Abstract
Background
Complex multimarker approaches to predict outcome after ST‐elevation myocardial infarction (STEMI) have only considered a single baseline sample, while neglecting easily obtainable peak creatine kinase and creatine kinase‐MB (CK‐MB) values during hospitalization.
Methods
We studied 476 patients undergoing primary percutaneous coronary intervention for STEMI and cardiac magnetic resonance imaging (CMRI) at 4‐6 months after STEMI. We determined the association with cardiac biomarkers (peak CK‐MB, peak troponin T, N‐terminal pro‐brain natriuretic peptide), clinical and angiographic characteristics with infarct size, and LVEF, followed by association with mortality in 1120 STEMI patients.
Results
Peak CK‐MB was the strongest predictor for infarct size (P<0.001, R 2=0.60) and LVEF (P<0.001, R 2=0.40). The additional value of clinical and angiographic characteristics was limited. The optimal peak CK‐MB cutpoints, for differentiation among small (<10% of the left ventricle), moderate (≥10%–<30%), and large infarct size (≥30%), were 210 U/L and 380 U/L, respectively. These cutpoints were associated with 90‐day mortality; the hazard ratio for moderate infarct was 2.99 (95% confidence interval [CI]: 1.51‐5.93, P=0.002) and for large infarct 6.53 (95% CI: 3.63‐11.76, P<0.001).
Conclusions
Classical peak CK‐MB measured during hospitalization for STEMI was superior to other clinical and angiographic characteristics in predicting CMRI‐defined infarct size and LVEF, and should be included and validated in future multimarker studies. Peak CK‐MB cutpoints differentiated among infarct size categories and were associated with increased 90‐day mortality risk.
Keywords: Ischemic heart disease, myocardial infarction, creatine kinase MB, Imaging, magnetic resonance imaging, LVEF, mortality
1. INTRODUCTION
The last decade, improvement of therapy has resulted in an overall decline of early and late mortality from ST‐segment myocardial infarction (STEMI) at the cost of an increasing number of patients eventually developing heart failure. Due to an ageing population and unhealthy lifestyles, the burden of coronary artery disease (CAD) and heart failure is anticipated to increase the next decade.1
Early identification of patients at increased risk to develop adverse outcome after STEMI is important. Myocardial infarct size and left ventricular ejection fraction (LVEF) are both strong and independent predictors of outcome and can be reliably determined by cardiac magnetic resonance imaging (CMRI)2, 3 several months after STEMI.4, 5 Multiple variables during myocardial infarction (MI) hospitalization have been studied as early predictors of infarct size and LVEF. These include clinical, biochemical, and angiographic factors, including troponin, N‐terminal pro‐brain natriuretic peptide (NT pro‐BNP), high sensitivity C‐reactive protein, duration of ischemia, location of STEMI and myocardial blush grade (MBG).6, 7, 8, 9, 10, 11, 12, 13 Whether these factors have independent value in addition to the more classical parameter creatine kinase‐MB (CK‐MB) to predict CMRI‐measured infarct size and LVEF has not been well established. Previous CMRI studies were hampered by small sample size, investigated only a single or selected number of cardiac specific biomarkers, and did not determine optimal cutpoints that might be of value in clinical care. In addition, previous (multimarker) outcome studies focused on admission blood biomarkers and neglected the values easily obtainable during hospitalization, including peak CK‐MB or peak troponin.14
The aim of this study was to determine the value of biochemical, clinical, and angiographic characteristics in relation to CMRI‐defined infarct size and LVEF at 4 to 6 months in STEMI patients treated with primary percutaneous coronary intervention (PCI). We determined optimal biomarker cutpoints to differentiate small from large MI and assessed their association with long‐term mortality.
2. METHODS
We pooled individual‐level data of the GIPS‐III (Glycometabolic Intervention as Adjunct to Primary Percutaneous Coronary Intervention in ST‐Segment Elevation Myocardial Infarction) and PREPARE (The PRoximal Embolic Protection in Acute myocardial infarction and Resolution of ST‐Elevation) randomized clinical trials, both described thoroughly in the Supporting Information in the online version of this article. Both studies evaluated LVEF and infarct size within 4 to 6 months after STEMI by CMRI. LVEF was categorized according to current guidelines.15 Categories moderately and severely abnormal LVEF were taken together because of small group size. Infarct size was categorized in small (<10% of the left ventricle), moderate (≥10%– < 30%), and large infarct size (≥30%) as recommended by the guidelines.16
The University Medical Centre Groningen (UMCG) STEMI registry was used to investigate association of biomarker cutpoints with long‐term mortality. In total, 1120 patients with STEMI in the period of January 1, 2011 until May 31, 2013, including subjects participating in the GIPS‐III trial, treated in the UMCG, were included. All patients with STEMI underwent diagnostic coronary angiography and PCI, and were 18 years of age and older. Patients were excluded when no significant CAD was found. The medical ethics committee of the UMCG approved the study.
2.1. Statistical analysis
Dichotomous variables are presented as percentages and continuous variables as medians with the interquartile range (IQR). Uni‐ and multivariable linear regression analyses were performed to determine correlates of infarct size and LVEF at 4 to 6 months and baseline variables. Age and sex were forced in all multiple linear regression models. Variables with P value ≤0.10 in univariable linear regression were included in the multiple linear regression analyses. Multiple linear regression analyses were performed by means of a forward stepwise algorithm (cutoff for entry ≤0.05) to identify independent predictors of infarct size and LVEF. To validate the consistency, this was repeated with a backward stepwise algorithm (cutoff for removal ≤0.10), and with the bootstrap method as implemented with the swboot command in Stata (StataCorp LP, College Station, TX). By bootstrapping, 1000 repetitions were made for model selection, and variables were reported when present in the majority of bootstrap attempts (>50%). The models were tested for collinearity using the variance inflation factor and the condition index. Goodness‐of‐fit of the final linear regression models were assessed with the adjusted R2 and the Mallow Cp statistic. The nonparametric Dunn test was performed for comparisons between categories of cardiac biomarkers. Optimal cutpoints for cardiac biomarkers were determined using the Youden index. Time to event analyses were performed with Cox regression models adjusted for risk factors available in our dataset and referred to in the European Society of Cardiology guidelines: age, sex, previous CAD (PCI, coronary artery bypass graft [CABG], and/or MI), and diabetes mellitus (DM).17 Model discrimination was tested with the Harrell C index. All reported P values are 2‐sided. A P < 0.05 was considered to indicate a significant difference. Analyses were performed with STATA/IC version 13.0 (StataCorp).
3. RESULTS
3.1. Baseline characteristics
Baseline characteristics of patients included in the GIPS‐III and PREPARE trials are presented in Table 1. The median age was 58 years, and the majority was male. The prevalence of smoking, diabetes, history of stroke, diastolic blood pressure, and single vessel disease was different between GIPS‐III and PREPARE. Peak values of CK‐MB were available in all patients; peak troponin T was available in 175 (85%) of patients participating in the PREPARE trial and in all patients of the GIPS‐III study. Peak troponin T, CK‐MB, and troponin T at admission were higher in the PREPARE than GIPS‐III. Infarct‐related arteries, Thrombolysis in Myocardial Infarction (TIMI) flow pre‐PCI and MBG were also distributed unevenly. In the UMCG STEMI registry, the majority of the patients was male (72%) and the median age was 62.5 years (IQR 52.9–71.3). Median peak CK‐MB was 153 U/L (IQR 65–305.5), median peak troponin T was 2.59 µg/L (IQR 0.89–5.82).
Table 1.
Baseline characteristics of GIPS‐III and PREPARE
| Factor | GIPS‐III, Median (IQR)/No. (%), n = 271 | PREPARE, Median (IQR)/No. (%), n = 205 |
|---|---|---|
| Age, y | 58 (49–65) | 58 (50– 66) |
| Male | 213 (79) | 172 (84) |
| Body mass index, kg/m2 | 26.5 (24.3–29.3) | 26.5 (24.3–28.6) |
| Ethnicity; Caucasian, Asian, black | 240 (95), 10 (4), 4 (2) | 0 |
| Hypertension | 71 (28) | 43 (21) |
| Diabetes | 0 | 14 (7) |
| Active smoker at randomization | 129 (51) | 127 (62) 1 |
| Stroke | 1 (0) | 5 (2) 1 |
| Previous PCI | 3 (1) | 8 (4) |
| Blood pressure, mmHg, systolic, diastolic | 132 (119–146), 84 (74–95) | 130 (114–153), 77 1 (68–88) |
| Heart rate, beats/min | 75 (64–84) | 70 (60– 86) |
| Ischemia time, min | 161 (107–242) | 158 (127–219) |
| Single vessel disease | 195 (72) | 141 (69) 1 |
| Infarct‐related artery–left main | 0 | 0 1 |
| LAD | 111 (41) | 64 (31) |
| LCX | 46 (17) | 22 (11) |
| RCA | 114 (42) | 119 (58) |
| TIMI flow pre‐PCI | ||
| 0 | 145 (57) | 190 (93) 1 |
| 1 | 19 (8) | 12 (6) |
| 2 | 44 (17) | 3 (1) |
| 3 | 46 (18) | 0 |
| TIMI flow post‐PCI | ||
| 0 | 0 | 1 (0) |
| 2 | 16 (6) | 16 (8) |
| 3 | 238 (94) | 188 (92) |
| Myocardial blush grade | ||
| 0 | 5 (2) | 4 (2) 1 |
| 1 | 17 (7) | 46 (23) |
| 2 | 53 (21) | 89 (45) |
| 3 | 177 (70) | 57 (29) |
| CK‐MB, U/L | 17 (13–25) | 5 (3.5–9) 1 |
| Troponin T, µg/L | 0.05 (0.02–0.15) | 0 (0–0.07) 1 |
| NT pro‐BNP, ng/L | 81 (38–200) | 84 (14–245) |
| HsCRP, mg/L | 1.8 (0.9–3.8) | 2.2 (1.2–4.5) |
| eGFR, mL/min | 96 (86–103) | 93 (80–103) |
| Peak CK‐MB, U/L | 163 (76–328) | 180 (108–325) |
| Peak Troponin T, µg/L | 2.99 (1.27–6.37) | 3.64 (1.35–7.91) 1 |
Abbreviations: CK‐MB, creatine kinase‐MB; eGFR, estimated glomerular filtration rate; GIPS‐III, Glycometabolic Intervention as Adjunct to Primary Percutaneous Coronary Intervention in ST‐Segment Elevation Myocardial Infarction; HsCRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LAD, left anterior descending artery; LCX, left circumflex artery; NT pro‐BNP, N‐terminal pro‐brain natriuretic peptide; PCI, percutaneous coronary intervention; PREPARE, The PRoximal Embolic Protection in Acute myocardial infarction and Resolution of ST‐Elevation; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial Infarction.
P ≤ 0.05 between GIPS‐III and PREPARE.
3.2. Infarct size and LVEF
Infarct size, as a percentage of the left ventricle, was available in 454 patients with a median of 8.9% (IQR 3.1–15.5), and this was higher in the PREPARE compared to the GIPS‐III trial (median 7.1% vs 10.2%, respectively, P = 0.0003). None of the patients in the GIPS‐III trial died during the first 4 months of follow‐up. In total, 7 patients of the PREPARE study died within 120 days of follow‐up, and therefore CMRI was not performed in these patients. LVEF was available in 476 patients, with a median of 54.2% (IQR 47.0–59.3) and was higher in the GIPS‐III compared to the PREPARE trial (median 55.4% vs 52.0%, respectively, P = 0.0003). Infarct size and LVEF were correlated (r = −0.69, P < 0.0001).
3.3. Univariable linear regression
The univariable regression of infarct size identified several predictors: patient‐related parameters (heart rate), angiographic‐related characteristics (infarct‐related artery, TIMI flow pre‐PCI, TIMI flow post‐PCI, and MBG) and cardiac biomarkers (CK‐MB, CK, NT pro‐BNP, and troponin T) (Table 2). The cardiac biomarkers were the strongest predictors for infarct size at 4 to 6 months. The same parameters were also predictive for LVEF at 4 to 6 months.
Table 2.
Univariable regression analyses data of GIPS‐III and PREPARE
| Factor | Infarct Size | LVEF | ||||
|---|---|---|---|---|---|---|
| P | Standardized β, SE | R 2 | P | Standardized β, SE | R 2 | |
| Age | 0.41 | 0.61 | ||||
| Sex | 0.98 | 0.20 | ||||
| Body mass index, kg/m2 | 0.06 | −0.09, 0.13 | 0.01 | 0.12 | ||
| Ethnicity | 0.23 | 0.80 | ||||
| Hypertension | 0.27 | 0.13 | ||||
| Diabetes | 1.00 | 0.19 | ||||
| Active smoker at randomization | 0.22 | 0.92 | ||||
| Stroke | 0.76 | 0.10 | ||||
| Previous PCI | 0.72 | 0.54 | ||||
| Systolic blood pressure | 0.61 | 0.90 | ||||
| Diastolic blood pressure | 0.45 | 0.36 | ||||
| Heart rate, beats/min | 0.002 | 0.14, 0.02 | 0.02 | 0.002 | −0.14, 0.02 | 0.02 |
| Ischemia time, min | 0.73 | 0.07 | −0.08, 0.003 | 0.01 | ||
| Single vessel disease | 0.16 | 0.51 | ||||
| Infarct‐related artery | <0.001 | 0.11 | <0.001 | 0.08 | ||
| LCX | 0.131 | −0.07, 1.27 | 0.34 | 0.05, 1.30 | ||
| RCA | <0.001 | −0.35, 0.90 | <0.001 | 0.30, 0.90 | ||
| TIMI flow pre‐PCI | <0.001 | 0.06 | <0.001 | 0.03 | ||
| TIMI 1 | 0.77 | 0.02, 0.98 | 0.16 | −0.07, 0.99 | ||
| TIMI 2 | 0.03 | −0.11, 1.41 | 0.13 | 0.07, 1.43 | ||
| TIMI 3 | <0.001 | −0.22, 1.49 | 0.009 | 0.13, 1.49 | ||
| TIMI flow post‐PCI | 0.006 | 0.02 | 0.002 | 0.03 | ||
| TIMI 2 | 0.50 | 0.17, 9.29 | 0.11 | −0.41, 9.49 | ||
| TIMI 3 | 0.92 | 0.03, 9.16 | 0.33 | −0.25, 9.36 | ||
| Myocardial blush grade | <0.001 | 0.09 | <0.001 | 0.09 | ||
| MBG 1 | 0.75 | 0.04, 3.13 | 0.67 | −0.05, 3.19 | ||
| MBG 2 | 0.12 | −0.24, 3.03 | 0.18 | 0.20, 3.08 | ||
| MBG 3 | 0.022 | −0.37, 2.99 | 0.03 | 0.36, 3.05 | ||
| CK‐MB, U/L | <0.001 | 0.18, 0.01 | 0.03 | 0.002 | −0.14, 0.01 | 0.02 |
| Troponin T, µg/L | 0.42 | 0.80 | ||||
| NT pro‐BNP, 1000 ng/L | <0.001 | 0.24, 0.001 | 0.06 | <0.001 | −0.26, 0.001 | 0.07 |
| eGFR, mL/min | 0.42 | 0.54 | ||||
| Peak CK‐MB, 100 U/L | <0.001 | 0.77, 0.001 | 0.60 | <0.001 | −0.63, 0.002 | 0.40 |
| Peak troponin T, µg/L | <0.001 | 0.67, 0.060 | 0.44 | <0.001 | −0.56, 0.07 | 0.32 |
Abbreviations: CK‐MB, creatine kinase‐MB; eGFR, estimated glomerular filtration rate; GIPS‐III, Glycometabolic Intervention as Adjunct to Primary Percutaneous Coronary Intervention in ST‐Segment Elevation Myocardial Infarction; IQR, interquartile range; LCX, left circumflex artery; LVEF, left ventricular ejection fraction; MBG, myocardial blush grade; NT pro‐BNP, N‐terminal pro‐brain natriuretic peptide; PCI, percutaneous coronary intervention; PREPARE, The PRoximal Embolic Protection in Acute myocardial infarction and Resolution of ST‐Elevation; RCA, right coronary artery; SE, standard error; TIMI, Thrombolysis in Myocardial Infarction.
3.4. Multivariable linear regression
The stepwise backward multiple linear regression model of infarct size identified peak CK‐MB, peak Troponin T, MBG 1, TIMI 1 flow pre‐PCI, and culprit vessel right coronary artery (RCA) as predictors (R2 = 0.66, P ≤ 0.017; Table 3). The forward model was identical. The bootstrap model, including variables present >50% of the time, also identified the same variables. The stepwise backward and forward models for LVEF identified peak CK‐MB, NT pro‐BNP, MBG, and culprit vessel RCA (R 2 = 0.46, P ≤ 0.049) as predictors. The bootstrap model for LVEF also identified the same variables, except peak troponin T was more frequently selected than MBG. Significant interactions (P ≤ 0.05) existed between age, gender, and cardiac biomarkers peak troponin T and peak CK‐MB, but including them in the models did not improve the model fit.
Table 3.
Multivariable regression models for infarct size and LVEF data of GIPS‐III and PREPARE
| Coefficient | SE | t | P | Standardized β | |
|---|---|---|---|---|---|
| Infarct size, R 2 = 0.66 | |||||
| Peak CK‐MB, 100 U/L | 2.81 | 0.22 | 13.05 | <0.001 | 0.63 |
| Peak troponin T, µg/L | 0.25 | 0.08 | 3.04 | 0.003 | 0.15 |
| MBG 1 | 3.00 | 0.81 | 3.71 | <0.001 | 0.11 |
| TIMI 1 pre‐PCI | 1.55 | 0.57 | 2.72 | 0.007 | 0.08 |
| RCA | −1.37 | 0.57 | −2.40 | 0.017 | −0.07 |
| LVEF, R 2 = 0.46 | |||||
| Peak CK‐MB (100 U/L) | −2.58 | 0.17 | −15.49 | <0.001 | −0.58 |
| NT pro‐BNP (1000 ng/L) | −1.64 | 0.66 | −2.49 | 0.013 | −0.09 |
| MBG 1 | −4.29 | 1.02 | −4.21 | <0.001 | −0.16 |
| MBG 2 | −1.48 | 0.75 | −1.97 | 0.049 | −0.07 |
| RCA | 1.68 | 0.70 | 2.41 | 0.016 | 0.09 |
Abbreviations: CK‐MB, creatine kinase‐MB; GIPS‐III, Glycometabolic Intervention as Adjunct to Primary Percutaneous Coronary Intervention in ST‐Segment Elevation Myocardial Infarction; LVEF, left ventricular ejection fraction; MBG, myocardial blush grade; NT pro‐BNP, N‐terminal pro‐brain natriuretic peptide; PCI, percutaneous coronary intervention; PREPARE, The PRoximal Embolic Protection in Acute myocardial infarction and Resolution of ST‐Elevation; RCA, right coronary artery; SE, standard error; TIMI, Thrombolysis in Myocardial Infarction.
3.5. Categories of infarct size and LVEF and optimal biomarker cutpoints
Categories of infarct size, as assessed by CMRI, strongly related to cardiac biomarkers (see Supporting Figure 1A in the online version of this article). Median peak CK‐MB was 105 U/L (IQR 50–160) in small infarct size, 316 U/L (IQR 222–473) in moderate infarct size, and 578 U/L (IQR 436–768) in large infarct size (P < 0.01 for all comparisons). Median peak troponin T was 1.79 µg/L (IQR 0.63–2.94) in small infarct size, 6.47 µg/L (IQR 3.93–8.93) in moderate infarct size, and 11 µg/L (IQR 7.95–18.81) in large infarct size (P = 0.11 for moderate vs large comparison and P < 0.001 for remaining comparisons). In logistic regression analysis, the predictive value to discriminate between infarct size categories using the multiple regression model for infarct size, peak CK‐MB, peak troponin T, or LVEF was assessed. The models were significantly different in their predictive ability (see Supporting Figure 2A–D in the online version of this article, P ≤ 0.001). The area under the curve (AUC) of the multiple regression model for infarct size and peak CK‐MB were high (0.82–0.95). When differentiating between moderate and large infarct size, the AUC of the multiple regression model was the highest (0.95) compared to AUC of 0.82 for peak CK‐MB, 0.78 for peak troponin T, and 0.89 for LVEF.
LVEF was categorized and related to cardiac biomarkers (see Supporting Figure 1B in the online version of this article). Comparisons between mildly (n = 142) or moderately abnormal (n = 51) vs severely abnormal LVEF (n = 11) were not significant. After taking into account group size, the categories moderately and severely abnormal LVEF were merged (P < 0.001 for all comparisons). Median peak CK‐MB was 131 U/L (IQR 56–196) in normal LVEF, 276 U/L (IQR 160–418) in mildly abnormal LVEF, and 499 U/L (IQR 345–666) in moderately to severely abnormal LVEF. Median peak troponin T was 2.07 µg/L (IQR 0.98–3.81) in normal LVEF, 5.18 µg/L (IQR 2.47–8.26) in mildly abnormal LVEF, and 9.67 µg/L (IQR 6.54–14.80) in moderately to severely abnormal LVEF. In logistic regression analysis, the predictive value to discriminate between LVEF categories using the multiple regression model for LVEF, peak CK‐MB, and peak troponin T were assessed. The AUC for LVEF categories was overall lower than reported for infarct size categories (see Supporting Figure 2A–D in the online version of this article).
For peak CK‐MB, the strongest univariate predictor of both infarct size and LVEF, the optimal cutpoint for differentiation between small vs moderate infarct was 210 U/L (sensitivity 0.79 and specificity 0.88). For moderate vs large infarct, the cutpoint was 380 U/L (sensitivity 0.90 and specificity 0.65). The optimal cutpoint for differentiation between normal vs mildly abnormal LVEF was 200 U/L (sensitivity 0.68 and specificity 0.77). For mildly abnormal vs moderately to severely abnormal LVEF the cutpoint was 361 U/L (sensitivity 0.74 and specificity 0.70).
3.6. Peak CK‐MB and its association with mortality
In the UMCG STEMI registry (n = 1120) 90‐day mortality was related to categories of predicted infarct size based on the defined two cutpoints of peak CK‐MB. The hazard ratio (HR) was 2.99 (95% confidence interval [CI]: 1.51‐5.93) for the moderate category infarct size and HR 6.53 (95% CI: 3.63‐11.76) for the large category infarct size (P < 0.01, Figure). After adjustment for age, sex, previous CAD (including history of PCI, CABG, or MI), and DM, the corresponding HRs remained similar (Harrell C index 0.78, Table 4). After stepwise backward multivariable Cox regression, including variables from the infarct size linear regression model, only peak CK‐MB was a significant predictor of 90‐day mortality. From the variables of the LVEF linear regression model, only peak CK‐MB and NT pro‐BNP predicted 90‐day mortality (peak CK‐MB per 100 U/L: adjusted HR 1.24 [95% CI: 1.19‐1.29], P < 0.001, NT pro‐BNP per 1000 U/L: adjusted HR 1.01 [95% CI: 1.00‐1.02], P = 0.007, Harrell C index 0.80).
Table 4.
Peak CK‐MB and NT pro‐BNP predicting 90‐day mortality in UMCG STEMI registry
| Factor | Hazard Ratio | P | Confidence Interval |
|---|---|---|---|
| Peak CK‐MB ≥210 and <380 U/L | 3.39 | 0.001 | 1.69‐6.80 |
| Peak CK‐MB >380 U/L | 7.47 | <0.001 | 4.05‐13.78 |
| Age per year | 1.04 | <0.001 | 1.02‐1.06 |
| Female | 0.64 | 0.16 | 0.34‐1.19 |
| Previous CAD 1 | 2.38 | 0.003 | 1.34‐4.21 |
| Diabetes | 1.10 | 0.768 | 0.59‐2.05 |
Abbreviations: CK‐MB, creatine kinase‐MB; CAD, coronary artery disease; NT pro‐BNP, N‐terminal pro‐brain natriuretic peptide; STEMI, ST‐segment elevation myocardial infarction; UMCG, University Medical Centre Groningen.
Previous CAD included history of PCI, coronary artery bypass surgery, and/or myocardial infarction.
Figure 1.
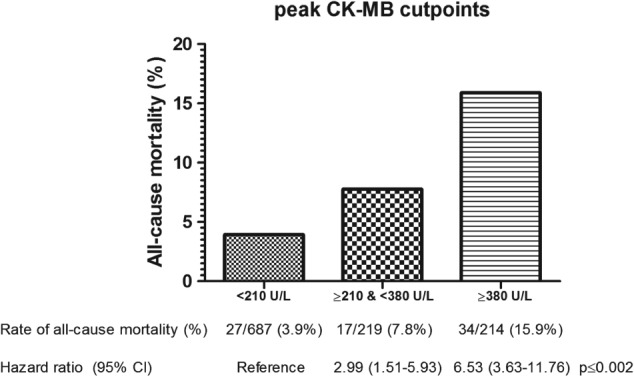
Peak CK‐MB categorization based on predicted small vs moderate vs large infarct size with 90‐day all‐cause mortality and hazard ratios (N = 1120). Abbreviations: CI, confidence interval; CK‐MB, creatine kinase‐MB.
4. DISCUSSION
We studied the contemporary value of cardiac biomarkers available during hospitalization and their optimal cutpoints to predict myocardial infarct size, LVEF, and mortality in STEMI patients. We demonstrated that peak CK‐MB is the strongest predictor of infarct size, LVEF and mortality and superior to clinical and angiographic characteristics. The optimal CK‐MB cutpoints to discern between small, moderate, and large infarct as measured by CMRI were defined, and these strongly predicted mortality.
Recently, several publications have reported the importance of a multimarker approach to predict long‐term outcome in patients presenting with STEMI.10, 14, 18, 19 Interestingly, many of these studies only considered available biomarkers derived from blood obtained at admission, and focused on the long‐term instead of short‐term outcome. A major limitation of these studies is therefore the ignorance of easily available short‐term data hours after hospital admission, including CK‐MB measurements. Several earlier CMRI studies have investigated biomarkers for infarct size and LVEF in smaller‐sized populations (see Supporting Table 1 in the online version of this article). Overall, cardiac biomarkers CK, CK‐MB, troponin I, and troponin T correlated strongly with infarct size and less with LVEF. Peak CK‐MB level was reported to be the superior predictor of histological infarct size in a postmortem study.20 AUC levels were not complete for both cohorts, and in the GIPS‐III trial, peak CK‐MB was superior to CK‐MB AUC. In contrast, some studies found that the AUC is superior to peak values for predicting infarct size and LVEF. This is likely due to differences in sample size and availability of data to calculate AUC accurately in all patients.
Comprehensive multiple regression analyses to predict infarct size and LVEF have been performed earlier in various small scale studies. Peak CK‐MB was also reported as the strongest predictor of infarct size at 30 days in a substudy of the INFUSE‐AMI (Intracoronary Abciximab Infusion and Aspiration Thrombectomy in Patients Undergoing Percutaneous Coronary Intervention for Anterior ST Segment Elevation Myocardial Infarction) trial, including anterior STEMI patients undergoing PCI.21 In this study multiple regression analysis peak CK‐MB, final MBG 0 or 1, and age were independent predictors of LVEF. In a similar substudy of the same trial, high leukocyte count at presentation, together with age, total abnormal wall motion score, time from symptom onset to first device, proximal left anterior descending location, and poor TIMI flow at baseline have also been reported as independent predictors of infarct size.22 In the INFUSE‐AMI substudies, R 2 of the multivariable models have not been reported, and direct comparison of the explained variance with the current study is therefore not possible. In a different study, the most important predictors of LVEF at 3 months were NT pro‐BNP, microvascular obstruction, and CMRI‐defined infarct size at baseline.23 However, this study did not include other cardiac biomarkers, such as troponin or CK‐MB, into their statistical models.
To our knowledge, cutpoints of peak CK‐MB have not been investigated previously. In our study, peak CK‐MB appeared to be a stronger predictor for infarct size than for LVEF. These cutpoints were clinically relevant as indicated by its strong association with 90‐day mortality. In a recent study, including a large pooled group of STEMI patients, CK‐MB levels were also independently associated with 3‐ and 6‐month mortality.24 The currently presented cutpoints might provide an easy tool for risk stratification in the early phase after STEMI, and should be considered in future multimarker studies that might provide further guidance for treatment.
In our study and previous studies, correlations with biomarkers and CMRI infarct size appear to be stronger than with LVEF. This might be explained by considerable interindividual variation of LVEF in the general population. NT pro‐BNP measured at admission was present in the LVEF multiple regression model only, and together with peak CK‐MB it was associated with 90‐day mortality. NT pro‐BNP might reflect a preexistent cardiac condition not related to the infarct event, and therefore possibly provides additional information on outcome.
5. CONCLUSION
This study demonstrated the strength of peak CK‐MB in the prediction of infarct size and LVEF after STEMI. When considering peak CK‐MB obtained during hospitalization, the additional value of other biomarkers and more complex multiple regression models was remarkably limited. We provided peak CK‐MB cutpoints that are associated with infarct size categories and mortality. Further studies are needed to validate our cutpoints to predict clinical outcome and guide therapy.
Supporting information
Table S1. overview literature uni‐ and multivariable predictors for infarct size and LVEF
Figure S1. Data is presented as median with IQR and adjacent lines are at 1.5*IQR below the 25th percentile and above the 75th percentile. A. Combined data of GIPS‐III & PREPARE: median peak CK‐MB and peak Troponin T levels in different categories of infarct size as assessed by CMR. Measures of peak CK‐MB and infarct size were available in n=454; peak Troponin T in n=425. P≤0.0057 for all comparisons of peak CK‐MB. P=0.11 for moderate vs. large comparison and p<0.0001 for remaining comparisons of peak Toponin T. B. Combined data of GIPS‐III & PREPARE: median peak CK‐MB and peak Troponin T levels in different categories of LVEF as assessed by CMR. Measures of peak CK‐MB and LVEF were available in n=476; for peak Troponin T n=446. P≤0.0006 for all comparisons of peak CK‐MB and peak Troponin T.
Figure S2. A. Combined data of GIPS‐III & PREPARE: ROC curves of the multiple regression model, peak CK‐MB, peak Troponin T and LVEF for prediction of small vs. moderate infarct size (N=397, p<0.0001). The multiple regression model includes peak CK‐MB, peak Troponin T, MBG1, TIMI 1 pre‐PCI and RCA. B. ROC curves of the multiple regression model, peak CK‐MB, peak Troponin T and LVEF for prediction of moderate vs. large infarct size (N=186, p=0.0001). C. ROC curves of the multiple regression model, peak CK‐MB, peak Troponin T for prediction of normal vs. mildly abnormal LVEF (N=380, p=0.0133). The multiple regression model includes peak CK‐MB, NT pro‐BNP, MBG1, MBG2, and RCA. D. ROC curves of the multiple regression model, peak CK‐MB, peak Troponin T for prediction of mildly abnormal vs. moderately to severely abnormal LVEF (N=182, p=0.0238).
ACKNOWLEDGMENTS
The authors thank the GIPS‐III and the PREPARE study groups for providing access to data to perform the current analyses.
5.1. Conflict of interests
The authors declare no potential conflicts of interest.
Hartman MHT, Eppinga RN, Vlaar PJJ, Lexis CPH, Lipsic E, Haeck JDE, van Veldhuisen DJ, van der Horst ICC and van der Harst P. The contemporary value of peak creatine kinase‐MB after ST‐segment elevation myocardial infarction above other clinical and angiographic characteristics in predicting infarct size, left ventricular ejection fraction, and mortality. Clin Cardiol. 2017;40:322–328. 10.1002/clc.22663
REFERENCES
- 1. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. [DOI] [PubMed] [Google Scholar]
- 2. Mahrholdt H, Wagner A, Holly TA, et al. Reproducibility of chronic infarct size measurement by contrast‐enhanced magnetic resonance imaging. Circulation. 2002;106:2322–2327. [DOI] [PubMed] [Google Scholar]
- 3. American College of Cardiology Foundation Task Force on Expert Consensus Documents , Hundley WG, Bluemke DA, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College Of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55:2614–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lonborg J, Vejlstrup N, Kelbaek H, et al. Final infarct size measured by cardiovascular magnetic resonance in patients with ST elevation myocardial infarction predicts long‐term clinical outcome: an observational study. Eur Heart J Cardiovasc Imaging. 2013;14:387–395. [DOI] [PubMed] [Google Scholar]
- 5. Roes SD, Kelle S, Kaandorp TA, et al. Comparison of myocardial infarct size assessed with contrast‐enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol. 2007;100:930–936. [DOI] [PubMed] [Google Scholar]
- 6. Norris RM, Whitlock RM, Barratt‐Boyes C, Small CW. Clinical measurement of myocardial infarct size. Modification of a method for the estimation of total creatine phosphokinase release after myocardial infarction. Circulation. 1975;51:614–620. [DOI] [PubMed] [Google Scholar]
- 7. Mayr A, Mair J, Klug G, et al. Cardiac troponin T and creatine kinase predict mid‐term infarct size and left ventricular function after acute myocardial infarction: a cardiac MR study. J Magn Reson Imaging. 2011;33:847–854. [DOI] [PubMed] [Google Scholar]
- 8. Mayr A, Mair J, Schocke M, et al. Predictive value of NT‐pro BNP after acute myocardial infarction: relation with acute and chronic infarct size and myocardial function. Int J Cardiol. 2011;147:118–123. [DOI] [PubMed] [Google Scholar]
- 9. Mather AN, Fairbairn TA, Artis NJ, Greenwood JP, Plein S. Relationship of cardiac biomarkers and reversible and irreversible myocardial injury following acute myocardial infarction as determined by cardiovascular magnetic resonance. Int J Cardiol. 2013;166:458–464. [DOI] [PubMed] [Google Scholar]
- 10. Damman P, Kuijt WJ, Woudstra P, et al. Multiple biomarkers at admission are associated with angiographic, electrocardiographic, and imaging cardiovascular mechanistic markers of outcomes in patients undergoing primary percutaneous coronary intervention for acute ST‐elevation myocardial infarction. Am Heart J. 2012;163:783–789. [DOI] [PubMed] [Google Scholar]
- 11. Husser O, Bodi V, Sanchis J, et al. White blood cell subtypes after STEMI: temporal evolution, association with cardiovascular magnetic resonance—derived infarct size and impact on outcome. Inflammation. 2011;34:73–84. [DOI] [PubMed] [Google Scholar]
- 12. De Luca G, Parodi G, Sciagra R, et al. Relation of gender to infarct size in patients with ST‐segment elevation myocardial infarction undergoing primary angioplasty. Am J Cardiol. 2013;111:936–940. [DOI] [PubMed] [Google Scholar]
- 13. De Luca G, Parodi G, Sciagra R, et al. Preprocedural TIMI flow and infarct size in STEMI undergoing primary angioplasty. J Thromb Thrombolysis. 2014;38:81–86. [DOI] [PubMed] [Google Scholar]
- 14. Damman P, Beijk MA, Kuijt WJ, et al. Multiple biomarkers at admission significantly improve the prediction of mortality in patients undergoing primary percutaneous coronary intervention for acute ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2011;57:29–36. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 16. Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 17. Task force on the management of ST‐segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) ; Steg PG, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 18. Westerhout CM, Fu Y, Lauer MS, et al. Short‐ and long‐term risk stratification in acute coronary syndromes: the added value of quantitative ST‐segment depression and multiple biomarkers. J Am Coll Cardiol. 2006;48:939–947. [DOI] [PubMed] [Google Scholar]
- 19. Garcia‐Paredes T, Aguilar‐Alonso E, Arboleda‐Sanchez JA, et al. Evaluation of prognostic scale thrombolysis in myocardial infarction and killip. An ST‐elevation myocardial infarction new scale. Am J Emerg Med. 2014;32:1364–1369. [DOI] [PubMed] [Google Scholar]
- 20. Costa TN, Cassaro Strunz CM, Nicolau JC, Gutierrez PS. Comparison of MB fraction of creatine kinase mass and troponin I serum levels with necropsy findings in acute myocardial infarction. Am J Cardiol. 2008;101:311–314. [DOI] [PubMed] [Google Scholar]
- 21. Dohi T, Maehara A, Brener SJ, et al. Utility of peak creatine kinase‐MB measurements in predicting myocardial infarct size, left ventricular dysfunction, and outcome after first anterior wall acute myocardial infarction (from the INFUSE‐AMI trial). Am J Cardiol. 2015;115:563–570. [DOI] [PubMed] [Google Scholar]
- 22. Palmerini T, Brener SJ, Genereux P, et al. Relation between white blood cell count and final infarct size in patients with ST‐segment elevation acute myocardial infarction undergoing primary percutaneous coronary intervention (from the INFUSE AMI trial). Am J Cardiol. 2013;112:1860–1866. [DOI] [PubMed] [Google Scholar]
- 23. Ezekowitz JA, Armstrong PW, Granger CB, et al. Predicting chronic left ventricular dysfunction 90 days after ST‐segment elevation myocardial infarction: an assessment of pexelizumab in acute myocardial infarction (APEX‐AMI) substudy. Am Heart J. 2010;160:272–278. [DOI] [PubMed] [Google Scholar]
- 24. Bagai A, Schulte PJ, Granger CB, et al. Prognostic implications of creatine kinase‐MB measurements in ST‐segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention. Am Heart J. 2014;168:503–511.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. overview literature uni‐ and multivariable predictors for infarct size and LVEF
Figure S1. Data is presented as median with IQR and adjacent lines are at 1.5*IQR below the 25th percentile and above the 75th percentile. A. Combined data of GIPS‐III & PREPARE: median peak CK‐MB and peak Troponin T levels in different categories of infarct size as assessed by CMR. Measures of peak CK‐MB and infarct size were available in n=454; peak Troponin T in n=425. P≤0.0057 for all comparisons of peak CK‐MB. P=0.11 for moderate vs. large comparison and p<0.0001 for remaining comparisons of peak Toponin T. B. Combined data of GIPS‐III & PREPARE: median peak CK‐MB and peak Troponin T levels in different categories of LVEF as assessed by CMR. Measures of peak CK‐MB and LVEF were available in n=476; for peak Troponin T n=446. P≤0.0006 for all comparisons of peak CK‐MB and peak Troponin T.
Figure S2. A. Combined data of GIPS‐III & PREPARE: ROC curves of the multiple regression model, peak CK‐MB, peak Troponin T and LVEF for prediction of small vs. moderate infarct size (N=397, p<0.0001). The multiple regression model includes peak CK‐MB, peak Troponin T, MBG1, TIMI 1 pre‐PCI and RCA. B. ROC curves of the multiple regression model, peak CK‐MB, peak Troponin T and LVEF for prediction of moderate vs. large infarct size (N=186, p=0.0001). C. ROC curves of the multiple regression model, peak CK‐MB, peak Troponin T for prediction of normal vs. mildly abnormal LVEF (N=380, p=0.0133). The multiple regression model includes peak CK‐MB, NT pro‐BNP, MBG1, MBG2, and RCA. D. ROC curves of the multiple regression model, peak CK‐MB, peak Troponin T for prediction of mildly abnormal vs. moderately to severely abnormal LVEF (N=182, p=0.0238).


