Abstract
Background
The benefit of aspirin among patients with stable atherosclerosis without a prior ischemic event is not well defined.
Hypothesis
Aspirin would be of benefit in outpatients with atherosclerosis with prior ischemic events, but not in those without ischemic events.
Methods
Subjects from the Reduction of Atherothrombosis for Continued Health registry were divided according to prior ischemic event (n =21 724) vs stable atherosclerosis, but no prior ischemic event (n = 11 872). Analyses were propensity score matched. Aspirin use was updated at each clinic visit and considered as a time‐varying covariate. The primary outcome was the first occurrence of cardiovascular death, myocardial infarction, or stroke.
Results
In the group with a prior ischemic event, aspirin use was associated with a marginally lower risk of the primary outcome at a median of 41 months (hazard ratio [HR]: 0.81, 95% confidence interval [CI]: 0.65‐1.01, P = 0.06). In the group without a prior ischemic event, aspirin use was not associated with a lower risk of the primary outcome at a median of 36 months (HR: 1.03, 95% CI: 0.73‐1.45, P = 0.86).
Conclusions
In this observational analysis of outpatients with stable atherosclerosis, aspirin was marginally beneficial among patients with a prior ischemic event; however, there was no apparent benefit among those with no prior ischemic event.
Keywords: Adverse Cardiovascular Events, Aspirin, Atherosclerosis, Coronary Artery Disease, Stable Ischemic Heart Disease, Myocardial Infarction
1. INTRODUCTION
Aspirin has been documented to be beneficial during acute myocardial infarction (MI) and acute ischemic stroke.1, 2 In a large meta‐analysis of multiple randomized trials, aspirin was confirmed to be efficacious in the secondary prevention of future cardiovascular events.3 Although not well studied, aspirin has been assumed to be of benefit and recommended in all forms of atherosclerosis, including those without a prior ischemic event.4, 5, 6 However, contemporary studies are limited. A post hoc analysis of hypertensive patients with coronary artery disease (CAD) enrolled in a clinical trial found that aspirin use was associated with a lower risk for cardiovascular events among those with a prior ischemic event; however, there was a lack of evidence for benefit in patients with stable CAD with no prior ischemic event.7 The Reduction of Atherothrombosis for Continued Health (REACH) registry is a large international, prospective registry that enrolled outpatients with atherothrombosis, where the use of aspirin was at the discretion of the treating physician. This provided a pragmatic opportunity to evaluate the efficacy of aspirin use across a wide variety of clinical practice settings in atherosclerosis patients with and without a prior ischemic event.
2. METHODS
The design and methods of the REACH registry have been previously published.8, 9, 10, 11 In brief, REACH is a prospective, observational registry that enrolled consecutive patients at least 45 years of age with CAD, cerebrovascular disease, peripheral artery disease, or ≥3 risk factors for atherosclerosis. Study participants were from 7 geographical regions. Enrollment occurred between December 2003 and June 2004. The last follow‐up data collection was April 2009. Written informed consent was obtained from all the participants, and the protocol was approved by the local institutional review board. Data were centrally collected using standardized case report forms.
For the purpose of the current analysis, aspirin use was updated at each clinic visit and considered as a time‐varying covariate. Patients using adenosine diphosphate receptor antagonists or anticoagulants at baseline were excluded from the analysis.12 Patients were categorized into 2 groups: prior ischemic event (defined as history of unstable angina, MI, transient ischemic attack [TIA], or stroke, irrespective of the timing of the event) and stable atherosclerosis, but no prior ischemic event (defined as stable angina requiring medication; history of percutaneous coronary intervention or coronary artery bypass grafting; ≥1 carotid plaque or asymptomatic carotid stenosis ≥70%, history of carotid angioplasty, stent, or surgery; ankle brachial index <0.9 or lower extremity angioplasty, stent, surgery, or amputation). Participants were followed prospectively for up to 4 years. The primary outcome was the first occurrence of cardiovascular death, MI, or stroke. The secondary outcome was the first occurrence of all‐cause death, MI, or stroke. The tertiary outcomes were cardiovascular death, all‐cause death, MI, and stroke individually. Bleeding was defined as any event resulting in hospitalization or transfusion.
Propensity score matching was constructed in an attempt to balance the different baseline characteristics between exposure groups.13 Propensity score matching was selected over other models due to its greater ability to eliminate bias.14 Propensity scores were calculated using a nonparsimonious multivariable logistic regression model,13, 14, 15 with the dependent variable of time‐varying aspirin use, and 22 baseline characteristics entered as covariates (sex, age, region, ethnic origin, current smoking, congestive heart failure, hypercholesterolemia, hypertension, diabetes, atrial fibrillation/flutter, use of diuretics, β‐blockers, calcium channel antagonists, nitrates/other antiangina agents, lipid‐lowering agents, non–steroidal anti‐inflammatory drugs, insulin, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, other antihypertensives, aortic stenosis, body mass index, and peripheral arterial claudication medications). To assess the propensity‐score effectiveness of matching cohorts, we estimated absolute standardized differences between aspirin and nonaspirin users, for each variable and in both study groups. An absolute standardized difference <10% for a given covariate indicated a relatively small imbalance.
Descriptive statistics including frequencies and percentages for categorical variables, and mean and standard deviation for continuous variables were presented. Comparative statistics (Student t test for continuous variables and χ2 test for categorical variables) were used to compare baseline characteristics. The risk of an outcome in aspirin users vs nonusers was compared using a Cox proportional hazard regression model conducted on the propensity score–matched cohort. To account for excluded patients in the propensity‐matched analysis, a sensitivity analysis using a propensity‐adjusted analysis was also performed.16, 17 Another sensitivity analysis for the propensity score–matched cohorts with aspirin status at baseline introduced in the models (instead of aspirin as a time‐varying covariate) was conducted. Subgroup analyses were also performed for the primary outcome for each cohort on the following variables: diabetes vs no diabetes, statin use vs no statin use, history of coronary revascularization vs no coronary revascularization, recent ischemic event (≤1 year) vs remote ischemic event (>1 year), and history of unstable angina/MI vs TIA/stroke. All analyses were conducted using SAS software version 9.3 (SAS Institute Inc., Cary, NC).
3. RESULTS
Among all participants in the REACH registry, 21 724 were classified into the prior ischemic event group, and 11 872 patients into the stable atherosclerosis, but no prior ischemic event group (Figure 1).
Figure 1.
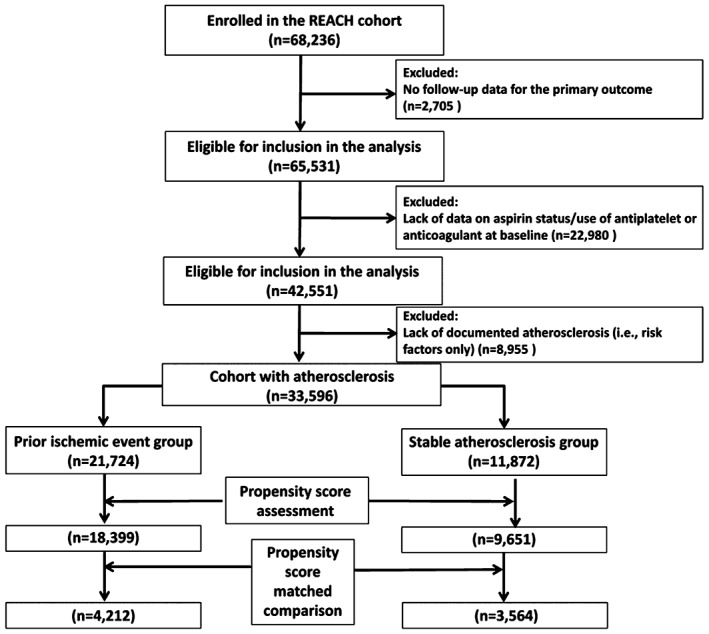
Flow diagram of study participants. Abbreviations: REACH, Reduction of Atherothrombosis for Continued Health.
3.1. Prior ischemic event group
In the prior ischemic event group, 19 228 patients (88.5%) reported using aspirin at baseline. Among aspirin users, aspirin dose was ≥325 mg in 16.2%. Out of the 18 324 patients who reported aspirin at baseline and had at least 1 aspirin status at any follow‐up), 16 072 patients (88%) reported aspirin at the latest available follow‐up. Out of the 2319 patients who did not report aspirin at baseline and had at least 1 aspirin status at any follow‐up), 793 patients (34%) reported aspirin at the latest available follow‐up. The mean number of follow‐up visits was 3.0. There were differences in the baseline characteristics comparing aspirin users with nonusers (Table 1). Propensity score matched 2106 aspirin users (11% of the cohort) with 2106 non‐users (84% of the cohort). After propensity score matching, the absolute standardized differences were <10% for all matched variables (see Supporting Figure 1 in the online version of this article). The risk of the first occurrence of cardiovascular death, MI, or stroke at a median of 41 months was marginally lower with aspirin users vs nonusers (15.2% vs 15.8%; hazard ratio [HR]: 0.81, 95% confidence interval [CI]: 0.65‐1.01, P = 0.06). Subgroup analysis for aspirin vs nonaspirin users, matched on their propensity score for the primary outcome, revealed no evidence for treatment interaction (Figure 2A).
Table 1.
Baseline characteristics of participants with a prior ischemic event
| Characteristic | Aspirin Users, n = 19 228 | Aspirin Nonusers, n = 2496 | P Value | Propensity Score Matched | P Value | |
|---|---|---|---|---|---|---|
| Aspirin Users, n = 2106 | Aspirin Nonusers, n = 2106 | |||||
| Age, y, mean (SD) | 67.7 (10.2) | 69.5 (10.3) | <0.01 | 69.7 (10.1) | 69.3 (10.3) | 0.21 |
| Body mass index, mean (SD) | 27.8 (5.2) | 27.6 (5.9) | <0.01 | 27.5 (5.7) | 27.6 (6.0) | 0.62 |
| Male sex, n (%) | 15 318 (79.7%) | 1982 (79.4%) | <0.01 | 1242 (59%) | 1221 (58%) | 0.51 |
| Region, n (%) | <0.01 | 0.79 | ||||
| North America | 6453 (33.6%) | 1003 (40.2%) | 825 (39.2%) | 862 (40.9%) | ||
| Latin America | 697 (3.6%) | 61 (2.4%) | 58 (2.8%) | 57 (2.7%) | ||
| Western Europe | 5153 (26.8%) | 373 (14.9%) | 211 (10%) | 226 (10.7%) | ||
| Eastern Europe | 2455 (12.8%) | 224 (9.0%) | 227 (10.8%) | 207 (9.8%) | ||
| Middle East | 332 (1.7%) | 9 (0.4%) | 6 (0.3%) | 8 (0.4%) | ||
| Asia | 1942 (10.1%) | 310 (12.4%) | 305 (14.5%) | 279 (13.2%) | ||
| Japan | 1396 (7.3%) | 317 (12.7%) | 294 (14%) | 294 (14%) | ||
| Australia | 800 (4.2%) | 199 (8.0%) | 180 (8.5%) | 173 (8.2%) | ||
| Medical history, n (%) | ||||||
| Unstable angina/MI <1 year | 10 233 (53.7%) | 1040 (42.5%) | <0.01 | — | — | — |
| Coronary revascularization | 9174 (48%) | 718 (29.0%) | <0.01 | — | — | — |
| Asymptomatic carotid stenosis ≥70% | 830 (4.3%) | 90 (3.6%) | 0.02 | — | — | — |
| Stroke/TIA <1 year | 4696 (24.7%) | 955 (38.8%) | <0.01 | — | — | — |
| Carotid revascularization | 1183 (6.3%) | 136 (5.6%) | 0.16 | — | — | — |
| ABI <0.9 in either leg at rest | 1378 (7.2%) | 163 (6.5%) | 0.16 | — | — | — |
| Lower extremity revascularization | 780 (4.1%) | 92 (3.7%) | 0.37 | — | — | — |
| Congestive heart failure | 2824 (14.9%) | 411 (16.9%) | 0.01 | 307 (14.6%) | 344 (16.3%) | 0.11 |
| Hypertension | 15318 (79.7%) | 1982 (79.4%) | 0.75 | 1663 (79.0%) | 1650 (78.3%) | 0.62 |
| Hypercholesterolemia | 13411 (69.8%) | 1367 (54.8%) | <0.01 | 1145 (54.4%) | 1141 (54.2%) | 0.90 |
| Diabetes mellitus | 6788 (35.3%) | 1006 (40.3%) | <0.01 | 864 (41%) | 862 (40.9%) | 0.95 |
| Current smoker | 2666 (14.2%) | 379 (15.8%) | 0.03 | 311 (14.8%) | 340 (16.1%) | 0.21 |
| Baseline medications, n (%) | ||||||
| NSAID | 1887 (10.0%) | 360 (14.7%) | <0.01 | 320 (15.2%) | 307 (14.6%) | 0.57 |
| Statin | 13 369 (69.6%) | 1275 (51.1%) | <0.01 | ‐ | ‐ | ‐ |
| Other lipid‐lowering agent | 1952 (10.2%) | 266 (10.7%) | 0.42 | ‐ | ‐ | ‐ |
| β‐Blocker | 10 617 (55.3%) | 931 (37.4%) | <0.01 | 767 (36.4%) | 783 (37.2%) | 0.60 |
| Calcium antagonist | 6158 (32.1%) | 897 (36.1%) | <0.01 | 781 (37.1%) | 756 (35.9%) | 0.42 |
| ACE‐inhibitor or ARB | 12 657 (65.9%) | 1386 (55.7%) | <0.01 | 1144 (54.3%) | 1147 (54.5%) | 0.92 |
| Nitrates or other antianginal agent | 5695 (30.0%) | 556 (22.7%) | <0.01 | 472 (22.4%) | 465 (22.1%) | 0.79 |
| Diuretics | 7158 (37.3%) | 916 (36.9%) | 0.65 | 737 (35%) | 748 (35.5%) | 0.72 |
Abbreviations: ABI, ankle brachial index; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; MI, myocardial infarction; NSAID, nonsteroidal anti‐inflammatory drug; SD, standard deviation; TIA, transient ischemic attack.
Figure 2.
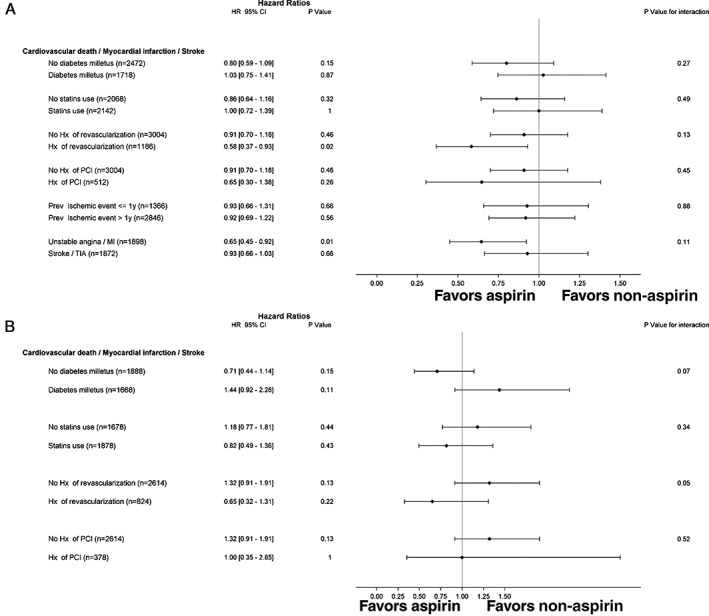
Propensity‐matched analysis for time‐varying use of aspirin vs no aspirin and association with cardiovascular death, myocardial infarction, or stroke according to various subgroups. (A) Prior ischemic event group. (B) Stable atherosclerosis but no prior ischemic event. Abbreviations: CI, confidence interval; HR, hazard ratio; Hx, history; MI, myocardial infarction, PCI, percutaneous coronary intervention, TIA, transient ischemic attack.
Aspirin use was associated with a lower risk of the first occurrence of all‐cause death, MI, or stroke (18.9% vs 20.6%; HR: 0.74, 95% CI: 0.61‐0.90, P < 0.01). The risks of cardiovascular death (HR: 0.65, 95% CI: 0.46‐0.91, P = 0.01) and all‐cause death were lower in the aspirin‐users group (HR: 0.57, 95% CI: 0.43‐0.74, P < 0.01), whereas the risk of MI (HR: 1.00, 95% CI: 0.68‐1.46, P = 1.00), total stroke (HR: 0.86, 95% CI: 0.61‐1.21, P = 0.38), and ischemic stroke (HR: 1.06, 95% CI: 0.65‐1.75, P = 0.81) were similar for aspirin users vs nonusers (Figure 3A). Bleeding events were similar between the groups (HR: 0.50, 95% CI: 0.19‐1.33, P = 0.17). Sensitivity analyses are provided in the Supporting Table in the online version of this article.
Figure 3.
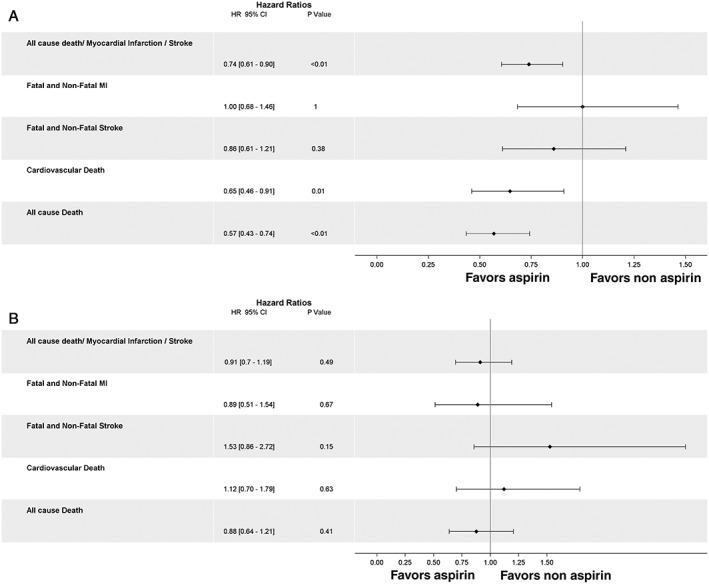
Propensity‐matched analysis for time‐varying use of aspirin vs no aspirin and association with cardiovascular events. (A) Prior ischemic event group. (B) Stable atherosclerosis but no prior ischemic event. Abbreviations: CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
3.2. Stable atherosclerosis, but no prior ischemic event
In the stable atherosclerosis, but no prior ischemic event group, 9526 patients (80%) were aspirin users at baseline. Among aspirin users, the dose of aspirin was ≥325 mg in 17.5%. Out of the 9003 patients who reported aspirin at baseline and had at least 1 aspirin status at any follow‐up), 7919 patients (88%) still reported aspirin at the latest follow‐up available. Out of the 2191 patients who did not report aspirin at baseline and had at least 1 aspirin status at any follow‐up, 765 patients (35%) reported aspirin at the latest follow‐up available. The mean number of follow‐up visits was 2.9. There were observed differences in the baseline characteristics between those with aspirin use vs nonusers prior to matching (Table 2). Propensity score matched 1782 aspirin users (19% of the cohort) with 1782 nonusers (76% of the cohort). After propensity score matching, the absolute standardized differences were <10% for all matched variables (see Supporting Figure 2 in the online version of this article). The risk of the first occurrence of cardiovascular death, MI, or stroke at a median of 36 months was similar for aspirin users versus nonusers (10.7% vs 10.5%; HR: 1.03, 95% CI: 0.73‐1.45, P = 0.86). Subgroup analysis for aspirin vs nonaspirin users, matched on their propensity score, for the primary outcome revealed evidence for treatment interaction favoring aspirin therapy with a history of coronary revascularization vs no coronary revascularization (P interaction = 0.05) (Figure 2B).
Table 2.
Baseline characteristics of participant with stable atherosclerosis but no prior ischemic event
| Characteristic | Aspirin Users, n = 9526 | Aspirin Nonusers, n = 2,346 | P Value | Propensity Score Matched | P Value | |
|---|---|---|---|---|---|---|
| Aspirin Users, n = 1,782 | Aspirin Nonusers, n = 1,782 | |||||
| Age, y, mean (SD) | 69.1 (9.7) | 69.9 (10.1) | <0.01 | 69.8 (10.1) | 70.1 (9.7) | 0.24 |
| Body mass index, mean (SD) | 28.2 (5.5) | 28.1 (6) | 0.30 | 28 (5.9) | 27.9 (5.7) | 0.66 |
| Male sex, n (%) | 6191 (65%) | 1242 (52.9%) | <0.01 | 915 (51.3%) | 895 (50.2%) | 0.50 |
| Region, n (%) | ||||||
| North America | 4,188 (44%) | 895 (38.2%) | <0.01 | 750 (42.1%) | 779 (43.7%) | 0.81 |
| Latin America | 206 (2.2%) | 32 (1.4%) | 27 (1.5%) | 28 (1.6%) | ||
| Western Europe | 2725 (28.6%) | 620 (26.4%) | 320 (18%) | 286 (16%) | ||
| Eastern Europe | 620 (6.5%) | 147 (6.3%) | 141 (7.9%) | 132 (7.4%) | ||
| Middle East | 124 (1.3%) | 7 (0.3%) | 6 (0.3%) | 5 (0.3%) | ||
| Asia | 671 (7%) | 166 (7.1%) | 142 (8%) | 142 (8%) | ||
| Japan | 434 (4.6%) | 299 (12.7%) | 243 (13.6%) | 241 (13.5%) | ||
| Australia | 558 (5.9%) | 180 (7.7%) | 153 (8.6%) | 169 (9.5%) | ||
| Medical history, n (%) | ||||||
| Unstable angina/MI | 0 (0%) | 0 (0%) | NA | — | — | — |
| Coronary revascularization | 4848 (51.2%) | 492 (21.2%) | <0.01 | — | — | — |
| Asymptomatic carotid stenosis ≥70% | 562 (5.9%) | 99 (4.2%) | <0.01 | |||
| Stroke/TIA | 0 (0%) | 0 (0%) | NA | — | — | — |
| Carotid revascularization | 661 (7.1%) | 107 (4.7%) | <0.01 | — | — | — |
| ABI <0.9 in either leg at rest | 1619 (17.0%) | 547 (23.3%) | <0.01 | |||
| Lower extremity revascularization | 997 (10.5%) | 186 (7.9%) | <0.01 | — | — | — |
| Congestive heart failure | 880 (9.4%) | 217 (9.5%) | 0.91 | 167 (9.4%) | 174 (9.8%) | 0.69 |
| Hypertension | 7747 (81.3%) | 1846 (78.7%) | <0.01 | 1396 (78.3%) | 1416 (79.5%) | 0.41 |
| Hypercholesterolemia | 7307 (76.7%) | 1390 (59.4%) | <0.01 | 1074 (60.3%) | 1093 (61.3%) | 0.51 |
| Diabetes mellitus | 3754 (39.4%) | 1116 (47.6%) | <0.01 | 847 (47.5%) | 833 (46.7%) | 0.63 |
| Current smoker | 1336 (14.4%) | 425 (18.9%) | <0.01 | 323 (18.1%) | 324 (18.2%) | 0.96 |
| Baseline medications, n (%) | ||||||
| NSAID | 1115 (12%) | 372 (16.1%) | <0.01 | 303 (17%) | 323 (18.1%) | 0.37 |
| Statin | 7044 (74%) | 1200 (51.2%) | <0.01 | — | — | — |
| Other lipid‐lowering agent | 1206 (12.7%) | 309 (13.2%) | 0.50 | — | — | — |
| β‐Blocker | 4862 (51.2%) | 795 (34%) | <0.01 | 617 (34.6%) | 627 (35.2%) | 0.72 |
| Calcium antagonist | 5845 (61.5%) | 1295 (55.3%) | <0.01 | 641 (36%) | 657 (36.9%) | 0.57 |
| ACE‐inhibitor or ARB | 3370 (35.5%) | 852 (36.4%) | 0.44 | 996 (55.9%) | 1028 (57.7%) | 0.27 |
| Nitrates or other antianginal agent | 2385 (25.4%) | 472 (20.4%) | <0.01 | 372 (20.9%) | 374 (21%) | 0.93 |
| Diuretics | 3655 (38.5%) | 912 (38.9%) | 0.67 | 670 (37.6%) | 681 (38.2%) | 0.70 |
Abbreviations: ABI, ankle brachial index; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; MI, myocardial infarction; NSAID, nonsteroidal anti‐inflammatory drug; SD, standard deviation; TIA, transient ischemic attack.
The risk of the first occurrence of all‐cause death, MI, or stroke was similar (14.7% vs 16.1%; HR: 0.91, 95% CI: 0.70‐1.19, P = 0.49), as were the risks of cardiovascular death (HR: 1.12, 95% CI: 0.70‐1.79, P = 0.63), all‐cause death (HR: 0.88, 95% CI: 0.64‐1.21, P = 0.41), MI (HR: 0.89, 95% CI: 0.51‐1.54, P = 0.67), total stroke (HR: 1.53, 95% CI: 0.86‐2.72, P = 0.15), and ischemic stroke (HR: 0.83, 95% CI: 0.36‐1.94, P = 0.67) (Figure 3B). Bleeding events were similar between groups (HR: 1.22, 95% CI: 0.51‐2.95, P = 0.66). Sensitivity analyses are provided in the Supporting Table in the online version of this article.
4. DISCUSSION
In this large observational analysis from the REACH registry, we found that aspirin was marginally beneficial for the primary outcome among patients with a prior ischemic event; however, no benefit was observed among those with no prior ischemic event. In the cohort with a prior ischemic event, aspirin use was associated with a significantly lower rate of all‐cause death, MI, or stroke. The difference in all‐cause mortality was more apparent than the difference in cardiovascular mortality, which some have made the case as the preferred outcome in the evaluation of cardiovascular disease patients.18 Benefit was not observed among the cohort with stable atherosclerosis, but no prior ischemic event; however, subgroup analysis suggested that individuals with prior coronary revascularization might derive benefit from aspirin.
Several large randomized trials have demonstrated the benefit of aspirin during acute ischemic events. In the Second International Study of Infarct Survival, 17 187 acute MI patients were randomized in 2 × 2 factorial design to aspirin alone, streptokinase alone, both active treatments, or both placebos for 30 days.1 Aspirin reduced the risk of all‐cause mortality.1 In the International Stroke Trial, 19 435 acute ischemic stroke patients were randomized to aspirin vs no aspirin and to heparin vs no heparin.2 Aspirin was associated with a reduction in early stroke; however, there was no mortality benefit.2 A recent meta‐analysis of randomized trials suggests that aspirin is associated with a reduction in the risk of early recurrent strokes; however, this benefit diminishes with long‐term use.19
Among the prior ischemic event group, aspirin was associated with a lower risk of all‐cause mortality and cardiovascular mortality, although there was no apparent reduction in MI. The lack of benefit for aspirin on the outcome of MI among subjects with a prior ischemic event was similar to another observational study and may have to do with a lack of adjudication of events in this registry.7 Although the short‐term use of aspirin initiated during a MI was shown to reduce the risk of recurrent MI in a large trial,1 other trials evaluating the long‐term use of aspirin in patients with prior MI had shown conflicting results on the risk of MI with aspirin compared with placebo.20, 21 In addition, up to 50% of incident MIs are clinically silent, which makes complete ascertainment of this complex outcome difficult during registry follow‐up.22 Among those with a prior ischemic event, aspirin may be associated with a survival advantage by attenuating the severity of recurrent thrombosis‐mediated events, rather than reducing the occurrence of MI per se. Also, the reduction in risk of all‐cause mortality could be driven by other factors such as reduction in sudden cardiac death or cancer‐related mortality.23
Among the stable atherosclerosis, there was no strong evidence that aspirin was beneficial, except in a sensitivity analysis (propensity‐adjusted model with the time‐varying use of aspirin). On subgroup analysis, there was suggestion of lack of benefit for aspirin among those with no coronary revascularization versus prior coronary revascularization.
A meta‐analysis of 6 randomized trials of individuals with stable CAD concluded that low‐dose aspirin reduced the risk of adverse cardiovascular events and all‐cause mortality.24 However, in that meta‐analysis, only 1 of the included trials actually examined the benefits of aspirin in patients similar to our stable atherosclerosis but no prior ischemic event cohort. The remainder of the trials in that analysis enrolled patients with a prior ischemic event. Although the Swedish Angina Pectoris Aspirin Trial and an analysis of the Physicians' Health Study demonstrated that aspirin reduced the risk of MI in patients with chronic stable angina, patients enrolled in these trials were not on contemporary medical therapy (eg, statins).25, 26 An observational analysis of the more contemporary INVEST: INternational VErapamil SR/Trandolapril STudy demonstrated that aspirin use among hypertension patients with CAD, but no ischemic event, was not associated with fewer adverse events.7 A previous analysis of the REACH registry had indicated that stable patients with previous MI did not benefit from β‐blocker use,27 which had been the recommendation for decades in patients with prior MI.28 The present analysis using data from the REACH registry further evolves our understanding of aspirin in atherosclerosis patients.
4.1. Limitations
It is possible that the lack of benefit of aspirin therapy among the atherosclerosis, but no prior ischemic event, group reflects insufficient power to detect a difference in outcomes. However, with the current conditions (HR: 0.90, and event rate of 16.1% in the control group), we estimated from survival analysis that 1112 patients would be needed in each group to achieve 80% power for the primary outcome.27 Because 1782 patients were propensity matched in each group, there should have been sufficient power to detect a difference between treatment groups. Outcomes in REACH were not adjudicated, although that is not unusual in registries.8 Moreover, lack of adjudication is unlikely to impact hard outcomes such as all‐cause mortality, but may have led to under‐reporting of MI. Among aspirin users, the majority used a dose <325 mg; however, higher doses have not been shown to offer further ischemic benefit.29 In addition, the total number of major bleeding events was small, which limited our ability to make firm conclusions on this outcome. Although propensity score matching was employed and use of aspirin was introduced as a time‐varying covariate in the model, other unmeasured confounders cannot be entirely excluded.
5. CONCLUSION
In this observational analysis of outpatients with stable atherosclerosis, the use of aspirin was marginally beneficial only among atherosclerosis patients with a prior ischemic event. Future studies are needed before concluding that aspirin could be omitted among atherosclerosis patients with no coronary revascularization.
Conflicts of interest
Dr. Anthony A. Bavry discloses the following relationship: honorarium from the American College of Cardiology. Dr. Philippe Gabriel Steg discloses the following relationships: research grants from Merck, Sanofi, and Servier; speaking or consulting fees from Amarin, AstraZeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myers‐Squibb, CSL‐Behring, Daiichi‐Sankyo, GlaxoSmithKline, Janssen, Lilly, Merck Novartis, Pfizer, Regeneron, Sanofi, Servier, The Medicines Company. Dr. Deepak L. Bhatt discloses the following relationships: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor‐in‐Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor‐in‐Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Vice‐Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Coinvestigator: Biotronik, Boston Scientific, St. Jude Medical; Trustee: American College of Cardiology; Unfunded Research: FlowCo, PLx Pharma, Takeda. The other authors have no conflicts of interest to declare.
Supporting information
Figure S1. Absolute standardized differences (%, aspirin vs. non aspirin users) before and after propensity score matching, prior ischemic event group.
Figure S2. Absolute standardized differences (%, aspirin vs. non aspirin users) before and after propensity score matching, stable atherosclerosis but no prior ischemic event group.
Table S1. Sensitivity analyses
Bavry AA, Elgendy IY, Elbez Y, Mahmoud AN, Sorbets E, Steg PG, Bhatt DL and for the REACH Registry Investigators . Aspirin and the risk of cardiovascular events in atherosclerosis patients with and without prior ischemic events. Clin Cardiol. 2017;40:731–738. 10.1002/clc.22724
Funding information There was no funding for this analysis. The REACH Registry was sponsored by sanofiaventis, Bristol‐Myers Squibb, and the Waksman Foundation (Tokyo, Japan), and is endorsed by the World Heart Federation.
A complete list of the REACH Registry investigators appears in Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189.
REFERENCES
- 1. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS‐2 . ISIS‐2 (Second International Study of Infarct Survival) Collaborative Group. Lancet . 1988;2:349–360. [PubMed] [Google Scholar]
- 2. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke . International Stroke Trial Collaborative Group. Lancet . 1997;349:1569–1581. [PubMed] [Google Scholar]
- 3. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients . Antiplatelet Trialists' Collaboration. BMJ . 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 4. Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 5. Park K, Bavry AA. Aspirin: its risks, benefits, and optimal use in preventing cardiovascular events. Cleve Clin J Med. 2013;80:318–326. [DOI] [PubMed] [Google Scholar]
- 6. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. [DOI] [PubMed] [Google Scholar]
- 7. Bavry AA, Gong Y, Handberg EM, et al. Impact of aspirin according to type of stable coronary artery disease: insights from a large international cohort. Am J Med. 2015;128:137–143. [DOI] [PubMed] [Google Scholar]
- 8. Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 9. Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 10. Ohman EM, Bhatt DL, Steg PG, et al. The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events‐study design. Am Heart J . 2006;151:786.e1–e10. [DOI] [PubMed] [Google Scholar]
- 11. Steg PG, Bhatt DL, Wilson PW, et al. One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. [DOI] [PubMed] [Google Scholar]
- 12. Jackson LR II, Piccini JP, Cyr DD, et al. Dual antiplatelet therapy and outcomes in patients with atrial fibrillation and acute coronary syndromes managed medically without revascularization: insights from the TRILOGY ACS trial. Clin Cardiol. 2016;39:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rubin DB. Using propensity score to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 16. Hajage D, Tubach F, Steg PG, et al. On the use of propensity scores in case of rare exposure. BMC Med Res Methodol. 2016;16:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity‐based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–270. [DOI] [PubMed] [Google Scholar]
- 18. Lauer MS, Blackstone EH, Young JB, et al. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. 1999;34:618–620. [DOI] [PubMed] [Google Scholar]
- 19. Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time‐course analysis of randomised trials. Lancet. 2016;388:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aspirin in coronary heart disease . The Coronary Drug Project Research Group. J Chronic Dis . 1976;29:625–642. [PubMed] [Google Scholar]
- 21. Aspirin Myocardial Infarction Study Group . A randomized, controlled trial of aspirin in persons recovered from myocardial infarction. JAMA . 1980;243:661–669. [PubMed] [Google Scholar]
- 22. Zhang ZM, Rautaharju PM, Prineas RJ, et al. Race and sex differences in the incidence and prognostic significance of silent myocardial infarction in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2016;133:2141–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rothwell PM, Price JF, Fowkes FG, et al. Short‐term effects of daily aspirin on cancer incidence, mortality, and non‐vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. [DOI] [PubMed] [Google Scholar]
- 24. Berger JS, Brown DL, Becker RC. Low‐dose aspirin in patients with stable cardiovascular disease: a meta‐analysis. Am J Med. 2008;121:43–49. [DOI] [PubMed] [Google Scholar]
- 25. Juul‐Moller S, Edvardsson N, Jahnmatz B, et al. Double‐blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Lancet . 1992;340:1421–1425. [DOI] [PubMed] [Google Scholar]
- 26. Ridker PM, Manson JE, Gaziano JM, et al. Low‐dose aspirin therapy for chronic stable angina. A randomized, placebo‐controlled clinical trial. Ann Intern Med . 1991;114:835–839. [DOI] [PubMed] [Google Scholar]
- 27. Bangalore S, Steg G, Deedwania P, et al. REACH Registry Investigators. β‐Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA . 2012;308:1340–1349. [DOI] [PubMed] [Google Scholar]
- 28. Elgendy IY, Mahmoud A, Conti CR. Beta‐blockers in the management of coronary artery disease: are we on the verge of a new paradigm shift? Recent Pat Cardiovasc Drug Discov. 2014;9:11–21. [DOI] [PubMed] [Google Scholar]
- 29. Mehta SR, Tanguay JF, Eikelboom JW, et al. Double‐dose versus standard‐dose clopidogrel and high‐dose versus low‐dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT‐OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233–1243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Absolute standardized differences (%, aspirin vs. non aspirin users) before and after propensity score matching, prior ischemic event group.
Figure S2. Absolute standardized differences (%, aspirin vs. non aspirin users) before and after propensity score matching, stable atherosclerosis but no prior ischemic event group.
Table S1. Sensitivity analyses


