Abstract
BACKGROUND:
Malignant Ovarian Germ Cell Tumors (MOGCT) most commonly occur in young women in the reproductive age group. Timely antenatal diagnosis and treatment of the tumour to enhance maternal and perinatal outcomes are the main challenges confronting the obstetrician and the gyne-oncologist.
CASE PRESENTATION:
Here we present three cases of pregnancy complicated with MOGCTs. The first case (immature teratoma) was complicated by maternal psychological symptoms consistent with stress and histopathological examination confirmed the diagnosis of premature ovarian failure (POF). The second case (dysgerminoma) preterm labour occurred as an obstetric complication, but the baby was born in good condition without IUGR. The third case (yolk sac tumour) treated with docetaxel (brexel)-carboplatin chemotherapy administration there was no maternal or fetal complication. At the end of the pregnancy and delivery, complete surgical staging and cytoreduction were performed, and no metastases were found.
CONCLUSION:
Optimal management strategies centre on a multi-disciplinary comprehensive team approach is critical resulting in better outcomes for the mother and the baby by avoiding complications.
Keywords: Ovarian malignant germ cell tumours, Pregnancy, Chemotherapy, Surgical staging
Introduction
Germ cell tumours (GCTs) originate from ovarian primordial germinal cells [1]. Malignant Ovarian Germ cell tumours occur most commonly in young women, usually unilateral (84.3%) and diagnosed at stage I (76.4%) [2]. The most common is dysgerminoma, the second is immature teratoma, and the third is the Yolk sac tumour. These tumours can be pure or mixed. The worldwide cancer prevalence in 2012 according to the International Agency for Research on Cancer (IARC) was 6,657,518. The prevalence of ovarian cancer was 238,719 (3.6%) with overall mortality 151,917 (4.3%) [3]. Based on Dharmais Cancer Hospital data in 2010-2013, the incidence of ovarian cancer in Indonesia was 536, and 126 women died. There were 8 cases of MOGCTs recorded at Pathology Department of Sanglah Hospital in 2016 to 2017 which consisted of 1 case of dysgerminoma, 5 cases of immature teratoma, 1 case of yolk sac tumour, and 1 case of mixed GCTs. Three cases of MOGCTs in pregnancy will be described in this report. The incidence of MOGCTs in pregnancy is very rare which 1 in 12,500-25,000 pregnancies [4]. Prognosis is dependent on histopathological tumour grading [2]. Decision-making regarding timing, mode, and place of delivery should be made by a comprehensive team approach which will reduce maternal and perinatal morbidity and mortality [3], [4]. The incidence of gynaecological cancer over the past four decades has been rising associated with the trend of delayed childbearing [5]. As a result, the average age of primigravidae increased thus raising the risk of ovarian cancer and ovarian cancer during pregnancy.
We present three cases of pregnancy complicated with MOGCTs. All cases were delivered at term after conservative primary surgery performed involving unilateral salpingo-oophorectomy in late mid-trimester followed by chemotherapy until delivery and then surgical staging and debulking at relaparotomy after delivery of the baby. Surgical staging was conducted according to the FIGO Staging classification of ovarian tumours.
Case 1
A 31 y.o. Multigravida (G2P1A0) at 8 weeks 1 day (8w1d) single/life gestation with solid ovarian tumour referred by the obstetrician to Obstetric and Gynecologic Policlinic of Sanglah Hospital, Denpasar. The pelvic examination found an adnexal tumour which increased progressively in size from 15 x 15 cm at 8w1d gestational age (GA) to 30 x 30cm at 19w5d GA, which was solid, with an irregular surface, mobile, and painful. Tumor marker levels were raised, AFP: 699.9 IU/mL (normal level at 2nd trimester: 22-93 IU/mL), CA-125: 285.4 U/mL (normal level at 2nd trimester: < 35 U/mL), LDH: 579 U/L (normal level at 2nd trimester: 240-480). The patient had primary surgery (left oophorectomy, cytological test, and omentectomy) at 19w5d GA with minimal manipulation of the uterus. A midline abdominal incision performed, and an operation, a unilateral solid ovarian tumour identified 40 x 40cm in size with an irregular surface, arising from the left ovary with omental adhesions requiring adhesiolysis despite rupture of the tumour, it was successfully removed (Figure 1).
Figure 1.

Immature Teratoma Ultrasound (US) and Macroscopic Finding Durante Operation. CASE 1: A. US showed hypohyperechoic left adnexal mass which cannot reach totally inside probe range at 19w5d GA, irregular border, unsmooth surface; B. uterine size appropriate with 18-20 weeks GA, left Fallopian tube was normal, post-oophorectomy; and C. Lobulated solid mass with internal multiseptal cystic mass
Internal abdominal and genitalia organ evaluation of uterus, size/consistency appropriate 18-20 weeks GA, right and left Fallopian tube to seem normal, right ovarium seems normal, no nodules on the peritoneum, omentum, and liver.
Histopathological assessment of left ovary was a grade II immature teratoma with immature components consisting of neuro-epithelial tubule and immature fat tissue; there was no evidence of malignant cell/immature component cell spreading on peritoneal fluid cytology. The patient’s tumour was designated as a Stage IC1 grade II Ovarian immature teratoma (FIGO). An MRI conducted at 25w3d gestation without evidence of tumour spreading. After appropriate consultation of the multidisciplinary team, four courses of Bleomycin-Etoposide-cisplatin (BEP) chemotherapy regimen treatment was instituted (starting at 27w2d gestation), with two weeks follow up of fetal well-being with USG fetal scanning and fetal well-being every 2 weeks after chemotherapy was instituted along with routine NST after 34 weeks’ gestation. Chemotherapy was paused 2 weeks before labour at 37w 6d gestation. The pregnancy continued until term and at gestation age 40w2d a female baby, 2700 gram, was delivered vaginally following the spontaneous onset of labour. At delivery, the baby had an Apgar score 7-8, with no evidence of congenital abnormality. Secondary surgery (relaparotomy complete surgical staging) was performed on day 58 postpartum. The surgeon performs a Total Abdominal Hysterectomy (TAH), left salpingectomy, right salpingo-oophorectomy, bilateral pelvic and paraaortic lymphadenectomy, omentectomy and peritoneal biopsy (bilateral paracolic- and prevesical peritoneum). Histopathological examination revealed nodules containing only mature glial components on the peritoneum (prevesical and cavum Douglas), with no evidence of malignant cells or metastases.
Case 2
A 24-year old pregnant woman was sent by Karangasem Hospital to Sanglah Hospital Denpasar with a chief complaint of dyspnea with a swollen abdomen for 11 days before admission. The patient was in her third pregnancy at 29w gestation with a solid ovarian tumour. Tumour marker levels were raised, AFP: 135.48 IU/mL, CA-125: 113.4 U/mL, RMI: 340.32, Human Epididymis Protein 4 (HE4): 68.9, ROMA: 99.37%. Corticosteroids were administered before the planned surgical procedure to enhance fetal lung maturation, and tocolytics were also administered. Utrogestan orally 2 x 200 mg was given 24 hours before surgery and continued for 48 hours post-laparotomy.
The patient had primary surgery (Laparotomy left salpingo-oophorectomy and omentectomy, frozen section and cytological test) at 30w gestation through a midline abdominal incision. The operation revealed a solid ovarian mass admixed with a cystic component 19 cm x 16 cm x 13 cm in size. Internal abdominal evaluation of the uterus was consistent with 30w gestation, the right and left Fallopian tubes were normal, the right ovary was normal, and there were no nodules on the peritoneum, omentum, and liver (Figure 2). The result of the histopathological assessment of left ovary was a dysgerminoma with a round to oval shape atypical cells separated with complex thin fibrous tissue septal network. Ascitic fluid cytology was positive for malignant cells, but no malignant cells were found in the momentum. The patient was staged as a FIGO Stage IC dysgerminoma ovarian cancer. No fetal anomalies were detected on feto-maternal ultrasound examination before treatment.
Figure 2.

Dysgerminoma Ultrasound and Macroscopic Finding Durante Operation. Case 2. A. USG showed hypo-hyperechoic left adnexa mass 11.17x8.16 with papillae at 29w2d GA; B. and C. Durante operation: solid mass mixed with cystic 19 cm x 16 cm x 13 cm in size, arising from left ovarium
Following primary surgery, the patient received 4 courses of BEP regimen (starting at 33w1d gestation), and she was planned to have a re-laparotomy with surgical staging at the time of caesarean section at 38-weeks gestation age. However, the patient began in labour spontaneously at 35 weeks’ gestation and delivered vaginally with the baby, a male, 2100 g with Apgar scores of 7 and 8, and Ballard scores of 28 equivalent to 35-36w gestation. The placenta appeared normal at the time of delivery, and histopathological examination was not performed. Postpartum follow up was uneventful. No signs of myelosuppression of the baby were found, with haemoglobin level being 15,9 g/dL. The baby was not breastfed during the period of maternal chemotherapy. Abdominal USG was performed after delivery which demonstrated no spread of the tumour. Re-laparotomy surgical staging was performed at 42 days postpartum, and chemotherapy continued until completion of the 4 courses.
It was decided to perform TAH and right salpingo-oophorectomy. Histopathological examination revealed no evidence of malignancy. The patient advised to have a routine physical check-up, ultrasonography, CA 125 and AFP serum tumour markers every 3 months in the first year which normal. The baby is now 2 y.o., in good condition and growing normally.
Case 3
A 27-year old pregnant woman referred to Sanglah Hospital by a provincial hospital from West Nusa Tenggara with a diagnosis of 17–18 weeks’ gestation pregnancy and ovarian cyst suspicious of malignancy.
USG revealed a single fetus, fetal heart and movements were present, breech presentation, with 18w5d GA. At 19–20 weeks GA an ovarian cyst suspicious of malignancy (RMI: 400.80) confirmed and based on that finding the RMI was 400. A laparotomy was a right salpingo-oophorectomy, omentectomy and appendicectomy performed. Cytological examination of the ascitic fluid revealed seeding of mucinous adenocarcinoma, and the FZ result was mucinous adenocarcinoma. The result of histopathological examination of paraffin-embedded tissue was a Yolk Sac ovarian tumour (reticular and glandular pattern) (Figure 3). After pathological review, a Yolk Sac tumour of right ovary was confirmed with diagnosis G2P1001 21w3d gestation singleton pregnancy with an ovarian cancer FIGO stage IC post SOD, omentectomy, appendicectomy.
Figure 3.
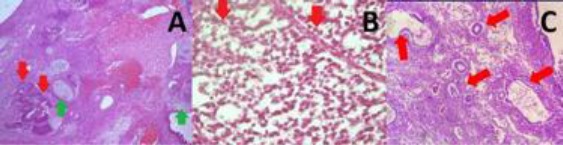
Microscopy of Immature Teratoma, Dysgerminoma, and Yolk Sac Tumor. A: Immature teratoma, consist of immature component/neuro-epithelial rosette and tubules (red arrow), mature glial tissue which pointed with green arrow, and another mature mesodermal component. B: Dysgerminoma, consist of round to oval atypical cell separated with complex thin fibrous tissue septal network which have rich lymphocyte infiltrate (red arrow). C: Yolk sac tumor, consist of cuboidal to columnar atypical cell forming gland-like structure, in part have clear cytoplasm forming glandular pattern (red arrow)
Docetaxel (Brexel)–carboplatin given for 6 courses. Follow up of fetal well being performed by the Feto-maternal Division every 2 weeks until chemotherapy was completed. Fetal scanning was performed at gestation age 26w 5d. No major congenital anomaly was found. Monitoring with NST was started at 35 weeks’ gestation and remained normal. Chemotherapy was ceased 3 weeks before a planned caesarean section. This was done to allow time for recovery of the bone marrow of mother and baby.
The final diagnosis was FIGO ovarian cancer stage IC post TAH + BSO + omentectomy + appendicectomy, and post-chemotherapy. Patient advised to undergo routine physical examination, ultrasonography, CA 125 and serum AFP every month for the first 3 months and then 6 monthly for 2 years.
Discussion
In our case series, the first case was a 31 y.o pregnant woman with immature teratoma, the second case was a 24 y.o pregnant woman with a dysgerminoma, and the third case was a 27 y.o pregnant woman with a yolk sac tumour — the characteristics of the cases described in Table 1.
Table 1.
Table of Case Illustration
| Criteria | Cases | ||
|---|---|---|---|
| First | Second | Third | |
| Maternal Age | 31 y.o | 24 y.o | 27 y.o. |
| Patient finding | Abdomen enlarged at 8w 1d gestation | Abdomen enlarged & dyspneu at age 29w gestation | Abdomen enlarged at 19-20w gestation |
| Ultrasonography Finding | Hypo-hyperechoic mass, sircuscribed, rough surface, papillary, with septa | Hypo-hyperechoic mass, papillary | Hypo-hyperechoic mass, with septa |
| Tumor Marker | (-) | CA 125: 113.4 | CA 125: 400.8; |
| (-) | RMI: 340.32 | RMI : 400.8 | |
| AFP: 699.9 | AFP: 135.48 | (-) | |
| Antenatal Surgery time/type | 19w 5d, left oophorectomy, omentectomy | 30 w, Left salphingo-oophorectomy - FZ | 21w, Right salphingo-oophorectomy-FZ, Omentectomy, Appendisectomy |
| Durante Operation Finding | Prominent solid component, 40x40 cm, lobulated, with cystic component, multisepta | Solid mass with cystic component, 19x16x13 cm | Solid mass with cystic component |
| FIGO Stage | IC1 | IC | IC |
| Histophatology Type | Immature Teratoma grade II | Dysgerminoma | Yolk sac tumor |
| Metastase Evaluation | MRI (no metastase) | USG (no metastase) | (-) |
| Chemotherapy time/type | BEP 4 series at 27w2d gestation | BEP 4 series at 33w gestation | Docetaxel (Brexel) – Carboplatin 6 series at 21w 3d |
| Complication maternal after chemotherapy | POF | (-) | (-) |
| Complication fetal after chemotherapy | (-) | Preterm delivery | (-) |
| Mode of delivery/time | Vaginal delivery At 40w 2d gestation | Vaginal delivery At 35w gestation | CS At 38w gestation, |
| Placental metastasis | (-) | Not assesed | Not assesed |
| Fetal Outcome | 2700 g (persentile 10-25), vigorous, no congenital abnormality | 2105 g (persentile 25-75), vigorous, no congenital abnormality | 3165 g (persentile 50-75), vigorous, no congenital abnormality |
| Surgical staging time/type | Complete surgical Staging at 58 d postpartum | Cytoreduction at 42 d postpartum, | Cytoreduction at the same with CS, at 39w |
| Breast feeding | (-) | (-) | (-) |
| Survival 5 years | 62% | >90% | 90% |
Diagnosis of MOGCTs in the young female is challenging using tumour markers (AFP, LDH, hCG, CA125-RMI) and the US, with histopathological as the gold standard. Increased tumour markers level occur during pregnancy. Hence tumour markers level should be cautiously interpreted, and treatment strategies should not be based on these markers alone [8]. AFP produced initially by the fetal yolk sac and continued by the liver and digestive tract with a high AFP level associated with the suspicion of neural defect abnormalities such as spina bifida, anencephaly, oesophageal defect, and fetal abdominal separation failure. The AFP level decreased exponentially after primary ovarian tumour removal, and it concluded that the increased AFP level in this patient, originated from the primary tumour, and similarly with the other 2 cases where no major structural abnormality found.
The first case complicated by maternal psychological symptoms consistent with stress. The FSH level was high at 50.01 mU/L, but FSH has poor accuracy as a diagnostic marker in pregnancy for ovarian failure. The AMH is more reliable, and this plus the right ovarian atrophy (contralateral ovary) demonstrated on histopathological examination confirmed the diagnosis of premature ovarian failure (POF) as a possible contributor to her symptoms. Following immunohistochemistry (IHC) to detect estrogen receptors (ER) and progesterone receptors (PR) on affected left ovary (ovary with immature teratoma) it was concluded that hormone replacement therapy (HRT) could be safely given due to ER (-) and PR (+) [6]. With the second case, preterm labour occurred as an obstetric complication, but the baby was born in good condition without IUGR (percentile 25-50). The third case (yolk sac tumour) treated with docetaxel (brexel)-carboplatin chemotherapy administration there was no maternal or fetal complication. Carboplatin a second generation platinum-based drug was shown by Mir et al., to be a safer drug compared to cisplatin with no apparent fetal malformation or maternal toxicity [2], [7].
In our case series, all the babies had normal weight. Laboratory findings at first week after birth not found any evidence of transient myelosuppression. It was suggested that routinely follow up of the baby every 6 months needed for the first 2 years as a minimum. At the end of pregnancy and delivery, complete surgical staging and cytoreduction were performed, and no metastases were found.
Management of Patient
Conservative Surgery
The treatment of MOGCTs in pregnancy is by primary conservative surgery and chemotherapy followed by a complete surgical staging and cytoreduction post-delivery. Removal of an adnexal mass at pregnancy as conservative surgery is best done through a midline abdominal incision with a unilateral oophorectomy and cystectomy, omentectomy, and peritoneal fluid cytology to confirm the histopathological diagnosis. An adnexal mass detected in the first trimester of pregnancy in a patient with an ovarian lesion of suspected low malignant potential can be treated with conservatively. However in women with an ovarian mass with septae, solid areas, papillae, nodules, or persisting until 16 weeks gestation, surgery may be postponed until the second trimester (16-18 weeks gestation), as it is a ‘safe period’ for the fetus and the best period to perform surgical intervention of an adnexal mass as the spontaneous abortion risk is low (up to 10%), and an acceptable operative field is still available, allowing minimal uterine manipulation and lowering the risk of obstetric complications. The surgery can be performed ideally at 24-26 weeks gestation [9].
Adnexal mass surgery in the third trimester increases the incidence of preterm labour up to 22% and is associated with adverse obstetric outcome. Adnexal masses detected after 35 weeks gestational age can be removed at caesarean section [9]. Other indications for operative management include acute abdominal symptoms such as pain, rupture, and torsion. Routine tocolytic administration is still controversial but can be considered if there are signs of preterm labour [10, [11].
There is still a chance for spontaneous vaginal delivery with surgical staging completed 3 to 6 weeks after delivery. If operative delivery chosen then cytoreduction may be done at the same time as caesarean section. Re-laparotomy to complete surgical staging or cytoreduction is recommended as these may be limited during the pregnancy period, and there are reports of rapid growth and recurrence of OMGCTs during the puerperium.
Chemotherapy
The most challenging problem of chemotherapy during pregnancy is to obtain optimal chemotherapy without disturbing fetal growth and development with gestation age as a consideration [7]. Malignant ovarian GCTs are sensitive to chemotherapy and radiotherapy. Chemotherapy combination is usually used as adjuvant if the choice to continue the pregnancy. The choice of treatment determined by tumour type and its histological features to obtain the best response and improve prognosis. Bleomycin-etoposide-cisplatin is the first line chemotherapy for MOGCTs with treatment every 3 weeks for 3-4 courses. Patients have a good prognosis after cisplatin-based chemotherapy combination [13].
One review of the literature found that BEP chemotherapy was not associated with increasing congenital malformations [11]. Another study the use of cisplatin suggested an association with hearing loss (2,7%), ventriculomegaly (2%), IUGR and prematurity (8.3%), oligohydramnios (5.6%), anaemia (5.6%), micro-ophthalmia (2.8%) [6], [12]. Additionally, BEP may cause an imbalance of procoagulant-anticoagulant associated with disseminated intravascular coagulation, thrombosis, and bleeding (cisplatin-associated with liver vessel endothelial damage which causes ischemia and infarction; bleomycin associated with disturbance of blood clotting cascade; etoposide associated with thrombocytopenia).
Labour and Delivery
The optimal labour time is after 35-37 weeks and 3 weeks post-chemotherapy to avoid chemotherapy accumulation and to allow recovery from possible bone marrow suppression of mother and baby [13]. Mode of delivery preference was spontaneous vaginal rather than caesarean section. The benefits of spontaneous delivery are less blood loss, less operative risk and low infection risk with shorter length of stay at the hospital. Women having chemotherapy not recommended breastfeeding due to the risk of chemotherapy excreted through breast milk which could result in neonatal pancytopenia. Placental metastases often mentioned as a curiosity but occurred very rarely with only 80 cases reported since 1866. The incidence of fetal metastases is even more rare with only 11 cases reported in the literature [15].
Prognosis
The staging system used was the International Federation of Gynecology and Obstetrics (FIGO) 2014 with three ovarian cancers being stage IC. Malignant germ cell tumour has a better prognosis (> 95%) if detected at stage I. Advanced MOGCTs very aggressive, but prognosis may still be good if not delay chemotherapy. Prognosis of immature ovarian teratoma is governed by grade and stage. Five-year survival rate overall for all stages is 70-80%, and 90-95% for stage I patients. The five-year survival rate is 82%, 62%, and 30% respectively in patients with grades 1, 2, and 3 treated with optimum chemotherapy. Recurrence occurs on 36% during 5 years follow up, and the survival rates of early and advanced dysgerminomas are 95 and > 80%, respectively. Stage 1a dysgerminoma after unilateral salpingo-oophorectomy has a relapse rate of 10 to 20% with overall survival rate 90-100%. Recurrence patients who undergo repeat chemotherapy still have a survival rate greater than 90% [5].
In conclusion, malignant ovarian germ cell tumours in pregnancy are rare but challenging. Optimal management strategies centre on a multi-disciplinary comprehensive approach about diagnosis, primary surgery, chemotherapy, delivery, complete surgical staging, and neonatal care. Such an approach is critical resulting in better maternal and fetal outcomes by avoiding complications.
Acknowledgements
The authors wish to acknowledge the invaluable assistance given to them by the nursing and theatre staff and Pathology Department of Sanglah Hospital in the management of the patients and for the advice offered by Prof John Svigos, Visiting Professor Department of Obstetrics and Gynecology, Sanglah Hospital and University of Udayana in the preparation of this paper for publication.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Akhtar K, Ahmad S S, Kumar A, Afshan N. Dysgerminoma with Pregnancy and Viable Baby:A Case Report. Oman Medical Journal. 2011;26:198–200. doi: 10.5001/omj.2011.48. https://doi.org/10.5001/omj.2011.48 PMid:22043416 PMCid:PMC3191700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert J K, Maria L C, Simon C H, Robert H Y. WHO Classification of Tumours of Female Reproductive Organs. 4th Edition. Lyon: International Agency for Research on Cancer; 2014. [Google Scholar]
- 3.Andrijono, Kanker Ovarium. Dalam:Sinopsis Kanker Ginekologi. Edisi Keempat. Jakarta: Balai Penerbit Fakultas Kedokteran Universitas Indonesia; 2013. [Google Scholar]
- 4.Peccatori, et al. Cancer, Pregnancy and Fertility:ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Annals of Oncology. 2013;24(6):vi160–70. doi: 10.1093/annonc/mdt199. https://doi.org/10.1093/annonc/mdt199. [DOI] [PubMed] [Google Scholar]
- 5.Gupta M, Saini V. Germ Cell Tumors and Their Association with Pregnancy. New Delhi: Hindu Rao Hospital &NDMC Medical College; 2018. https://doi.org/10.5772/intechopen.72556. [Google Scholar]
- 6.Morgan S. How Do Chemotherapeutic Agents Damage the Ovary? Edinburgh: The University of Edinburgh; 2014. [Google Scholar]
- 7.Berek J.S, Friedlander M.L, Hacker N.F. Berek and Hacker's Gynecologic Oncology. 6th ed. Philladelphia: Wolters Kluwer; 2015. Germ Cell and Nonepithelial Ovarian Cancer; pp. 541–2. [Google Scholar]
- 8.Voulgaris E, Pentheroudakis G, Pavlidis N. Cancer and Pregnancy:A Comprehensive Review. Surgical Oncology. 2011;20:175–85. doi: 10.1016/j.suronc.2011.06.002. https://doi.org/10.1016/j.suronc.2011.06.002 PMid:21733678. [DOI] [PubMed] [Google Scholar]
- 9.Gezginc K, Karatayli R, Yazici F, Acar A, Celik C, Capar M. Ovarian Cancer during Pregnancy. International Journal of Gynecology and Obstetrics. 2011;115:150–143. doi: 10.1016/j.ijgo.2011.05.025. https://doi.org/10.1016/j.ijgo.2011.05.025 PMid:21872237. [DOI] [PubMed] [Google Scholar]
- 10.Grimm D, et al. Clinical management of epithelial ovarian cancer during pregnancy. European Journal of Cancer. 2014;50:963–971. doi: 10.1016/j.ejca.2013.12.020. https://doi.org/10.1016/j.ejca.2013.12.020 PMid:24462638. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman L B, Schorge J O, Schaffer J I, Halvorson L M, Bradshaw K D, Cunningham F G. Williams Gynecology. 2nd Edition. New York: McGraw Hill Medical; 2012. Ovarian Germ Cell and Sex Cord-Stromal Tumors; pp. 879–97. [Google Scholar]
- 12.Robert J K, Lora H E, Brigitte M R. Blaustein's Pathology of the Female Genital Tract. Sixth Edition. New York: Springer; 2011. [Google Scholar]
- 13.Koren G, Carey N, Gagnon R, Maxwell C, Nulman I, Senikas V. Cancer Chemoterapy and Pregnancy. SOGC Clinical Practice Guideline. 2013;288:263–78. doi: 10.1016/S1701-2163(15)30999-3. [DOI] [PubMed] [Google Scholar]
- 14.Amant F, et al. Gynecologic cancer in pregnancy:Guideline of an international consensus meeting. 2009;19:51. doi: 10.1111/IGC.0b013e3181a1d0ec. [DOI] [PubMed] [Google Scholar]
- 15.Sebire N J, Jauniaux E. Fetal and Placental Malignancies:Prenatal Diagnosis and Management. Ultrasound Obstet Gynecol. 2009;33:235–4. doi: 10.1002/uog.6246. https://doi.org/10.1002/uog.6246 PMid:19009536. [DOI] [PubMed] [Google Scholar]


