Abstract
Background
The real‐world impact of remote pulmonary artery pressure (PAP) monitoring on New York Heart Association (NYHA) class improvement and heart failure (HF) hospitalization rate is presented here from a single center.
Hypothesis
Methods
Seventy‐seven previously hospitalized outpatients with NYHA class III HF were offered PAP monitoring via device implantation in a multidisciplinary HF‐management program. Prospective effectiveness analyses compared outcomes in 34 hemodynamically monitored patients to a group of similar patients (n = 32) who did not undergo device implantation but received usual care. NYHA class and 6‐minute walk testing were assessed at baseline and 90 days. All hospitalizations were collected after 6 months of the implantation date (average follow‐up, 15 months) and compared with the number of hospitalizations experienced prior to hemodynamic monitoring.
Results
Patients in both groups had similar distributions of age, sex, and ejection fraction. After 90 days, 61.8% of the monitored patients had NYHA class improvement of ≥1, compared with 12.5% in the controls (P < 0.001). Distance walked in 6 minutes increased by 54.5 meters in the monitored group (253.0 ± 25.6 meters to 307.4 ± 26.3 meters; P < 0.005), whereas no change was seen in the usual‐care group. After implantation, 19.4% of the monitored group had ≥1 HF hospitalization, compared with 100% who had been hospitalized in the year prior to implantation. The monitored group had a significantly lower HF hospitalization rate (0.16; 95% confidence interval: 0.06‐0.35 hospitalizations/patient‐year) compared with the year prior (1.0 hospitalizations/patient‐year; P < 0.001).
Conclusions
Hemodynamic‐guided HF management leads to significant improvements in NYHA class and HF hospitalization rate in a real‐world setting compared with usual care delivered in a comprehensive disease‐management program.
Keywords: Heart failure, cardiac transplantation, cardiomyopathy, myocarditis
1. INTRODUCTION
Heart failure (HF) is a complex clinical syndrome usually associated with multiple comorbid conditions that complicate long‐term management. It is not surprising that HF is cited as one of the most frequent reasons for hospitalization in the United States and Europe. Approximately 26 million people worldwide are affected by chronic HF.1 Significant HF disease modification can be achieved from drug and device therapies, shown to significantly improve clinical outcomes in randomized controlled trials, but implementation of this “layered” therapy is made difficult by polypharmacy, implantable electrical device therapies, and other medical needs these patients may have.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 Consensus exists from the medical community regarding using neurohormonal antagonists, implantable cardioverter‐defibrillators, cardiac resynchronization therapy devices, mechanical circulatory support, and transplant14 for selected patients with chronic HF. However, several prospective randomized clinical trials involving >6000 patients, evaluating telemonitoring of signs, symptoms, daily weights, or other implanted device–based technologies, along with frequent telephone communications with HF patients, have failed to impact the need for HF hospitalizations once guideline‐directed medical care is established.15, 16, 17, 18, 19, 20, 21
Clearly, new remote management strategies are needed to focus on maintaining stability of patients with chronic HF to decrease hospitalizations.22 Remote monitoring should account for several components of the pathophysiology contributing to decompensation, including detection of exogenous volume accumulation and endogenous volume redistribution along with pulmonary vascular remodeling, all of which result in increased pulmonary artery (PA) pressure, which then leads to dyspnea on exertion or more severe symptoms at rest, requiring intravenous medical therapy to return the patient to a less symptomatic state.
Monitored parameters must also provide actionable data that are useful not only for early detection of decompensation, but also to guide therapy.22 One such strategy, hemodynamic‐guided HF medical management using information from fully implantable sensors, is known to decrease hospitalization in previously hospitalized, persistently symptomatic patients. The CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial demonstrated 33% reduction in hospitalizations after an average of 31 months of clinical trial follow‐up.23, 24 Integration of this monitoring strategy, including the infrastructural needs and time dedicated to monitoring patients into actual clinical practice, was not well described in the CHAMPION trial. This study outlines implementation and initial outcomes of hemodynamic monitoring of patients with HF and an indication for the PA pressure‐sensor system used in the CHAMPION trial in an organized HF disease‐management program.
2. METHODS
2.1. Center description
The Northwell HF disease‐management program is a stand‐alone facility operating as part of a tertiary‐care hospital. The cardiology group consists of 20 cardiologists, mostly interventionalists and electrophysiologists, with one board‐certified advanced HF cardiologist. The advanced HF cardiologist's responsibility includes providing expert inpatient consultation for referred patients hospitalized with acutely decompensated HF, which usually averages about 30% of the 4000 annual acutely decompensated HF hospitalizations in the system. Two other full‐time registered nurses (RNs) are employed to support the advanced practice cardiologist in outpatient management. One RN assists full‐time in face‐to‐face office visits, and the second full‐time RN is dedicated to telephone triage of outpatients for remote management. This individual answers approximately 30 to 40 telephone calls daily and follows medication algorithms. Telephone calls after hours and on weekends are taken by group nurses with scripted interactions and email communication with the advanced HF cardiologist. Finally, the program offers a same‐day visit slots 3 days per week for acutely ill patients.
The center does not provide advanced therapies, such as left ventricular assist device implantation or cardiac transplantation. Electrophysiologic support is available for implantation of cardiac resynchronization therapy and implantable cardioverter‐defibrillator interventions. Device diagnostics are followed in both electrophysiology and HF clinical settings utilizing the common electronic health records.
2.2. Usual care
Patients who receive inpatient expert HF consultation are followed up with a visit within 7 days of discharge. Monthly auditing of compliance to this metric is monitored by the clinic's administration, with 100% compliance to 7‐day face‐to‐face follow‐up. All patients are assessed for guideline‐recommended medication and device therapies, and each is instituted in line with evidence supporting their use. Particularly, neurohormonal antagonists are titrated to doses used in clinical trials, when tolerated. All medical therapies are instituted for ≥3 months before device therapies are considered.
Once appropriate neurohormonal antagonism and device therapies are established at maximized dosing and settings, patients enter a chronic management phase in which frequent follow‐up and monitoring of weights and symptoms are performed utilizing both remote monitoring and face‐to‐face visits. The program currently has approximately 360 patient visits per month, usually follows ≥5 inpatient consultations daily, and receives approximately 30 to 40 telephone calls from outpatients daily. If intervention falls outside the standing‐order algorithm, information is then sent by email to the HF physician, who responds to the telephone triage RN for relaying information and action items back to the patient. Outpatient telephone calls made after normal office hours are received by central operators, who identify the patient as being followed in the HF disease‐management program. The treatment plan is then relayed back to the patient by the after‐hours operator. A copy of the email order is then placed in the patient's electronic health record.
Returning‐patient office visits are scheduled for 20 minutes each, whereas new patients are scheduled for 1 hour. Each new‐patient office visit includes a 6‐minute hall walk test (6MWT) and baseline Minnesota Living With Heart Failure Questionnaire. These assessments are periodically repeated and tabulated to determine progress.
Patient expectations in the long‐term management of their HF disease syndrome are clearly outlined, including the expectation for daily weight measurements, management of prescription refills, and instructions about when to call the office. Dietary consultations are offered to each new patient, along with psychological support to deal with the HF diagnosis. Additionally, the center emphasizes including family members in all educational activities, along with a social assessment.
2.3. Identifying hemodynamic‐monitoring candidates
Patients considered for long‐term hemodynamic monitoring are generally identified while being hospitalized for HF, especially if the patient has a history of predominantly New York Heart Association (NYHA) class III symptoms despite maximal medical and device therapies. Information on hemodynamic monitoring in general, with details about the implantation procedure, risks, benefits, and alternatives, is presented before discharge. A new discussion about recommendation for use of an implantable hemodynamic monitoring system is made if the patient persistently has class III HF symptoms during the postdischarge visit. In addition to identifying appropriate inpatients, some patients are referred to the center specifically for hemodynamic monitoring.
Details about implanting the hemodynamic‐monitoring sensor and the system for uploading information from the sensor are outlined in previous publications.23, 24, 25, 26, 27, 28, 29, 30, 31 At the time of implantation, details discussed during previous conversations are reiterated to both the patient and the family support group, including determining whether the patient resides in an area with cellular telephone coverage or if a landline will be required. Risks of the procedure are outlined based on results from the CHAMPION trial.23 In addition to procedural risks, patients and their families are informed of their responsibilities to upload daily information as part of their new routine for HF disease management. Medication lists are reviewed and verified with the patient, and specific medications most likely to be adjusted remotely are identified to the patient and the family (generally, diuretics and vasodilators). The patients are instructed that they will not hear from the center with every upload, but only when medications must be changed to maintain their stability. The family members and the patient must demonstrate proficiency in setting up the interrogation unit and successful interrogation of the implanted device prior to discharge. Telephone numbers for troubleshooting are provided to the patient and family. Patients’ anticoagulation needs are assessed and dual antiplatelet therapy (DAPT) is instituted for 30 days after implantation, if it was not already indicated. The concordant control group used for comparison in this study consisted of patients who were previously hospitalized and had persistent NYHA class III symptoms but had contraindication to sensor implantation—such as an active infection, inability to tolerate DAPT, or morbid obesity—or who did not provide informed consent. All control‐group patients received usual care in the organized HF disease‐management program.
2.4. Hemodynamic‐monitoring workflow
Medical management of hemodynamic information starts with information from the right heart catheterization, specifically evaluating the PA diastolic pressure, pulmonary capillary wedge pressure, right atrial pressure, and transpulmonary gradient as a marker of resistance. The goal of this evaluation is to determine what the baseline pressures are and what component, volume and/or resistance, would be targeted with subsequent medication changes.
After discharge, patients then upload daily from home to begin the process of chronic hemodynamic monitoring. Particular attention is given to hemodynamic trends in patients whose medications were recently changed to determine need for personalization of hemodynamically active medications, primarily diuretics and vasodilators. When reviewed pressures are stable and at goal, the management team reevaluates neurohormonal intervention to ensure maximal target dosing is achieved. Pressure targets and medication‐change criteria are outlined in the Table 1.
Table 1.
Improved QoL scores and exercise capacity with remote PAP monitoring in patients with chronic HF
| Monitored Group | Usual‐Care Group | P Value | |
|---|---|---|---|
| NYHA class improvement | −0.74 ± 0.67 | −0.13 ± 0.34 | <0.00011 |
| Patients with no NYHA improvement, % | 38.2 | 87.5 | |
| Patients with NYHA class improvement, % | 61.8 | 12.5 | |
| Patients with improvement of 1 class | 50.0 | 12.5 | |
| Patients with improvement of 2 classes | 11.8 | 0.0 | |
| HFH events, n | 1 | 10 | |
| Patients with a HFH, n (%) | 1 (2.9) | 6 (18.8) | |
| Rate of HFHs/patient‐year | 0.119 | 1.268 | IRR: 0.094, 95% CI: 0.002‐0.662, P = 0.00492 |
Abbreviations: CI, confidence interval; HF, heart failure; HFH, heart failure hospitalization; IRR, incidence rate ratio; NYHA, New York Heart Association; PAP, pulmonary artery pressure; QoL, quality of life.
P value from 2‐sample, 2‐sided t test.
IRR, CI, and P value from Poisson test of rates.
2.5. Time and resource dedication
No new employees have been added to the existing HF management program to accommodate hemodynamic‐monitoring information flow. At odds with the methods of the CHAMPION trial,25 which asked investigators to weekly review daily uploads, this center's routine is to review pressures daily. The RN assigned to assist in face‐to‐face clinic visits reviews pressures each weekday from the patient care network website (http://www.Merlin.net) of the hemodynamic sensor manufacturer (St. Jude Medical Inc., Sylmar, California) and transcribes the daily pressures into a spreadsheet. Pressures that are out of range or trending upward according to the protocol are identified. This spreadsheet is then reviewed with the advanced HF cardiologist, with new orders relayed to the nurse, who then communicates to the patient. Pressure trends are analyzed, and an actionable rise is considered present when PA diastolic pressures are persistently ≥3 mm Hg above baseline for 3 days. The manufacturer's website allows the user to determine pressure thresholds that trigger automatic email notification of pressure excursions above or below the threshold. Automatic‐notification emails are sent to the HF physician, who reviews them and calls patients, if needed, on weekends or holidays. The website also has a section to record medications, which is updated regularly, along with brief notations on the webpage of any change in medication or other intervention. All therapy delivery‐device diagnostics (cardiac resynchronization therapy or implantable cardioverter‐defibrillator) from the hemodynamic sensor's manufacturer are also displayed on the webpage for time‐synched review.
2.6. Patient population
For the current analyses, the first 34 hemodynamically monitored patients to reach 6 months of follow‐up were assessed and compared with 32 patients with an indication for hemodynamic monitoring but who were unable to undergo sensor implantation. The reasons an implant could not be offered were inability to tolerate DAPT agents, morbid obesity, glomerular filtration rate <25 mL/min/1.73 m2, and refusal to consent. This group received usual care delivered at the center as described and served as a comparison group.
2.7. Outcome parameters
All patients were assessed at baseline and functional parameters were compared after 90 days of follow‐up. Functional parameters including NYHA symptoms class, 6MWT, and renal function were evaluated using 2‐sided, 2‐sample t tests and paired t tests with a 5% level of significance. Hospitalizations were evaluated in the monitored group using patients as their own controls by comparing their rate of HF hospitalization over an average follow‐up of 15 months to their rate in the year prior to implantation using a 2‐sided, 1‐sample test of Poisson rates.
3. RESULTS
As of January 13, 2016, seventy‐seven patients who met the US Food and Drug Administration–approved indication for hemodynamic monitoring underwent sensor implantation. All patients were successfully implanted with the sensor, and no sensor failures have occurred. No short‐term or long‐term complications associated with implantation of the hemodynamic sensor have been identified after the first 77 implantations. The first implantation was in August 2014 and the last was on January 13, 2016, which equates to 1.05 implantations per week for a total of 73 weeks. During this time, the HF disease‐management program evaluated a total of 500 individual inpatients with acutely decompensated HF.
Demographic information concerning the first 34 hemodynamically monitored patients to reach 90 days of follow‐up and the concomitant usual‐care patients is presented in the Table 1. All patients had NYHA class III symptoms and a previous hospitalization in the past 12 months in accordance with FDA indications. More patients in the usual‐care group were female (41% control vs 24% treatment). Most patients had HF with reduced ejection fraction.
3.1. Hospitalization rates
All patients were hospitalized in the previous 12 months prior to sensor implantation. During the average of 15 months following implantation, in the 31 patients in the monitored group with complete pre‐ and post‐implant hospitalization records (37.3 patient‐years of follow‐up), only 6 monitored patients required rehospitalization for HF (0.161; 95% confidence interval: 0.06‐0.35 hospitalizations/patient‐year) compared with their rate in the year prior (1.0 hospitalizations/patient‐year; P < 0.001). This translated into an 84% lower HF hospitalization rate compared with the time period during which PA pressures were unmonitored (Figure 1).
Figure 1.
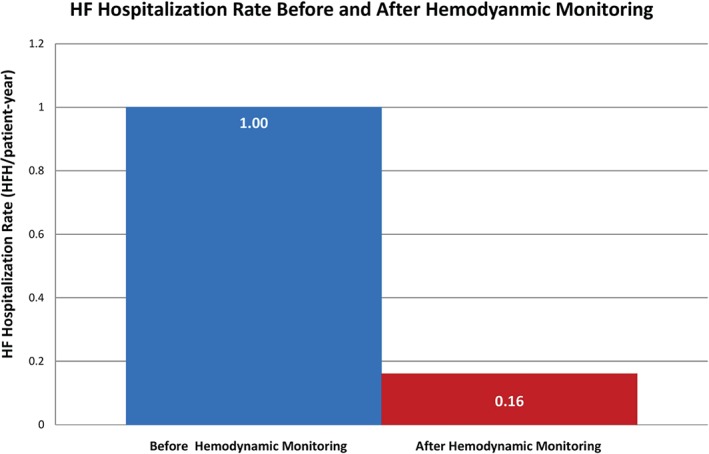
HF hospitalization rates before hemodynamic monitoring (blue bar, n = 31) and after hemodynamic monitoring (red bar, n = 31). Abbreviations: HF, heart failure; HFH, heart failure hospitalizations.
3.2. Functional assessments
NYHA symptom class improved significantly in the monitored group (−0.74 ± 0.67 average change) compared with the usual‐care group (−0.13 ± 0.34 average change, P < 0.0001; Table 1, Figure 2). Sixty‐three percent of monitored patients improved ≥1 NYHA classification, compared with 12.5% of the usual‐care group (P < 0.002). Conversely, 38% of the monitored group had no improvement in NYHA classification, which was lower than the 88% of usual‐care group patients with no improvement in symptoms class (P < 0.0001; Table 1). Fifty percent of monitored patients improved to NYHA class II, whereas 12% improved to NYHA class I. In contrast, 12% of patients in the usual‐care group were class II after 6 months and none were class I (Figure 2).
Figure 2.
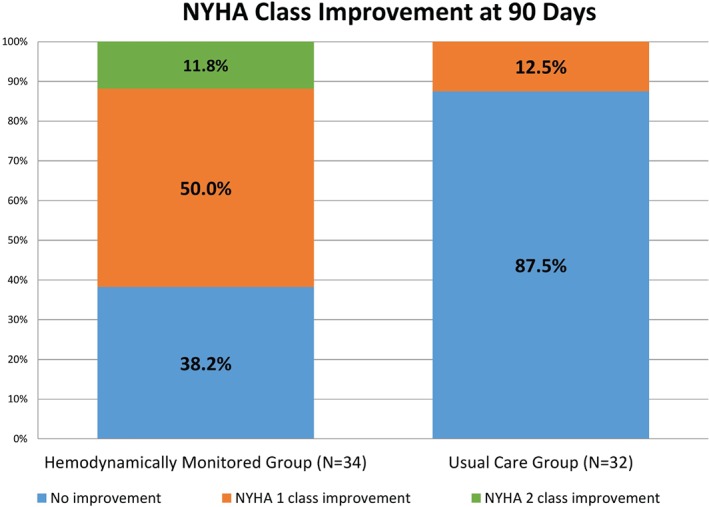
Change in NYHA symptom classification in patients with hemodynamic monitoring (left panel) compared with those receiving usual care (right panel). Abbreviations: NYHA, New York Heart Association.
No difference in 6MWT was seen at baseline between the monitored (253.0 ± 25.6 meters; range, 31–472 meters) and usual‐care (276 ± 18 meters; range, 90–457 meters; P = 0.41) groups. The 6MWT distances increased by 54.5 meters in the monitored group after 90 days of treatment (307.4 ± 26.3 meters; range of increase, 38–600 meters; P < 0.005), whereas the usual‐care group had no functional improvement (278.3 ± 19 meters, difference of +2.3 ± 8.4 meters; P = not significant; Figure 3).
Figure 3.
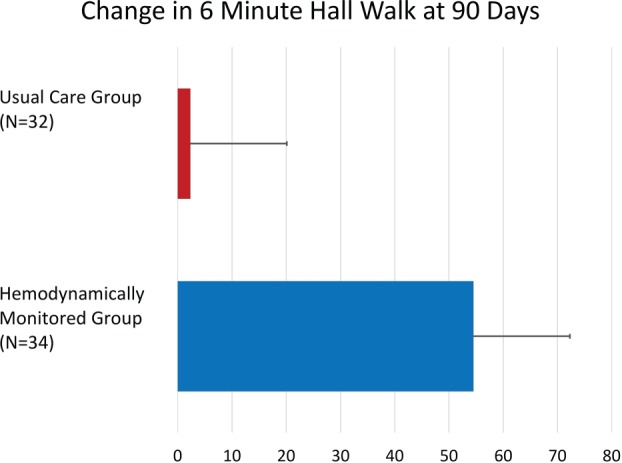
Changes in 6‐minute walk test after 90 days of usual care (n = 32) vs those patients who underwent hemodynamic monitoring (n = 32). The difference between groups was significant (P < 0.005).
3.3. Time allocation for hemodynamic monitoring
All implantations were performed by the HF physician in a hospital‐based cardiac catheterization laboratory. The typical implantation required approximately 30 minutes of catheterization laboratory time. Approximately 30 minutes of post‐implantation education was provided by nursing personnel to each patient and his or her family members. Daily reviews of the 77 monitored patients requires approximately 1 hour of dedicated RN time and 20 minutes of the HF physician's time. This time allocation was determined by sampling the first 10 reviews and multiplying by the exponential equivalent per additional 10 patients.
4. DISCUSSION
This case series with comparison to concomitant control demonstrates that integrating implantable hemodynamic monitoring into a comprehensive HF disease‐management program significantly reduces hospitalizations when compared with usual care. Data from this study are important, as they address several important questions not answered in the CHAMPION trial. In fact, the first‐year implant volumes at this center were higher than any single center achieved in the CHAMPION trial. Additionally, resources needed to perform hemodynamic monitoring as described in the protocol were not available from the CHAMPION trial. Methods in place at this center were developed based on the CHAMPION protocol25 and are expected to be reproducible at other centers.
Hemodynamic‐guided HF management was achieved in the Northwell system without adding new employees; it was accomplished by reallocation of time that was already being spent managing HF patients. The impact of hemodynamic‐guided HF management in this busy center resulted in significant reduction in subsequent hospitalizations, improved NYHA symptoms classification, and improved functional status as measured by the 6MWT. These data are important, as symptom‐change and functional‐status parameters were not reported in the CHAMPION trial.
Additionally, the CHAMPION trial was performed in 64 centers with locally constructed HF disease‐management programs. The exact metrics of these centers were not reported, which makes “usual care” difficult to quantify in the trial. The benefit of the current study is that “usual care” was clearly described in detail. Despite a rigorous program of monitoring weights and symptoms in this center, hemodynamic monitoring had a superior outcome. These findings seem consistent with large prospective HF‐management trials focusing on intense telemonitoring of weights, signs, and symptoms of worsening HF to provide an opportunity to intervene earlier and prevent hospitalizations. These trials consistently demonstrate no impact on HF hospitalizations, even after randomizing >6000 patients in prospective evaluation.15, 16, 17, 18, 19, 20, 21 It is not surprising, then, that intense monitoring of these parameters in the usual‐care group in this study was associated with a residual high risk for hospitalization.
4.1. Study limitations
Rigorous data collection and evaluation in the current study were employed for internal evaluation of the value of instituting a remote hemodynamic‐monitoring service at this institution. The concomitant control group was not the result of randomization, and the clinical reasons for not undergoing sensor implantation may limit comparison of hospitalization rates between groups. Event rates in the usual‐care group after 6 months of follow‐up (0.63 events/patient/6 months) were numerically higher than reported rates in the CHAMPION control group (0.44 events/patient/6 months).23 However, functional status assessed by 6MWT and NYHA classification, along with baseline demographics, were reasonably balanced between groups at baseline. The magnitude of event‐rate reduction noted should be interpreted with these limitations in mind.
5. CONCLUSION
Implementing remote hemodynamic monitoring in a real‐world setting of a comprehensive HF‐management program is efficient and can be accomplished with thoughtful reallocation of infrastructural resources already in place to manage these patients. Important clinical outcomes in a real‐world setting can be of similar magnitude, as predicted by the results of the CHAMPION clinical trial. Hemodynamic monitoring of indicated patients is a novel technology that aids in the successful management of HF patients with persistent NYHA class III symptoms and a previous hospitalization.
Conflicts of Iinterest
The authors declare no potential conflicts of interest.
Jermyn R, Alam A, Kvasic J, Saeed O and Jorde U. Hemodynamic‐guided heart‐failure management using a wireless implantable sensor: Infrastructure, methods, and results in a community heart failure disease‐management program. Clin Cardiol. 2017;40:170–176. 10.1002/clc.22643
REFERENCES
- 1. Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. SOLVD Investigators . Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 3. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet . 1999;353:2001–2007. [PubMed] [Google Scholar]
- 4. Pitt B, Zannad F, Remme WJ, et al; Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med . 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 5. Packer M, Poole‐Wilson PA, Armstrong PW, et al; ATLAS Study Group . Comparative effects of low and high doses of the angiotensin converting enzyme inhibitor lisinopril on morbidity and mortality in chronic heart failure. Circulation. 1999;100:2312–2318. [DOI] [PubMed] [Google Scholar]
- 6. Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group . Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med . 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 7. Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators . A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med . 2001;345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 8. Maggioni AP, Anand I, Gottlieb SO, et al; Val‐HeFT Investigators . Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin‐converting enzyme inhibitors. J Am Coll Cardiol . 2002;40:1414–1421. [DOI] [PubMed] [Google Scholar]
- 9. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees . Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM‐Overall programme [published correction appears in Lancet. 2009;(9703):1744]. Lancet . 2003;362:759–766. [DOI] [PubMed] [Google Scholar]
- 10. Moss AJ, Zareba W, Hall WJ, et al; MADIT‐II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med . 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 11. Cleland JG, Daubert JC, Erdmann E, et al; CARE‐HF Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med . 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 12. Bristow MR, Saxon LA, Boehmer J, et al; COMPANION Investigators . Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med . 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 13. Bardy GH, Lee KL, Mark DB, et al; SCD‐HeFT Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure [published correction appears in N Engl J Med. 2005;352:2146]. N Engl J Med . 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 14. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 15. Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure [published corrections appear in N Engl J Med. 2011;364:490 and N Engl J Med. 2013;369:1869]. N Engl J Med . 2010;363:2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koehler F, Winkler S, Schieber M, et al; TIM‐HF Investigators . Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the Telemedical Interventional Monitoring in Heart Failure Study (TIM‐HF). Circulation . 2011;123:1873–1880. [DOI] [PubMed] [Google Scholar]
- 17. Cleland JG, Louis AA, Rigby AS, et al; TEN‐HMS Investigators . Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans‐European Network‐Home‐Care Management System (TEN‐HMS) study. J Am Coll Cardiol . 2005;45:1654–1664. [DOI] [PubMed] [Google Scholar]
- 18. Black JT, Romano PS, Sadeghi B, et al; BEAT‐HF Research Group . A remote monitoring and telephone nurse coaching intervention to reduce readmissions among patients with heart failure: study protocol for the Better Effectiveness After Transition–Heart Failure (BEAT‐HF) randomized controlled trial. Trials. 2014;15:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Angermann CE, Störk S, Gelbrich G, et al. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ Heart Fail. 2012;5:25–35. [DOI] [PubMed] [Google Scholar]
- 20. van Veldhuisen DJ, Braunschweig F, Conraads V, et al; DOT‐HF Investigators . Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation . 2011;124:1719–1726. [DOI] [PubMed] [Google Scholar]
- 21. Böhm M, Drexler H, Oswald H, et al. Effect of implanted device‐based impedance monitoring with telemedicine alerts on mortality and morbidity in heart failure: results from the OptiLink HF study. Presented at: European Society of Cardiology (ESC) 2015 Congress; September 1, 2015; London, UK. Abstract 5057.
- 22. Desai A, Stevenson LW. Connecting the circle from home to heart‐failure disease management. N Engl J Med. 2010;363:2364–2367. [DOI] [PubMed] [Google Scholar]
- 23. Abraham WT, Adamson PB, Bourge RC, et al; CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial [published correction appears in Lancet. 2012;379:412]. Lancet . 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 24. Abraham WT, Stevenson LW, Bourge RC, et al; CHAMPION Trial Study Group . Sustained efficacy of pulmonary artery pressures to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet. 2016;387:453–461. [DOI] [PubMed] [Google Scholar]
- 25. Adamson PB, Abraham WT, Aaron M, et al. CHAMPION trial rationale and design: the long‐term safety and clinical efficacy of a wireless pulmonary artery pressure monitoring system. J Card Fail. 2011;17:3–10. [DOI] [PubMed] [Google Scholar]
- 26. Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–944. [DOI] [PubMed] [Google Scholar]
- 27. Benza RL, Raina A, Abraham WT, et al. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant. 2015;34:329–337. [DOI] [PubMed] [Google Scholar]
- 28. Krahnke JS, Abraham WT, Adamson PB, et al. Heart failure and respiratory hospitalizations are reduced in heart failure subjects with chronic obstructive pulmonary disease using an implantable pulmonary artery pressure monitoring device. J Card Fail. 2014;21:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raina A, Abraham WT, Adamson PB, et al. Limitations of right heart catheterization in the diagnosis and risk stratification of patients with pulmonary hypertension related to left heart disease: insights from a wireless pulmonary artery pressure monitoring system. J Heart Lung Transplant. 2015;34:438–447. [DOI] [PubMed] [Google Scholar]
- 30. Castro PF, Concepción R, Bourge RC, et al. A wireless pressure sensor for monitoring pulmonary artery pressure in advanced heart failure: initial experience. J Heart Lung Transplant. 2007;26:85–88. [DOI] [PubMed] [Google Scholar]
- 31. Verdejo HE, Castro PF, Concepción R, et al. Comparison of a radiofrequency‐based wireless pressure sensor to Swan‐Ganz catheter and echocardiography for ambulatory assessment of pulmonary artery pressure in heart failure. J Am Coll Cardiol. 2007;50:2375–2382. [DOI] [PubMed] [Google Scholar]


