tertiary care teaching hospital in Gujarat. International Journal of Scientific Research. 2014;3(1):333–335. https://doi.org/10.15373/22778179/JAN2014/112. [Google Scholar]
- 13.Darshan J Dave, Ajita Pillai, Dimple V Shah, Sneha Agrawal, Anilkumar Goel. An analysis of Utilization Pattern of Anticancer drugs in diagnosed cases of Carcinoma in a tertiary care teaching hospital. International Journal of Basic and Applied Medical Sciences. 2014;4(1):251–259. [Google Scholar]
- 14.Hesketh PJ. Defining the emetogenicity of cancer chemotherapy regimens:relevance to clinical practice. Oncologist. 1999;4:191–6. PMid:10394587. [PubMed] [Google Scholar]
- 15.Wolters Kluwer Health-Up-to-date. Cisplatin nephrotoxicity. 2013. Available from: http://www.uptodate.com/contents/cisplatin-nephrotoxicity .
- 16.Losonczy G, Mathe C, Muller V, Szondy K, Moldvay J. Incidence, risk factors and prevention of cisplatin induced nephrotoxicity in patients with lung cancer. Magy Onkol. 2010;54:289–96. doi: 10.1556/MOnkol.54.2010.4.3. https://doi.org/10.1556/MOnkol.54.2010.4.3 PMid:21163759. [DOI] [PubMed] [Google Scholar]
- 17.Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecologic oncology. 1993;50(2):147–58. doi: 10.1006/gyno.1993.1184. https://doi.org/10.1006/gyvno.1993.1184 PMid:8375728. [DOI] [PubMed] [Google Scholar]
- 18.Lehane D, Winston A, Gray R, Daskal Y. The effect of diuretic pre-treatment on clinical, morphological and ultrastructural cis-platinum induced nephrotoxicity. International Journal of Radiation Oncology*Biology*Physics. 1979;5(8):1393–9. doi: 10.1016/0360-3016(79)90677-1. https://doi.org/10.1016/0360-3016(79)90677-1. [DOI] [PubMed] [Google Scholar]
- 19.Lajer H, Daugaard G. Cisplatin and hypomagnesemia. Cancer Treat Rev. 1999;25:47–58. doi: 10.1053/ctrv.1999.0097. https://doi.org/10.1053/ctrv.1999.0097 PMid:10212589. [DOI] [PubMed] [Google Scholar]
- 20.Harada T, Doi M, Yamada Y, Akase T. Evaluation of short-time premedication with d-chlorpheniramine maleate injection for paclitaxel-induced hypersensitivity reaction. Gan to kagaku ryoho. Cancer &chemotherapy. 2008;35(8):1347–51. PMid:18701846. [PubMed] [Google Scholar]
- 21.The Scott Hamilton CARES initiative. Heartburn and chemotherapy. Available from: http://chemocare.com/chemotherapy/side-effects/heartburn.aspx#.U0sDNPmSwd4 .
- 22.Kumar BS, Maria S, Shejila CH, Udaykumar P. Drug Utilization Review and Cost Analysis of Anticancer Drugs Used in a Tertiary Care Teaching Hospital. Indian Journal of Pharmaceutical Sciences. 2018;80(4):686–93. https://doi.org/10.4172/pharmaceutical-sciences.1000408. [Google Scholar]
- 23.Khan MK, Thapa RK, Adhikari DS, Rajbhandari M, Dwa P, Shrestha S, et al. Evaluation of cancer prevalence and cytotoxic medication prescribing in central region of Nepal. Kathmandu Univ J Sci Eng Technol. 2013;9:189–99. [Google Scholar]


