Abstract
Vitamin K antagonists (VKAs) are known to increase vascular calcification, suggesting increased cardiovascular disease events. Apixaban is an oral direct factor Xa inhibitor superior to warfarin at preventing stroke or systemic embolism and may stabilize coronary atherosclerosis. The potential benefits of avoiding VKA therapy and the favorable effects of factor Xa inhibitors could contribute to cardiovascular disease event reduction. We hypothesized that apixaban inhibits vascular calcification and coronary atherosclerosis progression compared with warfarin in patients with atrial fibrillation (AF). This study is a single‐center, prospective, randomized, open‐label study. From May 2014 to December 2015, 66 patients with nonvalvular AF who experienced VKA therapy were enrolled. Patients were randomized into either warfarin or apixaban cohorts and followed for 52 weeks. The primary objective is to compare the rate of change in coronary artery calcification (CAC) from baseline to follow‐up in apixaban vs warfarin cohorts. The key secondary objective is to compare the rate of incident plaques and quantitative changes in plaque types between patients randomized to either warfarin or apixaban cohorts using serial coronary computed tomography angiography. Expert readers will blindly assess CAC and coronary artery plaques. It is thought that this trial will result in significant differences in CAC and coronary artery plaque progression between the VKA and apixaban. The results are anticipated to provide a novel insight into treatment selection for AF patients. The study is registered at http://www.clinicaltrials.gov (NCT 02090075).
Keywords: coronary artey calcium, warfarin, apixaban, coronary artery plaque
1. INTRODUCTION
Warfarin and other vitamin K antagonists (VKAs) have been widely used for many decades to achieve a reduced thrombotic risk. However, evidence indicates that VKAs inhibit not only post‐translational activation of vitamin K–dependent coagulation factors, but also synthesis of functional extrahepatic vitamin K–dependent proteins, thereby eliciting undesired side effects.1, 2 Several studies have revealed that calcification of the coronary arteries and heart valves increased in patients on VKA, whereas intake of vitamin K was associated with less progression of coronary artery calcification (CAC).3, 4, 5 CAC is strongly associated with atherosclerotic burden and predicts cardiovascular disease (CVD) events and mortality.6, 7 VKAs have recently been shown to not only increase calcification but also contribute to atherosclerotic plaques in both animals and humans, which may impact long‐term risk.8, 9
Apixaban is an oral direct factor Xa inhibitor that is superior to warfarin in preventing stroke or systemic embolism. The rate of myocardial infarction (MI) was reported to be 12% lower in the apixaban group compared with the warfarin group in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial.10 A study using rivaroxaban, which is also a direct factor Xa inhibitor, reported an increase in the stability of atherosclerotic plaques in apolipoprotein E–deficient mice, as suggested by the presence of thicker protective fibrous caps and decreased plaque erosion.11 Factor Xa inhibition reduces the expression of inflammatory mediators, such as tumor necrosis factor‐α TNF‐α and matrix metallopeptidase 9 (MMP‐9), suggesting atherosclerotic plaque stabilization.12 The antiatherosclerotic effects of factor Xa inhibitors could be effective at reducing CAC. The potential benefit of avoiding VKA therapy and the favorable effects of factor Xa inhibitors may also contribute to a reduction in CVD events.
The hypothesis of the current study is that use of apixaban inhibits progression of vascular calcification and coronary atherosclerosis compared with warfarin therapy in patients with atrial fibrillation (AF).
2. METHODS
2.1. Overall study design
This study is a single‐center, prospective, randomized, open‐label study designed to compare apixaban 5 mg oral twice daily (with a dose of 2.5 mg b.i.d. in selected patients) with warfarin (target international normalized ratio [INR], 2.0–3.0) for 52 weeks on coronary calcification and coronary plaque composition and volume in patients with nonvalvular AF. This study is registered at http://www.clinicaltrials.gov (NCT 02090075).
2.2. Study objectives
The hypothesis of our research is that treatment with apixaban therapy will slow the progression of calcified plaques, as determined by CAC score, and coronary artery atherosclerosis, as assessed using coronary computed tomography angiography (CCTA), compared with warfarin therapy in patients with AF.
The primary objective is to examine the rate of change in CAC from baseline to follow‐up in apixaban vs warfarin cohorts. This analysis is performed using CAC score. The key secondary objective is to examine the rate of incident plaques and quantitative changes in plaque types in patients randomized to either warfarin or apixaban cohorts using CCTA at 12 months after an initial evaluation. We will determine whether participants treated with apixaban have slower rates of progression compared with participants treated with warfarin, after controlling for all cardiovascular risk factors and demographics. Further, we will assess the differential effects of the 2 agents on inflammatory, serologic, and lipid parameters including total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglycerides, very low‐density lipoprotein, matrix Gla protein (MGP), and high‐sensitivity C‐reactive protein and their associations with incident plaques or quantitative plaque progression or regression. For assessment of MGP, we will measure desphospho‐uncarboxylated MGP, which could serve as a biomarker of vascular vitamin K status.13
2.3. Ethical considerations
The investigator followed all appropriate requirements, including Good Clinical Practice, International Conference on Harmonization, and Code of Federal Regulations. The study was conducted by qualified personnel and was approved by the local institutional review board/institutional ethics committee. All participants provided written informed consent for inclusion.
2.4. Targeted population
The targeted population included patients age 18 to 84 years with AF/flutter at enrollment or ≥2 episodes of AF (as documented by electrocardiography) ≥2 weeks apart in the 12 months before enrollment. Inclusion and exclusion criteria are shown in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
|---|
| 1. Age 18 to 84 years, willingness to participate in the study, and ability to sign informed consent |
| 2. Patients with AF/flutter at enrollment or ≥2 episodes of AF, as documented by ECG, ≥2 weeks apart in the 12 months before enrollment |
| 3. On a stable dose of warfarin for 6 months prior to enrollment |
| Exclusion criteria |
| 1. AF due to a reversible cause, moderate or severe mitral stenosis, or conditions other than AF that require anticoagulation |
| 2. A need for ASA at a dose of >165 mg/d or for both ASA and a P2Y inhibitor |
| 3. Serious bleeding event in the previous 6 months or a high risk of bleeding (eg, active peptic ulcer disease, a platelet count of <100 000/mm3 or Hgb <10 g/dL, stroke within the previous 10 days, documented hemorrhagic tendencies, or blood dyscrasias) |
| 4. Renal insufficiency (sCr of 12.5 mg/dL or calculated CrCl <50 mL/min) |
| 5. Weight >325 pounds |
| 6. Resting hypotension (SBP <90 mm Hg) or resting HTN (SBP >170 mm Hg or DBP >110 mm Hg) |
| 7. History of active malignancy requiring concurrent chemotherapy |
| 8. Any unstable medical, psychiatric, or substance‐abuse disorder that, in the opinion of the principal investigator, is likely to affect the subject's ability to complete the study |
| 9. Known allergy to iodinated contrast material |
| 10. Pregnancy, women of childbearing potential unwilling to use adequate contraception |
Abbreviations: AF, atrial fibrillation; ASA, acetylsalicylic acid (aspirin); CrCl, creatinine clearance; DBP, diastolic blood pressure; ECG, electrocardiography; Hgb, hemoglobin; HTN, hypertension; SBP, systolic blood pressure; sCr, serum creatinine.
2.5. Participating centers
All participants were enrolled at Los Angeles Biomedical Research Institute at Harbor UCLA (Torrance, CA). Data‐collection activity began in May 2014 with the goal of enrolling 66 patients. This was achieved in December 2015.
2.6. Patient recruitment and evaluation
The study randomized patients currently taking warfarin into 2 groups, one group to continue on warfarin and the other group to be switched to apixaban. The Figure 1 shows the schema of this study. Baseline examination includes patient demographics, coronary risk factors, serum biomarkers, coronary calcification, and coronary plaque volume/composition. All participants were educated on a low‐cholesterol diet at entry to the study. Standard lipid‐lowering therapy and/or antiplatelet therapy was provided based on risk assessment and lipid parameters at baseline according to current guidelines14, 15 before randomization. Baseline information was gathered regarding risk factors for atherosclerotic CVD (cigarette‐smoking status, systemic hypertension, family history of premature atherosclerosis, menopausal and hormone‐replacement status in women, sedentary lifestyle, current medications, chest‐pain questionnaire, and measures of obesity). After the observation period, eligible participants were randomized by computer‐generated numbers to one of 2 groups, either the apixaban group or the warfarin group. Patients in the apixaban group received 5 mg apixaban oral twice daily, with a dose of 2.5 mg twice daily for a subset of patients with ≥2 of the following: age ≥80 years, body weight ≤60 kg, and serum creatinine ≥1.5 mg/dL (133 µmol/L). Patients in the warfarin group simply stayed on warfarin at their current dose. When significant coronary stenosis was confirmed by CCTA at baseline, invasive treatment was performed according to the judgment of the attending physician. Baseline characteristics are shown in Table 2. The warfarin group had a higher proportion of males. There was no significant difference in the duration of warfarin treatment between the 2 groups.
Figure 1.
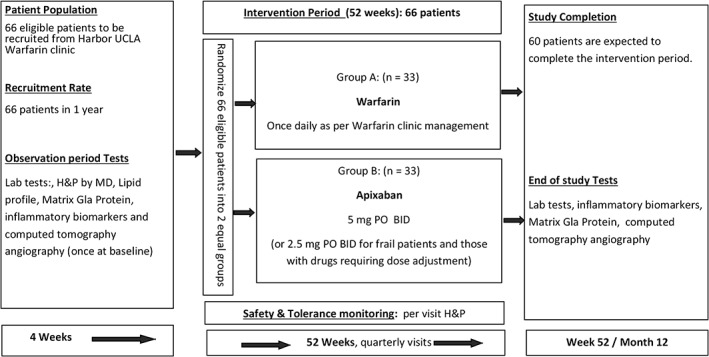
Study schema. Abbreviations: BID, twice daily; H&P, history and physical; MD, medical doctor; PO, orally.
Table 2.
Patient characteristics at baseline
| All, N = 66 | Warfarin Group, n = 33 | Apixaban Group, n = 33 | P Value | |
|---|---|---|---|---|
| Age, y | 57.8 ± 11.8 | 55.4 ± 13.0 | 60.1 ± 10.0 | 0.12 |
| Male sex | 44 (67) | 25 (76) | 19 (58) | 0.04 |
| BMI, kg/m2 | 32.8 ± 9.3 | 34.6 ± 11.1 | 30.9 ± 6.7 | 0.11 |
| HTN | 64 (97) | 31 (94) | 33 (100) | 0.15 |
| Dyslipidemia | 43 (38) | 22 (67) | 21 (64) | 0.80 |
| DM | 25 (38) | 11 (33) | 14 (42) | 0.45 |
| Current smoking | 6 (9) | 4 (12) | 2 (6) | 0.39 |
| Family history of CAD | 24 (36) | 10 (30) | 14 (42) | 0.31 |
| Medication use | ||||
| Lipid‐lowering agents | 39 (59) | 19 (58) | 20 (61) | 0.80 |
| Antihypertensive agents | 60 (91) | 29 (88) | 31 (94) | 0.39 |
| Antidiabetic agents | 22 (33) | 11 (33) | 11 (33) | 1.00 |
| ASA | 19 (29) | 10 (30) | 11 (33) | 0.79 |
| Duration of warfarin use, y | 6.3 ± 5.3 | 5.7 ± 4.6 | 6.8 ± 5.9 | 0.41 |
Abbreviations: ASA, acetylsalicylic acid (aspirin); BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; HTN, hypertension; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
2.7. Patient follow‐up
After randomization, participants returned quarterly (at 13, 26, 39, and 52 weeks) to assess compliance with medication and to receive any additional supply of medicine. Patients randomized to the warfarin group continued their monthly study visits focusing on control of the INR at 2–3 at the warfarin clinic, with research study visits every 3 months to include an INR check and an assessment of clinical outcomes and adverse events. At 52 weeks’ follow‐up, CCTA was scheduled to evaluate coronary calcification and coronary plaque volume/composition by readers blind to the randomization and clinical activities.
2.8. Safety
The primary safety outcome was major bleeding, which is defined by International Society on Thrombosis and Haemostasis criteria16 as clinically overt bleeding accompanied by a decrease in the hemoglobin level of ≥2 g/dL or transfusion of ≥2 units of packed red cells, occurring at a critical site, or resulting in death. An additional safety outcome was clinically relevant nonmajor bleeding, defined as clinically overt bleeding that did not satisfy the criteria for major bleeding and that led to hospital admission, physician‐guided medical or surgical treatment, or a change in antithrombotic therapy. Other safety measures included any bleeding, other adverse events, and liver‐function abnormalities.
2.9. The CCTA scan protocol
All CT scans were performed with a 64‐slice CT scanner (Lightspeed VCT; GE Healthcare, Milwaukee, WI) or a 256‐slice CT scanner (Revolution CT; GE Healthcare). Before CCTA, a prospective nonenhanced coronary calcium scan was performed. For quantitative assessment of CAC, the Agatston score was calculated using a 3‐mm CT slice thickness and a detection threshold of ≥130 HU involving ≥1 mm2 area/lesion (3 pixels).17 The CCTA was performed and interpreted by consensus of experts blind to all clinical data.
2.10. Assessment of CCTA
The CT images were transferred to a dedicated workstation for image analysis. Images were postprocessed in an independent workstation (AW 4.6; GE Healthcare) by the reader independently during a dynamic reading process. Curved maximum intensity projection multiplanar reconstructions were performed for each coronary artery segment at the end‐diastolic frame or the frame with the least motion artifacts. Multiplanar reformatting was also used to generate cross‐sectional images of coronary segments.
2.11. Assessment of coronary artery plaques
All coronary images were transferred to the workstation using a dedicated software tool for semi‐automated plaque analysis software (QAngioCT Research Edition, version 2.1.2; Medis Medical Imaging Systems, Leiden, the Netherlands). Each coronary segment was assessed for the presence of plaques, plaque type, plaque features, and plaque attenuation pattern. Coronary plaques are categorized as calcified plaques, noncalcified plaques (fibrous, fibrous‐fatty, and low‐attenuation plaques). Studies were blind to warfarin or apixaban use, and expert readers assessed all coronary arteries. The protocol for quantitative plaque assessment has been previously published.18 According to a modified 17‐segment American Heart Association coronary tree model, detected plaques were allocated according to plaque location.19 Vessel and plaque volumes were measured in segments with sufficient image quality and ≥1.5 mm in lumen diameter. Segments with coronary artery stents were excluded for plaque analysis. Coronary plaques as well as vessel and lumen volumes at baseline and follow‐up were measured, and plaque changes over time between warfarin and apixaban groups were evaluated.
2.12. Statistical analysis
Baseline variables are compared using an independent t test (2‐tailed) after performing the Levene test for equality of variances for all normally distributed continuous variables and the Mann‐Whitney test (2‐tailed) for all non–normally distributed variables. ANCOVA analysis, controlling for baseline demographics and baseline CAC score values, was used to assess the effect of apixaban and warfarin on calcium score. Models both unadjusted and adjusted for age, sex, race/ethnicity, and baseline cholesterol, lipid‐lowering medication, hypertension medication, smoking, diabetes, family history, and baseline CAC score were used to compare 2 treatment conditions between apixaban and warfarin. Post hoc power calculations were performed at a 2‐tailed significance level of 0.05. Power calculations strictly depend on the proposed sample size and assumed calcification progression difference ranges. As a result, 65% power will detect annualized relative change differences in the noncalcified plaque for 20% (10% vs 30%) based on the proposed study sample size. All statistical analyses use SAS 9.4 statistical software (SAS Institute, Inc., Cary, NC). A P value <0.05 is considered statistically significant.
2.13. Data Safety Monitoring Board
A Data Safety Monitoring Board (DSMB) comprising individuals with expertise in each of the efficacy areas (anticoagulation therapy, AF, and cardiac CT) as well as in anticoagulation trials and statistics are established. The DSMB chair prepares interim reports for the DSMB on a regular basis: every 6 months or more often, as deemed appropriate. Interim reports to the DSMB focus on analyses of the safety data: primarily bleeding, but other safety‐related outcomes, such as CT‐related adverse effects, are also reviewed in a nonblind fashion. The DSMB decides on the basis of these data whether to recommend modifying or stopping the trial and makes recommendations to the sponsor and the study leadership.
2.14. Study limitations
This study will have some limitations. First, it is a single‐center, open‐label study with a small number of patients. Second, no differentiation between atherosclerotic calcification and vascular (both medial and intimal) calcification can be made with CCTA. Thus, the effect of the 2 trial drugs on these 2 types of CAC will remain to be elucidated in the study. Third, the study enrolled and randomized patients currently taking VKA therapy. It potentially may be better to have randomized VKA‐naïve patients because the difference in duration of VKA therapy before randomization could become a source of bias. However, in this study, there is no significant difference in duration of prior VKA therapy between VKA and apixaban cohorts from patients’ baseline characteristics. Finally, we will use 65% power for calculating the sample size, which could be relatively small. A 0.35 type II error is based on the mean difference value of calcification progression between the 2 different groups from the reference; however, if progression rates are more divergent, the power will be better to see differences between groups.
3. DISCUSSION
This study aims to assess the rate of change in CAC and incident plaques and quantitative changes in different plaque types from baseline to follow‐up in apixaban vs warfarin cohorts. The results could be used to power an outcome study looking at the effect of apixaban and warfarin on cardiovascular outcomes with AF and established CVD and encourage clinicians to make a comprehensive decision on anticoagulation therapy based on both stroke outcome and coronary atherosclerosis progression, regression, and stabilization under the influence of the 2 different therapies.
VKA use is known to be associated with increased vascular calcification, which may impact long‐term risk. The potential mechanisms promoting calcification by VKA may be explained by inhibition of γ‐glutamylation of matrix Gla protein, which is a vitamin K–dependent protein for preventing arterial calcification,20 and its effects could be blocked by VKA and result in excessive calcification of the arteries.21, 22 In the experimental data, VKA treatment also tends to show more valvular calcification,23 and both increased peripheral artery calcification24 and CAC.25 A larger CAC indicates a greater risk of coronary heart disease (CHD) and CVD. Long‐term follow‐up data of >10 years for CAC recently have been validated in multiple studies, demonstrating the significant association between increased CAC and a higher incidence of future adverse outcomes.26, 27 CAC progression also shows a strong association with CHD events and total mortality.28 The current study investigates if patients with VKA treatment have a higher CAC score and greater CAC progression after 52 weeks of follow‐up, which may mean that VKA treatment is high‐risk compared with apixaban treatment for major adverse cardiac events.
CAC progression is associated with adverse clinical outcomes. The Multi‐Ethnic Study of Atherosclerosis (MESA) reported a linear relationship between CAC progression and risk for cardiovascular events; it identified a 3‐ to 6‐fold increased rate of events in those with an annual progression ≥300 units.29 CAC progression parallels overall plaque progression, leading to increased atherosclerosis and greater cardiovascular risk. In contrast, high‐intensity statin treatment, which can stabilize coronary artery plaques, promotes a significant increase in CAC.30 A key factor to resolving these paradoxical results might be each component of the CAC score, including volume and density of CAC. CAC density score showed a significantly stronger predictive value compared with CAC volume score for CHD and CVD.31 In our subanalysis, we will investigate the association between the 2 different therapies with CAC density or volume score.
Several studies have performed serial assessment of coronary artery plaque progression or regression with intravascular ultrasound (IVUS)32, 33 and optical coherence tomography,34, 35 which potentially require invasive coronary angiography. In contrast, CCTA can assess coronary artery plaque composition in a noninvasive manner. Several studies have revealed the potential of CCTA in quantifying plaque burden to quantify progression or regression of coronary artery disease.18, 36 CCTA has been shown to accurately determine atherosclerotic plaque size, remodeling, and plaque morphology compared with IVUS as a reference standard.37 For quantitative atherosclerotic plaque analysis, we will use a semiautomated method with novel plaque‐analysis software for the assessment of progression/regression of CAC and noncalcified plaques. The strength of semiautomated quantitative plaque software is its excellent reliability and reproducibility.36 We previously reported good correlations between total plaque volume measurements and 2 observers (correlation coefficient: 0.94, 95% confidence interval: 0.80‐0.98).38
Papadopoulou et al reported the natural history of coronary artery plaques on CCTA at a median follow‐up of 39 months in 32 patients with known coronary artery disease.36 The researchers found that plaque progression closely fitted the regression line illustrated by numerous studies examining the relationship between mean low‐density lipoprotein cholesterol levels and plaque volume changes on IVUS, indicating the potential of serial CCTA to assess plaque progression as a substitute for IVUS. Importantly, the population of the current study includes patients who all have a history of AF, which may potentially affect image quality of CCTA. However, technical improvements in CCTA can provide sufficient image quality, and its use has expanded into assessing coronary atherosclerosis, cardiac volumes, or treatment strategy before ablations in AF patients.39, 40 The current study will be the first to evaluate the quantitative assessment of coronary artery plaque burden using serial CCTA in apixaban vs warfarin cohorts.
4. CONCLUSION
This study will investigate differences between VKA and apixaban on CAC progression in a randomized controlled trial. We hypothesize that apixaban will show less CAC progression and fewer CT angiographic plaques compared with VKA in patients with AF. The study might provide a novel direction for treatment selection for AF patients. The results of this study are expected in late 2017.
Conflicts of interest
Matthew J. Budoff discloses work for the National Institutes of Health and General Electric Healthcare. The authors declare no other potential conflicts of interest.
Acknowledgments
The authors thank R. Terrazas and S. Hamal for participating in the collection and management of the data, and S. Susarla, E. Jayawardena, and C. Dailing for their technical support.
Osawa K, Nakanishi R, Win TT, et al. Rationale and design of a randomized trial of apixaban vs warfarin to evaluate atherosclerotic calcification and vulnerable plaque progression. Clin Cardiol. 2017;40:807–813. 10.1002/clc.22746
Funding information This study was supported by grants from Bristol‐Myers Squibb.
REFERENCES
- 1. Danziger J. Vitamin K–dependent proteins, warfarin, and vascular calcification. Clin J Am Soc Nephrol. 2008;3:1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–1407. [DOI] [PubMed] [Google Scholar]
- 3. Koos R, Mahnken AH, Mühlenbruch G, et al. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol. 2005;96:747–749. [DOI] [PubMed] [Google Scholar]
- 4. Shea MK, O'Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89:1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schurgers LJ, Aebert H, Vermeer C, et al. Oral anticoagulant treatment: friend or foe in cardiovascular disease? Blood. 2004;104:3231–3232. [DOI] [PubMed] [Google Scholar]
- 6. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 7. Budoff MJ, Shaw LJ, Liu ST, et al. Long‐term prognosis associated with coronary calcification: observations from a registry of 25 253 patients. J Am Coll Cardiol. 2007;49:1860–1870. [DOI] [PubMed] [Google Scholar]
- 8. Schurgers LJ, Joosen IA, Laufer EM, et al. Vitamin K antagonists accelerate atherosclerotic calcification and induce a vulnerable plaque phenotype. PLoS One. 2012;7:e43229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schurgers LJ, Spronk HM. Differential cellular effects of old and new oral anticoagulants: consequences to the genesis and progression of atherosclerosis. Thromb Haemost. 2014;112:909–917. [DOI] [PubMed] [Google Scholar]
- 10. Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 11. Zhou Q, Bea F, Preusch M, et al. Evaluation of plaque stability of advanced atherosclerotic lesions in apo E–deficient mice after treatment with the oral factor Xa inhibitor rivaroxaban. Mediators Inflamm. 2011;2011:432080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hara T, Fukuda D, Tanaka K, et al. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE‐deficient mice. Atherosclerosis. 2015;242:639–646. [DOI] [PubMed] [Google Scholar]
- 13. Cranenburg EC, Koos R, Schurgers LJ, et al. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. 2010;104:811–822. [DOI] [PubMed] [Google Scholar]
- 14. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in J Am Coll Cardiol. 2014;63(25 part B):3024–3025 and J Am Coll Cardiol. 2015;66:2812]. J Am Coll Cardiol. 2014;63(25 part B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 15. Goff DC Jr, Lloyd‐Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014;63(25 part B):3026]. J Am Coll Cardiol. 2014;63(25 part B):2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 17. Rumberger JA, Brundage BH, Rader DJ, et al. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons [published correction appears in Mayo Clin Proc. 1999;74:538]. Mayo Clin Proc. 1999;74:243–252. [DOI] [PubMed] [Google Scholar]
- 18. Nakanishi R, Ceponiene I, Osawa K, et al. Plaque progression assessed by a novel semi‐automated quantitative plaque software on coronary computed tomography angiography between diabetes and non‐diabetes patients: a propensity‐score matching study. Atherosclerosis. 2016;255:73–79. [DOI] [PubMed] [Google Scholar]
- 19. Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:342–358. [DOI] [PubMed] [Google Scholar]
- 20. Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla‐protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. 2008;100:593–603. [PubMed] [Google Scholar]
- 21. Tantisattamo E, Han KH, O'Neill WC. Increased vascular calcification in patients receiving warfarin. Arterioscler Thromb Vasc Biol. 2015;35:237–242. [DOI] [PubMed] [Google Scholar]
- 22. Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. [DOI] [PubMed] [Google Scholar]
- 23. Chatrou ML, Winckers K, Hackeng TM, et al. Vascular calcification: the price to pay for anticoagulation therapy with vitamin K antagonists. Blood Rev. 2012;26:155–166. [DOI] [PubMed] [Google Scholar]
- 24. Han KH, O'Neill WC. Increased peripheral arterial calcification in patients receiving warfarin. J Am Heart Assoc. 2016;5:pii:e002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weijs B, Blaauw Y, Rennenberg RJ, et al. Patients using vitamin K antagonists show increased levels of coronary calcification: an observational study in low‐risk atrial fibrillation patients. Eur Heart J. 2011;32:2555–2562. [DOI] [PubMed] [Google Scholar]
- 26. Valenti V, Ó Hartaigh B, Heo R, et al. A 15‐year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow‐up of 9715 individuals. JACC Cardiovasc Imaging . 2015;8:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shaw LJ, Giambrone AE, Blaha MJ, et al. Long‐term prognosis after coronary artery calcification testing in asymptomatic patients: a cohort study. Ann Intern Med. 2015;163:14–21. [DOI] [PubMed] [Google Scholar]
- 28. Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all‐cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–1236. [DOI] [PubMed] [Google Scholar]
- 29. Budoff MJ, Young R, Lopez VA, et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;61:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. [DOI] [PubMed] [Google Scholar]
- 31. Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicholls SJ, Tuzcu EM, Wolski K, et al. Lowering the triglyceride/high‐density lipoprotein cholesterol ratio is associated with the beneficial impact of pioglitazone on progression of coronary atherosclerosis in diabetic patients: insights from the PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) study. J Am Coll Cardiol. 2011;57:153–159. [DOI] [PubMed] [Google Scholar]
- 33. Hiro T, Kimura T, Morimoto T, et al; JAPAN‐ACS Investigators . Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN‐ACS [Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome] study). J Am Coll Cardiol. 2009;54:293–302. [DOI] [PubMed] [Google Scholar]
- 34. Komukai K, Kubo T, Kitabata H, et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY‐FIT study. J Am Coll Cardiol. 2014;64:2207–2217. [DOI] [PubMed] [Google Scholar]
- 35. Habara M, Nasu K, Terashima M, et al. Impact on optical coherence tomographic coronary findings of fluvastatin alone versus fluvastatin + ezetimibe. Am J Cardiol. 2014;113:580–587. [DOI] [PubMed] [Google Scholar]
- 36. Papadopoulou SL, Neefjes LA, Garcia‐Garcia HM, et al. Natural history of coronary atherosclerosis by multislice computed tomography. JACC Cardiovasc Imaging . 2012;5(3 suppl):S28–S37. [DOI] [PubMed] [Google Scholar]
- 37. Voros S, Rinehart S, Qian Z, et al. Prospective validation of standardized, 3‐dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions: results from the ATLANTA (assessment of tissue characteristics, lesion morphology, and hemodynamics by angiography with fractional flow reserve, intravascular ultrasound and virtual histology, and noninvasive computed tomography in atherosclerotic plaques) I study. JACC Cardiovasc Interv . 2011;4:198–208. [DOI] [PubMed] [Google Scholar]
- 38. Fahmy M., Nakanishi R., Matsumoto S., et al. Interobserver reproducibility in a novel semi‐automated coronary plaque quantification software [abstract]. J Cardiovasc Comput Tomogr. 2014. (suppl). [Google Scholar]
- 39. Vorre MM, Abdulla J. Diagnostic accuracy and radiation dose of CT coronary angiography in atrial fibrillation: systematic review and meta‐analysis. Radiology. 2013;267:376–386. [DOI] [PubMed] [Google Scholar]
- 40. Malchano ZJ, Neuzil P, Cury RC, et al. Integration of cardiac CT/MR imaging with three‐dimensional electroanatomical mapping to guide catheter manipulation in the left atrium: implications for catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:1221–1229. [DOI] [PubMed] [Google Scholar]


