Abstract
Background
The utility of rotor ablation using commercially available systems as an adjunct to pulmonary vein isolation (PVI) is controversial. Variable results may stem from heterogeneous practice patterns. We investigated whether a prespecified protocol to determine temperospatial rotor stability improved acute and intermediate outcomes following rotor ablation.
Hypothesis
Protocolized rotor mapping and ablation, with prespecified metrics to determine temporal rotor stability prior to ablation, will improve short‐ and long‐term PVI/rotor ablation outcomes.
Methods
Patients undergoing PVI plus rotor ablation at Johns Hopkins during 2015 were included. The first cohort underwent rotor mapping and ablation at the operator's discretion, whereas the second cohort underwent protocolized rotor mapping, with ablation limited to temperospatially stable rotors. Both cohorts underwent PVI. Acute results (rotor elimination, atrial fibrillation [AF] termination), procedural data, and 1‐year outcomes were assessed.
Results
Twenty‐seven patients underwent ablation (mean age, 64.4 ± 9 years, male 81.5%, persistent AF 85.2%, long‐standing persistent AF 14.8%, mean AF duration 4.4 ± 4 years, repeat cases 51.8%, and mean LA size 4.6 ± 0.8 cm). In the protocolized cohort, rotors were reproducible in 83% (10/12) of cases in at least 1 chamber. Acute rhythm change was achieved in 8/27 (29.6%) patients. Sinus rhythm on presentation (62.5% vs 15.8%, P = 0.03) and higher total targeted rotors (3.8 ± 1.7 vs 2.5 ± 1.0, P = 0.02) predicted acute change. At 12 months, freedom from AF/atrial tachycardia was achieved in 5/15 (33.3%) patients in the first cohort and 5/11 patients in the protocolized cohort (45.5%; P = 0.53 for comparison).
Conclusions
Acute and intermediate results did not change with protocolized mapping designed to identify temperospatially stable rotors. Outcomes at 12 months were similar in both groups.
Keywords: Atrial fibrillation, Arrhythmia, Catheter ablation, Focal impulse and rotor modulation
1. INTRODUCTION
Pulmonary vein isolation (PVI) is the cornerstone of catheter ablation for drug‐refractory atrial fibrillation (AF).1 However, the single‐procedure success rate for PVI remains modest, particularly in patients undergoing ablation for persistent AF.2 Additional ablation targets including complex fractionated atrial electrogram, non–pulmonary vein (PV) triggers, ganglionic plexi, and AF‐sustaining rotors are all subjects of active investigation, though consensus on extra‐PVI approaches is still lacking.
Ablation of focal impulse and rotor modulation (FIRM)–identified AF‐sustaining rotors using phase mapping has recently been proposed as an adjunct or alternative to PVI, with early investigations reporting significant improvement in AF‐free survival with these approaches.3, 4, 5, 6 Subsequent data from independent groups, however, have shown more limited success.7, 8, 9 One factor that may impact success is rigorous identification and targeting of stable rotor activity. In this study, we investigated whether a protocolized approach to identify temperospatially stable rotors by sequential FIRM mapping, when compared to a physician‐preference approach, would improve acute and intermediate‐term outcomes in patients undergoing FIRM‐guided ablation and PVI for persistent AF.
2. METHODS
2.1. Study population
Patients who underwent clinically indicated AF ablation with PVI plus FIRM‐guided rotor ablation at our institution during 2 periods in 2015 (February–April and September–December) were included in the study through serial enrollment into initial and protocolized cohorts; patients were not randomized. All patients provided written informed consent, and the study was approved by The Johns Hopkins Hospital institutional review board.
2.2. Procedural details
Briefly, patients were brought into the electrophysiology laboratory and placed under general anesthesia. A transesophageal echocardiogram was performed at the discretion of the operator. A 3‐dimensional bi‐atrial map was constructed with an electroanatomic mapping system (CARTO; Biosense Webster, South Diamond Bar, CA) and was merged onto a preexisting atrial computed tomography (CT) or magnetic resonance imaging (MRI). AF was induced in patients presenting in sinus rhythm by atrial burst pacing and isoproterenol infusion. Reference catheters were placed in the superior vena cava and coronary sinus.
Rotor mapping was performed with a 64‐pole basket mapping catheter (FIRMap; Abbott, Chicago, IL) of the appropriate size positioned in the right and the left atrium, with care taken to optimize electrode–tissue contact (Figure 1A,B). Anticoagulation with heparin was performed for a target activated clotting time of >350 seconds. Unipolar electrograms were recorded and exported for processing on the commercially available Topera system (Rhythm View; Topera Medical, Menlo Park, CA). Rotors were identified (see Mapping approaches below), projected onto the CARTO electroanatomical map (Figure 1C), and ablated.
Figure 1.
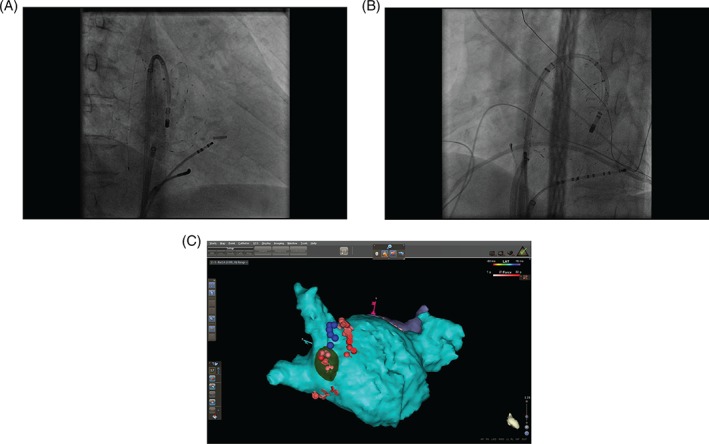
Right anterior oblique (panel A) and left anterior oblique (panel B) fluoroscopic images showing basket catheter deployment in the left atrium. Panel C illustrates projection of an identified rotor (green) onto a CARTO computed tomography–merged image of the left atrium. Blue dots indicate phrenic nerve capture sites; ablation lesions are shown in red.
Once rotor elimination was achieved in both atria, conventional PVI was performed as previously reported. Briefly, wide‐area circumferential lesion sets were delivered, with electrical PV isolation demonstrated with lasso–catheter mapping. Ablation (rotor and PVI) was performed with a 4‐mm, irrigated, force‐sensing ablation catheter, with 25 to 30 W (posterior LA) and 30 to 40 W (nonposterior LA) power delivery and goal contact force of 10 to 30g.
2.3. Mapping approaches
Patients in the first cohort (n = 15) underwent FIRM‐guided rotor ablation and PVI at the discretion of the operator, without predefined parameters addressing rotor stability, appropriate ablation targets, endpoints, or order of ablation (rotor and PVI). Patients in the second cohort (n = 12) underwent a predefined mapping protocol for rotor identification that was performed prior to PVI. Basket deployment was rigorously assessed by fluoroscopy and electrograms analysis to ensure optimal tissue contact. Mapping in each atrium was performed a minimum of 3 times, with no adjustment of the basket between data collection, and with mapping performed during an uninterrupted period of AF. Rotors in a spatially contiguous area seen on at least 2 out of 3 maps were targeted, with rotor elimination on subsequent mapping as the prespecified outcome. Rotors demonstrating temperospatial instability were not targeted.
2.4. Follow‐up
All patients were observed overnight in the hospital for hemodynamic monitoring and resumption of anticoagulation. Routine follow‐up with electrocardiograms (ECGs) and clinical assessment was performed at 3, 6, and 12 months. Additional follow‐up for symptomatic patients was performed if necessary, including Holter monitoring in symptomatic patients. Any recurrence of AF/atrial tachycardia (AT) documented by ECG or a device‐recording system lasting ≥30 seconds, outside of a 3‐month postprocedure blanking period, was considered recurrence.
2.5. Statistical analysis
Continuous variables were expressed as mean and standard deviation, whereas categorical variables are expressed as number and percentage. Univariate analysis was done using a t test or Wilcoxon rank sum test for continuous variables, and χ2 or Fisher exact test for categorical data where appropriate. A P value of <0.05 was considered significant. All statistical analyses were done using Stata version 12 (StataCorp, College Station, TX).
3. RESULTS
3.1. Patient and procedural characteristics
Fifteen patients underwent FIRM‐guided ablation with targets and outcomes defined by physician preference. Twelve subsequent patients underwent protocolized FIRM mapping and ablation. Baseline characteristics and procedural details of the 2 cohorts are presented in Table 1. Between the 2 groups, there was no significant difference in mean age (64.3 ± 10.4 years vs 64.6 ± 7.2 years, P = 0.94), male patients (73.3% vs 91.7%, P = 0.34), CHA2DS2VASC (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category) score (1.9 ± 1.2 vs 2.1 ± 1.8, P = 0.80), persistent AF (86.7% vs 83.3%, P = 0.81), and AF duration in years (4.7 ± 4.0 vs 4.0 ± 14.0, P = 0.56). Similarly, repeat procedure rate (60% vs 41.7%, P = 0.45) and LA size in centimeters (4.8 ± 0.9 vs 4.5 ± 0.7, P = 0.50) did not have any statistically significant difference. Only left ventricular ejection fraction percentage was lower in group 2 and was statistically significant (56.9 ± 2.5 vs 48.7 ± 8.7, P = 0.005).
Table 1.
Comparison of baseline characteristics of two groups
| Variable | Group 1, n = 15 | Group 2, n = 12 | P Value, Significance <0.05 |
|---|---|---|---|
| Age, y | 64.3 ± 10.4 | 64.6 ± 7.2 | 0.94 |
| Male sex | 11 (73.3%) | 11 (91.7%) | 0.34 |
| BMI, kg/m2 | 29.3 ± 5.7 | 31.9 ± 5.8 | 0.25 |
| CHA2DS2VASC | 1.9 ± 1.2 | 2.1 ± 1.8 | 0.80 |
| Hypertension | 11 (73.3%) | 7 (58.3%) | 0.45 |
| Diabetes mellitus | 0 (0%) | 1 (8.3%) | 0.26 |
| CHF | 1 (6.7%) | 1 (8.3%) | 1.00 |
| CAD | 2 (13.3%) | 3 (25%) | 0.44 |
| OSA | 3 (20%) | 4 (33.3%) | 0.43 |
| AF duration, y | 4.7 ± 4.0 | 4.0 ± 4.0 | 0.56 |
| Persistent AF | 13 (86.7%) | 10 (83.3%) | 0.81 |
| Long‐standing persistent AF | 2 (13.3%) | 2 (16.7%) | 0.45 |
| LVEF, % | 56.9 ± 2.5 | 48.7 ± 8.7 | 0.005 |
| LA size, cm | 4.8 ± 0.9 | 4.5 ± 0.7 | 0.50 |
| Redo procedure | 9 (60%) | 5 (41.7%) | 0.45 |
| Procedure time, min | 361 ± 69.2 | 389 ± 72.6 | 0.24 |
| Fluoroscopy time, min | 57.5 ± 11.8 | 62.9 ± 10.2 | 0.23 |
| RF time, min | 44.6 ± 15.5 | 44.4 ± 13.9 | 0.97 |
| Total no. of rotors | 2.7 ± 1.4 | 3.2 ± 1.0 | 0.13 |
| Rotors in RA | 1 ± 0.7 | 1.1 ± 1.1 | 0.94 |
| Rotors in LA | 1.7 ± 1.4 | 2.1 ± 1.0 | 0.18 |
| Intraprocedure cardioversion | 13 (86.7%) | 9 (75%) | 0.63 |
| Early recurrence | 6 (66.7%) | 3 (33.3%) | 0.68 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CHA2DS2VASC, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category; CHF, congestive heart failure; LA, left atrium; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnea; RA, right atrium; RF, radiofrequency.
Procedure time, fluoroscopy time, and ablation time did not differ between the 2 groups. In both groups, the majority of patients underwent cardioversion to normal sinus rhythm (NSR) (86.7% vs 75%, P = 0.63). The average number of total rotors among patients was 2.7 ± 1.4 in the first cohort vs 3.2 ± 1.0 in the protocolized cohort (P = 0.13), with 1.0 ± 0.7 vs 1.1 ± 1.1 (P = 0.94) rotors in the right atrium (RA) and 1.7 ± 1.4 vs 2.1 ± 1.0 (P = 0.18) rotors in left atrium (LA) for the initial and protocolized cohorts, respectively. There was no difference between cohorts in the number or location of rotors identified. In a subset of patients (n = 8), the rotor area was mapped from CARTO projections of the Topera basket (Abbott) using electroanatomic mapping to determine atrial area circumscribed by electrodes defining the rotor borders. The average rotor area was 3.1 ± 1.6 cm2, with no differences in RA and LA rotor areas. The rotor area comprised 1.2% ± 0.8% of total RA surface area, and 1.7% ± 0.8% of total LA surface area.
3.2. Temporal stability and acute rhythm changes
Of the patients assessed by protocolized mapping to determine temperospatial rotor stability, 10 out of 12 patients showed reproducibility in at least 1 atrium on a minimum of 2 out of 3 consecutive maps. In these 10 patients, a total of 33 stable rotors (mean, 3.3 ± 1.1) were mapped and ablated (13 [39.4%] in RA, 20 [60.6%] in LA). During ablation of these 33 stable rotors, there were 4 acute changes in rhythm (1 patient with conversion to AT during rotor ablation, 3 patients converting to NSR during subsequent catheter manipulation and PVI). In the 12 patients undergoing protocolized mapping, 17 rotors were found to be unstable (ie, not reproducible on sequential maps); these rotors were located in the RA (n = 12) and LA (n = 5).
In the entire cohort of 27 patients, we observed acute change in rhythm from AF to either AT or NSR in 8 patients. There was no significant difference in acute procedural change with presence or absence of temporal stability (4/10 [40%] vs 4/17 [23.5%], P = 0.42). When comparing patients with acute procedural change in rhythm to those without, sinus rhythm on presentation (62.5% vs 15.8%, P = 0.03), higher number of targeted rotors in LA (2.8 ± 1.2 vs 1.5 ± 1.0, P = 0.01), and higher total targeted rotors (3.8 ± 1.7 vs 2.5 ± 1.0, P = 0.02) were statistically significant on univariable analysis (Table 2).
Table 2.
Comparison of patients with acute procedural change versus those without any acute procedural change
| Variable | Acute Change, n = 8 | No Acute Change, n = 19 | P Value, Significance <0.05 |
|---|---|---|---|
| Age, y | 65.9 ± 6.4 | 63.8 ± 9.9 | 0.60 |
| Male sex | 8 (100%) | 14 (73.7%) | 0.28 |
| BMI, kg/m2 | 31.1 ± 6.1 | 30.2 ± 5.8 | 0.67 |
| CHA2DS2VASC | 2.1 ± 1.0 | 1.9 ± 1.6 | 0.36 |
| Hypertension | 5 (62.5%) | 13 (68.4%) | 1.00 |
| Coronary artery disease | 3 (37.5%) | 2 (10.5%) | 0.14 |
| Prior CVA | 2 (25%) | 1 (5.3%) | 0.20 |
| OSA | 2 (25%) | 5 (26.3%) | 1.00 |
| AF duration, y | 4.0 ± 4.4 | 4.6 ± 3.8 | 0.75 |
| AF on presentation | 3 (37.5%) | 15 (83.3%) | 0.06 |
| SR on presentation | 5 (62.5%) | 3 (15.8%) | 0.03 |
| LVEF, % | 54.1 ± 5.6 | 52.5 ± 8.3 | 0.64 |
| LA size, cm | 4.2 ± 0.6 | 4.8 ± 0.8 | 0.06 |
| Redo procedure | 3 (37.5%) | 11 (57.9%) | 0.42 |
| Fluoroscopy time, min | 62.3 ± 11.0 | 58.9 ± 11.4 | 0.49 |
| RF time, min | 47.1 ± 13.2 | 43.6 ± 15.2 | 0.60 |
| Total no. of rotors | 3.8 ± 1.7 | 2.5 ± 1.0 | 0.02 |
| Rotors in RA | 1 ± 1.2 | 1.1 ± 0.8 | 0.89 |
| Rotors in LA | 2.8 ± 1.2 | 1.5 ± 1.0 | 0.01 |
| Early recurrence (in blanking period) | 1 (12.5%) | 8 (42.1%) | 0.20 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CHA2DS2VASC, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category; CVA, cerebrovascular accident; LA, left atrium; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnea; RA, right atrium; RF, radiofrequency; SR, sinus rhythm.
3.3. Ablation outcomes
All patients had successful rotor elimination and PVI at the conclusion of the case. There were no procedure‐related complications reported in any patients. Early recurrence of AF/AT during the blanking period occurred in 9 (33.3%) patients, including 6 out of 15 (40%) in the first and 3 out of 12 (25%) in the second cohort. All patients were assessed a 6‐month follow‐up. Freedom from AT/AF at 6 months for all patients was 19 out of 27 (70.4%), including success rates of 11 out of 15 (73.3%) in the first cohort and 8 out of 12 (66.7%) in the protocolized cohort (P = 0.71). Two patients in the first cohort were still on an antiarrhythmic drug (AAD) at the time of the 6‐month assessment. All patients were assessed at the 12‐month follow‐up. One patient from group 2 was lost to follow‐up after the 6‐month visit and is excluded from the 1‐year outcome analysis. Freedom from AT/AF at 12 months for all patients was 10 out of 26 (38.5%), including success rates of 5 our of 15 (33.3%) in the first cohort and 5 out of 11 (45.5%) in the protocolized cohort (P = 0.53; Figure 2). Two patients in the first cohort and 1 patient in the protocolized cohort were still on an AAD at the time of assessment. Recurrent arrhythmias in the first cohort included AF (80%) and AT/flutter (20% of recurrence). In the second cohort, 4 patients experienced recurrent AF (66.6%) and 2 experienced AT/flutter (33.3%).
Figure 2.
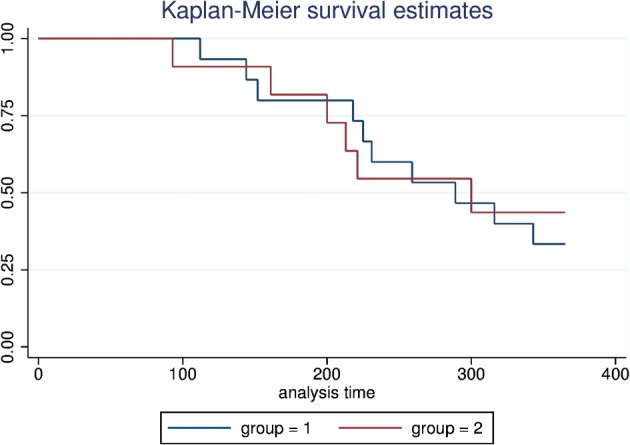
Kaplan‐Meier curves showing atrial fibrillation–free survival following pulmonary vein isolation and rotor ablation for the physician‐preference (blue) and protocolized (red) cohorts, with no statistically significant difference in freedom from recurrent arrhythmia at 1 year.
Univariate comparison for patients with and without recurrence of AF at 12 months for the entire cohort (n = 26, 1 patient lost to follow‐up) is presented in Table 3. Those with recurrence (n = 16) had larger LA size (5.0 ± 0.7 cm vs 4.1 ± 0.6 cm, P = 0.01), and more often presented in AF (87.5% vs 50%, P = 0.04). The duration of AF was longer in patients with recurrence but not statistically significant (5.3 ± 4.5 years vs 3.4 ± 2.7 years, P = 0.25). There was no difference in the number of rotors ablated (2.9 ± 1.4 vs. 2.8 ± 1.3, p = 0.80) and incidence of AF/AT during the blanking period (43.8% vs 30%, P = 0.48) in both the groups.
Table 3.
Univariate comparison of patients with and without recurrence at one year: includes patients without recurrence on antiarrhythmic drugs
| Variable | Patients With Recurrence, n = 16 | Patients Without Recurrence, n = 10 | P Value, Significance <0.05 |
|---|---|---|---|
| Age, y | 65.7 ± 9.8 | 61.7 ± 7.5 | 0.28 |
| Male sex | 12 (75%) | 9 (90%) | 0.35 |
| BMI, kg/m2 | 30.8 ± 6.5 | 29.4 ± 4.7 | 0.57 |
| CHA2DS2VASC | 2.1 ± 1.7 | 1.7 ± 1.1 | 0.49 |
| Hypertension | 12 (75%) | 5 (50%) | 0.19 |
| Diabetes mellitus | 0 (0%) | 1 (10%) | 0.19 |
| CHF | 2 (12.5%) | 0 (0%) | 0.25 |
| CAD | 2 (12.5%) | 2 (20%) | 0.61 |
| OSA | 3 (18.7%) | 3 (30%) | 0.51 |
| AF duration, y | 5.3 ± 4.5 | 3.4 ± 2.7 | 0.25 |
| LVEF, % | 53.0 ± 6.5 | 52.6 ± 9.8 | 0.92 |
| LA size, cm | 5.0 ± 0.7 | 4.1 ± 0.6 | 0.01 |
| Redo procedure | 9 (56.2%) | 5 (50%) | 0.75 |
| AF on presentation | 14 (87.5%) | 5 (50%) | 0.04 |
| Fluoroscopy time, min | 60.4 ± 10.4 | 57.4 ± 12 | 0.50 |
| RF time, min | 44.8 ± 16.1 | 44.0 ± 13 | 0.91 |
| Intraprocedure cardioversion | 15 (93.8%) | 7 (70%) | 0.10 |
| Total no. of rotors | 2.9 ± 1.4 | 2.8 ± 1.3 | 0.80 |
| Rotors in RA | 1.2 ± 0.8 | 0.7 ± 0.9 | 0.18 |
| Rotors in LA | 1.8 ± 1.1 | 2.1 ± 1.4 | 0.50 |
| Early recurrence | 7 (43.8%) | 3 (30%) | 0.48 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CHA2DS2VASC, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category; CHF, congestive heart failure; LA, left atrium; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnea; RA, right atrium; RF, radiofrequency.
4. DISCUSSION
In the current investigation, we report our initial experience with FIRM mapping and ablation of AF‐sustaining rotors in patients undergoing PVI. Our report is notable for 3 principle findings: (1) Establishing a protocol for identification of temperospatially stable rotors and for predetermined outcomes of ablation had no appreciable effect on acute or intermediate‐term procedure success. (2) Targeting increased numbers of rotors correlated with acute change in rhythm. (3) Short‐term outcomes at 6 months in this cohort was acceptable; however, longer follow‐up is needed to assess the true utility of this modality.
4.1. Rationale of formalized approach
Early reports assessing the utility of rotor mapping and ablation as an adjunctive or even an alternative strategy to PVI alone for AF patients have been encouraging.3, 4, 5, 6 Those positive results have not been reproduced in other investigations, 7, 8, 9 however, leading to impassioned public debates about the utility of rotor mapping and ablation.10, 11 Because a theme in this debate involves proper use of the basket catheter and mapping system for identification of stable or precessing rotors,12 we sought to take advantage of 2 trial periods with the Topera system. During the first epoch of Topera use, physicians were free to use the system at their discretion. During the second epoch, a uniform mapping protocol designed to identify stable rotor activity was adopted, as were standardized ablation outcomes (elimination of rotor activity with subsequent mapping). Our study was informed in part by the observation that in recurrent AF, organized sources have been suggested to be spatially stable over a time period of 1 to 2 years, and if these sources are carefully identified on initial ablation, this may lead to improved outcomes.13 Operators were the same during both phases of our study, and all are experienced ablationists; care was taken throughout to choose appropriately sized mapping basket catheters, and to use imaging liberally to ensure optimal basket positioning.
4.2. Outcomes
Despite these efforts, we had disappointing results. We found that in relatively few patients did targeting of rotor activity have any appreciable effect on rhythm. Acute procedural change including AF termination and slowing was achieved in 86% of patients in the CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Impulse and Rotor Modulation) trial.3 Other investigators have reported acute procedural success in up to 47% of patients.7, 8 We observed acute change in 8 out of 27 (29.6%) patients, with presentation in sinus rhythm and an increased total number of rotors targeted as the sole predictors of acute rhythm change. Protocolized mapping and (ideally) identification of true rotor activity had little effect on acute outcomes. This may be due to targeting of true rotors, and that rotor activity observed once, but not subsequently, represents a viable ablation target. An alternative explanation is that increased ablation burden—lesions that may be targeting ganglionic plexi, non‐PV triggers, complex fractionated electrograms sites, or bystander sites—leads to increased rates of rhythm change.
Our 12‐month follow‐up showed freedom from AF/AT achieved in 10 out of 26 (38.5%) patients, with 3 of them on an AAD at the time of assessment. There was no difference between cohorts in terms of freedom from AT/AF at 12 months. Assessment of arrhythmia recurrence in our cohort was performed at prespecified clinic visits, prompted by patient symptoms; routine Holter or implanted monitors were not used. This may lead to an underestimation of AT/AF recurrence, making our numbers even more sobering. Other investigators using the Topera system have achieved similar rates of success; Steinberg and colleagues found long‐term freedom from AF after TOPERA‐guided rotor ablation and PVI of 21% in a mix of paroxysmal and persistent patients (21% and 72%, respectively).9 Our groups of patients uniformly had advanced atriopathy, with either persistent or long‐standing persistent AF, a mean AF duration of 4.4 ± 4 years, large LA size (4.6 ± 0.8 cm), and a history of prior ablation procedures in 14 out of 27 (51.9%) patients. This may explain our relatively poor results.
Our investigation is confounded by the learning‐curve phenomenon. One might have expected that procedure times in the protocolized arm to be longer, given mandated repeated mapping and ablation endpoints. The fact that procedural time between the 2 groups was similar suggests that operator familiarity with the Topera system countered that effect, keeping procedural times in the second cohort similar to the first. In terms of procedural efficacy, operator learning‐curve effects should have contributed toward positive findings, with improved outcomes in the second cohort. No such improvement was seen, however, suggesting either that we are still early in our learning curve, that identification of temperospatially stable rotors is sufficiently achieved with single mapping runs, or that we do not fully understand the pathophysiological nature of the targets suggested by the Topera system, and whether ablating them has an appreciable, salutary effect.
4.3. Limitations
The principle limitation of this observational study is small sample size and lack of a control group. Outcomes may be impacted by selection bias, with FIRM mapping reserved for sicker patients not representative of the general patient population referred for AF ablation. Finally, operator variability may have played a role in outcomes, though this was addressed explicitly as much as possible through protocolization.
5. CONCLUSION
Focal impulse and rotor modulation–guided ablation in addition to PVI had unfavorable short‐ term outcomes in our cohort of sicker patients, but longer follow‐up is needed to fully assess its utility. Acute change in rhythm was achieved more often with a higher number of ablated rotors and in patients presenting in sinus rhythm. Protocolization to identify temperospatially stable rotors did not have an appreciable impact in short‐ and intermediate‐term outcomes.
Conflicts of interest
The authors declare no potential conflicts of interest.
Balouch M, Ipek EG, Chrispin J, Bajwa RJ, Zghaib T, Berger RD, Ashikaga H, Nazarian S, Marine JE, Calkins H and Spragg DD. Impact of rotor temperospatial stability on acute and one‐year atrial fibrillation ablation outcomes. Clin Cardiol. 2017;40:383–389. 10.1002/clc.22674
Funding information Edward St. John Foundation; Roz and Marvin H. Weiner and Family Foundation; Dr. Francis P. Chiaramonte Foundation; Marilyn and Christian Poindexter Arrhythmia Research Fund; Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund.
REFERENCES
- 1. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9:632–696.22386883 [Google Scholar]
- 2. Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow up? J Am Coll Cardiol. 2011;57:160–166. [DOI] [PubMed] [Google Scholar]
- 3. Narayan SM, Krummen DE, Shivkumar K et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller JM, Kowal RC, Swarup V, et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol. 2014;25:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Narayan SM, Baykaner T, Clopton P, et al. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow‐up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol. 2014;63:1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Narayan SM, Krummen DE, Clopton P, et al. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on‐treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation). J Am Coll Cardiol. 2013;62:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gianni C, Mohanty S, Di Biase L, et al. Acute and early outcomes of focal impulse and rotor modulation (FIRM)‐guided rotors‐only ablation in patients with nonparoxylsmal atrial fibrillation. Heart Rhythm. 2016;13(4):830–835. [DOI] [PubMed] [Google Scholar]
- 8. Buch E, Share M, Tung R, et al. Long‐term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: a multicenter experience. Heart Rhythm. 2016;13:636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steinberg JS, Shah Y, Bhatt A, et al. Focal impulse and rotor modulation: Acute procedural observations and extended clinical follow up [published online November 5, 2016]. Heart Rhythm. doi: 10.1016/j.hrthm.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 10. Jalife J, Filgueiras Rama D, Berenfeld O. Letter by Jalife et al regarding article, quantitative analysis of localized sources identified by focal impulse and rotor modulation mapping in atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8:1296–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buch E, Benharash P, Frank P, et al. Response to letter by Jalife et al regarding article, quantitative analysis of localized sources identified by focal impulse and rotor mapping in atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8:1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swarup V1, Baykaner T, Rostamian A, et al. Stability of rotors and focal sources for human atrial fibrillation: focal impulse and rotor mapping (FIRM) of AF sources and fibrillatory conduction. J Cardiovasc Electrophysiol. 2014;25:1284‐1292. [DOI] [PubMed] [Google Scholar]
- 13. Lalani GG, Coysh T, Baykaner T, et al. Organized sources are spatially conserved in recurrent compared to pre‐ablation atrial fibrillation: further evidence from non‐random electrical substrates. J Cardiovasc Electrophysiol. 2016;27:661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]


